Abstract
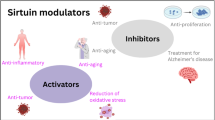
Sirtuins supercede their genre of histone deacetylases such that the seven mammalian sirtuins have been implicated in processes as diverse as the regulation of energy metabolism, gene expression, cell survival and even aging. Given this plethora of sirtuin functions, there is considerable energy being devoted to harnessing the activities of the various sirtuins because of their potential to address a broad range of diseases including obesity, diabetes, cancer, inflammation, and cardiovascular, neuronal and age-related diseases. In this chapter, we review the activities of the mammalian sirtuins in the context of their subcellular localization and follow this with a review of the compounds currently known to activate or inhibit sirtuins. Finally, we discuss the degree to which existing data support the use of sirtuin-based therapies for the treatment of human diseases.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Adams JD Jr, Klaidman LK (2007) Sirtuins, nicotinamide and aging: a critical review. Lett Drug Design Disc 4:44–48
Ahuja N, Schwer B, Carobbio S et al. (2007) Regulation of insulin secretion by SIRT4, a mitochondrial ADP-ribosyltransferase. J Biol Chem 282:33583–33592
Araki T, Sasaki Y, Milbrandt J (2004) Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science 305:1010–1013
Ashrafn N, Zino S, MacIntyre A et al. (2006) Altered sirtuin expression is associated with node-positive breast cancer. Br J Cancer 95:1056–1061
Barger JL, Kayo T, Pugh TD et al. (2008a) Short-term consumption of a resveratrol-containing nutraceutical mixture mimics gene expression of long-term caloric restriction in mouse heart. Exp Gerontol 43:859–866
Barger JL, Kayo T, Vann JM et al. (2008b) A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS ONE 3:e2264
Barzilai N, Banerjee S, Hawkins M et al. (1998) Caloric restriction reverses hepatic insulin resistance in aging rats by decreasing visceral fat. J Clin Invest 101:1353–1361
Baur JA, Pearson KJ, Price NL et al. (2006) Resveratrol improves health and survival of mice on a high calorie diet. Nature 444:337–342
Bedalov A, Gatbonton T, Irvine WP et al. (2001) Identification of a small molecule inhibitor of Sir2p. Proc Natl Acad Sci USA 98:15113–15118
Bellizzi D, Rose G, Cavalcante P et al. (2005) A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is associated with survival at oldest ages. Genomics 85:258–263
Biacsi R, Kumari D, Usdin K (2008) SIRT1 inhibition alleviates gene silencing in Fragile X mental retardation syndrome. PLoS Genet 4:e1000017
Bordone L, Motta MC, Picard F et al. (2006) Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic β-cells. PLoS Biol. 4:210–220
Borra, MT, Smith BC, Denu JM (2005) Mechanism of human SIRT1 activation by resveratrol. J Biol Chem 280:17187–17195
Bouras, T, Fu, M, Sauve, AA et al. (2005) SIRT1 deacetylation and repression of p300 involves lysine residues 1020/1024 within the cell cycle regulatory domain 1. J Biol Chem 280:10264–10276
Breen DM, Sanli T, Giacca A, Tsiani E (2008) Stimulation of muscle cell glucose uptake by resveratrol through sirtuins and AMPK. Biochem Biophys Res Commun 374:117–122
Brittain D, Weinmann H, Ottow E (2007) Recent advances in the medicinal chemistry of hystone deacetylase inhibitors. Ann Rep Med Chem 42:337–348
Brunet A, Sweeney LB, Sturgill JF et al. (2004) Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303:2011–2015
Catoire H, Pasco MY, Abu-Baker A et al. (2008) Sirtuin inhibition protects from the polyalanine muscular dystrophy protein PABPN1. Hum Mol Genet 17:2108–2117
Celotti E, Ferrarini R, Zironi R, Conte, LS (1996) Resveratrol content of some wines obtained from dried Valpolicella grapes: Recioto and Amarone. J Chromatogr A 730:47–52
Chen D, Bruno J, Easlon E et al. (2008) Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev 22:1753–1757
Civitarese AE, Carling S, Heilbronn LK et al. (2007) Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med 4:484–494
Cohen HY, Miller C, Bitterman KJ et al. (2004) Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305:390–392
Crujeiras AB Parra D, Goyenechea E, Martínez JA (2008) Sirtuin gene expression in human mononuclearcells is modulated by caloric restriction. Eur J Clin Invest 38: 672–678
Crunkhorn S, Dearie F, Mantzoros C et al. (2007) Peroxisome proliferator activator receptor gamma coactivator-1 expression is reduced in obesity: potential pathogenic role of saturated fatty acids and p38 mitogen-activated protein kinase activation. J Biol Chem 282:15439–15450
Csiszar A, Labinskyy N, Pinto JT et al. (2009) Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol 297:H13–20
Csiszar A, Labinskyy N, Podlutsky A et al. (2008) Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol 294:H2721-H2735
Das S, Das DK (2007) Resveratrol: a therapeutic promise for cardiovascular diseases. Recent Patents Cardiovasc Drug Discov 2:133–138
Denu JM (2005) Vitamin B3 and sirtuin function. Trends Biochem Sci 30:479–483
Dong F, Ren J (2007) Fidarestat improves cardiomyocyte contractile function in db/db diabetic obese mice through a histone deacetylase Sir2-dependent mechanism. J Hypertens 25: 2138–2147
Elliott PJ, Jirousek M (2008) Sirtuins: Novel targets for metabolic disease. Curr Opin Inves Drugs 9:371–378
Feige JN, Lagouge M, Canto C et al. (2008) Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab 8:347–358
Finnin MS, Donigian JR, Pavletich NP (2001) Structure of the histone deacetylase SIRT2. Nat Struct Biol 8:621–625
Firestein R, Blander G, Michan S et al. (2008) The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS ONE 3:e2020
Ford E, Voit R, Liszt G et al. (2006) Mammalian Sir2 homolog SIRT7 is an activator of RNA polymerase I transcription. Genes Dev 20:1075–1080
Frye RA (2000) Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Comunn 273:793–798
Gan L (2007) Therapeutic potencial of sirtuin-activating compounds in alzheimer’s disease. Drug News Perspect 20:233–239
Gray SG, Ekström TJ (2001) The human histone deacetylase family. Exp Cell Res 262:75–83
Grob A, Roussel P, Wright JE et al. (2009) Involvement of SIRT7 in resumption of rDNA transcription at the exit from mitosis. J Cell Sci 122:489–498
Grozinger CM, Chao ED, Blackwell HE et al. (2001) Identification of a class of small molecule inhibitors of the sirtuin family of NAD-dependent deacetylases by phenotypic screening. J Biol Chem 276:38837–38843
Haberland M, Montgomery RL, Olson EN (2009) The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet 10 32–42
Haigis MC, Mostoslavsky R, Haigis KM et al. (2006) SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell 126:941–954
Hallows WC, Lee S, Denu JM (2006) Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc Natl Acad Sci USA 103:10230–10235
Heltweg B, Gatbonton T, Schuler AD et al. (2006) Antitumor activity of a small-molecule inhibitor of human silent information regulator 2 enzymes. Cancer Res 66:4368–4377
Hirao M, Posakony J, Nelson M et al. (2003) Identification of selective inhibitors of NAD+-dependent deacetylases using phenotypic screens in yeast. J Biol Chem 278:52773–52782
Hiratsuka M, Inoue T, Toda T et al. (2003) Proteomics-based identification of differentially expressed genes in human gliomas: down-regulation of SIRT2 gene. Biochem Biophys Res Commun 309:558–566
Howitz KT, Bitterman KJ, Cohen HY et al. (2003) Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425:191–196
Inoue T, Hiratsuka M, Osaki M et al. (2007) SIRT2, a tubulin deacetylase, acts to block the entry to chromosome condensation in response to mitotic stress. Oncogene 26:945–957
Kaeberlein M (2008) The ongoing saga of sirtuins and aging. Cell Metab 8:4–5
Kanfi Y, Shalman R, Peshti V et al. (2008) Regulation of SIRT6 protein levels by nutrient availability. FEBS Lett 582:543–548
Kawahara TL, Michishita E, Adler AS et al. (2009) SIRT6 links histone H3 lysine 9 deacetylation to NF-κB dependent gene expression and organismal lifespan. Cell 136:62–74
Kim EJ, Kho JH, Kang MR, Um SJ (2007a) Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Mol Cell 2:277–290
Kim D, Nguyen MD, Dobbin MM et al. (2007b) SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J 26:3169–3179
Lagouge M, Argmann C, Gerhart-Hines Z et al. (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 127:1109–1122
Lain S, Hollick JJ, Campbell J et al. (2008) Discovery, in vivo activity, and mechanism of action of a small-molecule p53 activator. Cancer Cell 13:454–463
Lara E, Calvanese V, Altucci L et al. (2009) Salermide, a Sirtuin inhibitor with a strong cancer-specific proapoptotic effect. Oncogene 28:781–791
Li W, Zhang B, Tang J et al. (2007) Sirtuin 2, a mammalian homolog of yeast silent information regulator-2 longevity regulator, is an oligodendroglial protein that decelerates cell differentiation through deacetylating α-tubulin. J Neurosci 27:2606–2616
Lim CS. (2006) SIRT1: tumor promoter or tumor suppressor? Med Hypotheses 67: 341–344
Lin SJ, Ford E, Haigis M et al. (2004) Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev 18:12–16
Liszt G, Ford E, Kurtev M, Guarente L. (2005) Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J Biol Chem 280:21313–21320
Liu FC, Day YJ, Liou JT et al. (2008a) Sirtinol attenuates hepatic injury and pro-inflammatory cytokine production following trauma-hemorrhage in male Sprague-Dawley rats. Acta Anaesthesiol Scand 52:635–640
Liu L, Wang Y, Lam KS, Xu A (2008b) Moderate wine consumption in the prevention of metabolic syndrome and its related medical complications. Endocr Metab Immune Disord Drug Targets 8:89–98
Liu Y, Dentin R, Chen D et al. (2008c) A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature 456:269–273
Lombard DB, Alt FW, Cheng HL et al. (2007) Mammalian Sir2 Homolog SIRT3 Regulates Global Mitochondrial Lysine Acetylation. Mol Cell Biol 27:8807–8814
Mai A, Massa S, Lavu S, et al. (2005) Design, synthesis, and biological evaluation of sirtinol analogues as class III histone/protein deacetylase (Sirtuin) inhibitors. J Med Chem 48:7789–7795
Mattson MP, Duan W, Guo Z (2003) Meal size and frequency affect neuronal plasticity and vulnerability to disease: cellular and molecular mechanisms. J Neurochem 84:417–431
Medda F, Russell RJ, Higgins M et al. (2009) Novel cambinol analogs as sirtuin inhibitors: synthesis, biological evaluation, and rationalization of activity. J Med Chem 52:2673–2682
Michan S, Sinclair D (2007) Sirtuins in mammals: insights into their biological function. Biochem J 404:1–13
Michishita E, McCord RA, Berber E (2008) SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature 452:492–496
Michishita E, Park JY, Burneskis JM et al. (2005) Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell 16:4623–4635
Milne JC, Lambert PD, Schenk S et al. (2007) Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 50:712–716
Milne JC, Denu JM (2008) The Sirtuin family: therapeutic targets to treat diseases of aging. Curr Opin Chem Biol 12:11–17
Milne JC, Lambert PD, Smith JJ et al. (2008) Activation of the protein deacetylase SIRT1 blunts pro-inflammatory pathways in vivo. In: Abstracts of the American Diabetes Association’s 68th Annual Scientific Sessions, San Francisco, CA, June 2008
Min J, Landry J, Sternglanz R, Xu RM (2001) Crystal structure of a SIR2 homolog-NAD complex. Cell 105:269–279
Mostoslavsky R, Chua KF, Lombard DB et al. (2006) Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124:315–329
Nakamura Y, Ogura M, Tanaka D, Inagaki N (2008) Localization of mouse mitochondrial SIRT proteins: shift of SIRT3 to nucleus by co-expression with SIRT5. Biochem Biophys Res Commun 366:174–179
Nayagam VM, Wang X, Tan YC et al. (2006) SIRT1 modulating compounds from high-throughput screening as anti-inflammatory and insulin-sensitizing agents. J Biomol Screen 11:959–967
Nemoto S, Fergusson MM, Finkel T (2005) SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1 α. J Biol Chem 280:16456–16460
Neugebauer RC, Uchiechowska U, Meier R et al. (2008) Structure-activity studies on splitomicin derivatives as sirtuin inhibitors and computational prediction of binding mode. J Med Chem 51:1203–1213
North BJ, Marshall BL, Borra MT et al. (2003) The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell 11:437–444
North BJ, Verdin E (2004) Sirtuins: Sir2-related NAD-dependent protein deacetylases. Genome Biol. 5:224
Onyango, P. Celic, I McCaffery, JM et al. (2002) SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc Natl Acad Sci USA 99:13653–13658
Ota H, Tokunaga E, Chang K et al. (2006) Sirt1 inhibitor, Sirtinol, induces senescence-like growth arrest with attenuated Ras-MAPK signaling in human cancer cells. Oncogene 25:176–185
Outeiro TF, Kontopoulos E, Altmann SM et al. (2007) Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science 317:516–519
Outeiro TF, Marques O, Kazantsev A (2008) Therapeutic role of sirtuins in neurodegenerative disease. Biochim Biophys Acta 1782:363–369
Pagans S, Pedal A, North BJ et al. (2005) SIRT1 regulates HIV transcription via Tat deacetylation. PLoS Biol 3:e41
Pearson KJ, Baur JA, Lewis KN et al. (2008) Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 8:157–168
Perabo FG, Müller SC (2005) New agents in intravesical chemotherapy of superficial bladder cancer. Scand J Urol Nephrol 39:108–116
Picard F, Kurtev M, Chung N et al. (2004) Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 429:771–776
Posakony J, Hirao M, Stevens S et al. (2004) Inhibitors of Sir2: evaluation of splitomicin analogues. J Med Chem 47:2635–2644
Qin W, Yang T, Ho L et al. (2006) Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem 281:21745–21754
Reiling E, van Vliet-Ostaptchouk JV, van’t Riet E et al. (2009) Genetic association analysis of 13 nuclear-encoded mitochondrial candidate genes with type II diabetes mellitus: the DAMAGE study. Eur J Hum Genet doi: 10.1038/ejhg.2009.4
Rodgers JT, Lerin C, Haas WG. et al. (2005) Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434:113–118
Rose G, Dato S, Altomare K et al. (2003) Variability of the SIRT3 gene, human silent information regulator Sir2 homologue, and survivorship in the elderly. Exp Gerontol 38:1065–1070
Sauve AA, Moir RD, Schramm VL, Willis IM (2005) Chemical activation of Sir2-dependent silencing by relief of nicotinamide inhibition. Mol Cell 17:595–601
Scher MB, Vaquero A, Reinberg D (2007) SirT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress. Genes Dev 21:920–928
Schlicker C, Gertz M, Papatheodorou P et al. (2008) Substrates and regulation mechanisms for the human mitochondrial sirtuins sirt3 and sirt5. J Mol Biol 382:790–801
Schuetz A, Min J, Antoshenko T et al. (2007) Structural basis of inhibition of the human NAD+-dependent deacetylase SIRT5 by suramin. Structure 15:377–389
Schwer B, Verdin E (2008) Conserved metabolic regulatory functions of sirtuins. Cell Metab 7:104–112
Shi T, Wang F Stieren E, Tong Q (2005) SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Chem Biol 280:13560–13567
Smith JS (2002) Human Sir2 and the “silencing” p53 activity. Trends Cell Biol 12:404–406
Smith JS, Avalos J, Celic I et al. (2002) SIR2 family of NAD+-dependent. Methods Enzymol 353:282–300
Smith JJ, Kenney RD, Gagne DJ (2009) Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. BMC Syst Biol 10:3–31
Stünkel W, Peh BK, Tan YC et al. (2007) Function of the SIRT1 protein deacetylase in cancer. Biotechnol J 2:1360–1368
Sun Y, Sun D, Li F et al. (2007) Downregulation of Sirt1 by antisense oligonucleotides induces apoptosis and enhances radiation sensitization in A549 lung cancer cells. Lung Cancer 58:21–29
Sundaresan NR, Samant SA, Pillai VB et al. (2008) SIRT3 is a stress responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku-70. Mol Cell Biol 28:6384–6401
Suzuki K, Koike T (2007) Mammalian Sir2-related protein (SIRT) 2-mediated modulation of resistance to axonal degeneration in slow Wallerian degeneration mice: a crucial role of tubulin deacetylation. Neuroscience 147:599–612
Taunton J, Hassig CA, Schreiber SL (1996) A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science 272:408–411
Trapp J, Meier R, Hongwiset D et al. (2007) Structure-activity studies on suramin analogues as inhibitors of NAD+-dependent histone deacetylases (sirtuins). ChemMedChem 2:1419–1431
Vakhrusheva O, Smolka C, Gajawada P et al. (2008) Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res 102:703–10
Vaquero A, Scher M, Lee D et al. (2004) Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol Cell 16:93–105
Vaziri H, Dessain SK, Eaton EN et al. (2001) hSIR2SIRT1 Functions as an NAD-Dependent p53 Deacetylase. Cell 107:149–159
Vergnes B, Vanhille L, Ouaissi A, Sereno D (2005) Stage-specific antileishmanial activity of an inhibitor of SIR2 histone deacetylase. Acta Trop 94:107–115
Voelter-Mahlknecht S, Ho, AD, Mahlknecht, U (2005) FISH-mapping and genomic organization of the NAD-dependent histone deacetylase gene, Sirtuin 2 (Sirt2). Int J Oncol 27:1187–1196
Voelter-Mahlknecht S, Ho AD, Letzel S, Mahlknecht U (2006a) Assignment of the NAD-dependent deacetylase sirtuin 5 gene (SIRT5) to human chromosome band 6p23 by in situ hybridization. Cytogenet Genome Res 112:208–212
Voelter-Mahlknecht S, Ho AD, Mahlknecht, U (2006b) Chromosomal organization and fluorescence in situ hybridization of the human Sirtuin 6 gene. Int J Oncol 28:447–456
Voelter-Mahlknecht S, Mahlknecht, U (2006) Cloning, chromosomal characterization and mapping of the NAD-dependent histone deacetylases gene sirtuin 1. Int J Mol Med 17:59–67
Voelter-Mahlknecht S, Ho AD, Letzel S, Mahlknecht U (2006d) Fluorescence in situ hybridization and chromosomalorganization of the human Sirtuin 7 gene. Int J Oncol 28:899–908
Westphal CH, Dipp MA, Guarente L (2007) A therapeutic role for sirtuins indiseases of aging? Trends Biochem Sci 32:555–560
Walle T, Hsieh F, DeLegge MH et al. (2004) High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos 32:1377–1382
Wang F, Nguyen M, Qin FX, Tong Q (2007) SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell 6:505–514
Weindruch R, Walford RL (1982) Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science 215:1415–1418
Werner HB, Kuhlmann K, Shen S et al. (2007) Proteolipid protein is required for transport of sirtuin 2 into CNS myelin. J Neurosci 27:7717–7730
Witt O, Deubzer HE, Milde T, Oehme I (2009) HDAC family: What are the cancer relevant targets? Cancer Lett 277:8–21
Wood JG, Rogina B, Lavu S et al. (2004). Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430:686–689
Yang H, Baur JA, Chen A et al. (2007) Design and synthesis of compounds that extend yeast replicative lifespan. Aging Cell 6:35–43
Yang T, Sauve AA (2006) NAD metabolism and sirtuins: metabolic regulation of protein deacetylation in stress and toxicity. AAPS J 8:E632–643
Yang XJ, Seto E (2008) The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat Rev 9:206–218
Yeung F, Hoberg JE, Ramsey CS et al. (2004) Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23:2369–2380
Zhang Y, McLaughlin R, Goodyer C, LeBlanc A (2002) Selective cytotoxicity of intracellular amyloid beta peptide1–42 through p53 and Bax in cultured primary human neurons. J Cell Biol 156: 519–522
Zhang Y, Au Q, Zhang M et al. (2009) Identification of a small molecule SIRT2 inhibitor with selective tumor cytotoxicity. Biochem Biophys Res Commun 386:729–733
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Alcaín, F.J., Minor, R.K., Villalba, J.M., de Cabo, R. (2010). Small Molecule Modulators of Sirtuin Activity. In: Fahy, G.M., West, M.D., Coles, L.S., Harris, S.B. (eds) The Future of Aging. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-3999-6_10
Download citation
DOI: https://doi.org/10.1007/978-90-481-3999-6_10
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-90-481-3998-9
Online ISBN: 978-90-481-3999-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)