Abstract
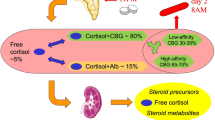
Endogenous Cushing’s syndrome (CS) is a rare disease, and usually characterized by hypertension, diabetes, obesity, osteoporosis, facial rounding, dorsocervical fat pad, thin skin, purple striae, hirsutism, and mood disorders. Efficient diagnostic and screening strategies lead to the diagnosis of a significantly higher number of cases of CS. As a screening test for CS, the Endocrine Society’s Clinical Practice Guidelines recommend a single test with a high diagnostic accuracy, among the 1-mg dexamethasone suppression test (1-mg DST), late night salivary cortisol (LNSC), and 24 h urinary free cortisol (UFC). In normal subjects, administering a higher than physiological dose of glucocorticoids prompts the suppression of cortisol secretion. The 1-mg DST explores this normal feedback reaction from the hypothalamic–pituitary–adrenal axis (HPA). It is a simple dynamic test, usually performed in outpatients. A morning serum cortisol level <50 nmol/L suffices to exclude CS, unless there is a strong clinical suspicion to suggest otherwise. The HPA axis reaches a nadir just after a person has fallen asleep, but its circadian rhythm is impaired in CS patients, who feature higher cortisol values at night, which are easy to measure in saliva (the LNSC assay). Saliva collection is also suitable for outpatients since cortisol is stable at room temperature and the collection device can be mailed to the laboratory for analysis. UFC levels reflect the integrated tissue exposure to free cortisol over 24 h, and thus provide a particular picture of endogenous hypercortisolism. In most cases, high UFC levels coincide with severe hypercortisolism. UFC is used not only to diagnose CS, but also to monitor its response to medical treatment. All screening tests have procedural snares: some drugs can interfere with the DST; false-positive or false-negative LNSC results may be due to an inadequate soaking of the device or to cyclic CS; and in the case of UFC it is important to ensure that patients provide complete urine collections with appropriate total volumes. Measuring cortisol with antibody-based immunoassays can also generate false-positive results due to cross-reactivity between cortisol, cortisone and other metabolites. Structurally-based assays, such as liquid chromatography with tandem mass spectrometry, only measure cortisol and have only recently become available for use in routine clinical practice. This review summarizes the recent literature on the clinical and biochemical aspects of CS diagnostics with a view to helping physicians choose the best screening test for diagnosing endogenous hypercortisolism.



Similar content being viewed by others
References
Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93(5):1526–40. doi:10.1210/jc.2008-0125.
Boscaro M, Arnaldi G. Approach to the patient with possible Cushing’s syndrome. J Clin Endocrinol Metab. 2009;94(9):3121–31. doi:10.1210/jc.2009-0612.
Chiodini I, Mascia ML, Muscarella S, et al. Subclinical hypercortisolism among outpatients referred for osteoporosis. Ann Intern Med. 2007;147(8):541–8.
Terzolo M, Reimondo G, Chiodini I, et al. Screening of Cushing’s syndrome in outpatients with type 2 diabetes: results of a prospective multicentric study in Italy. J Clin Endocrinol Metab. 2012;97(10):3467–75. doi:10.1210/jc.2012-1323.
Ilias I, Torpy DJ, Pacak K, Mullen N, Wesley RA, Nieman LK. Cushing’s syndrome due to ectopic corticotropin secretion: twenty years’ experience at the National Institutes of Health. J Clin Endocrinol Metab. 2005;90(8):4955–62.
Ejaz S, Vassilopoulou-Sellin R, Busaidy NL, Hu MI, Waguespack SG, Jimenez C, Ying AK, Cabanillas M, Abbara M, Habra MA. Cushing syndrome secondary to ectopic adrenocorticotropic hormone secretion: the University of Texas MD Anderson Cancer Center Experience. Cancer. 2011;117(19):4381–9. doi:10.1002/cncr.26029.
Anderson GH Jr, Blakeman N, Streeten DH. The effect of age on prevalence of secondary forms of hypertension in 4429 consecutively referred patients. J Hypertens. 1994;12(5):609–15.
Omura M, Saito J, Yamaguchi K, Kakuta Y, Nishikawa T. Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens Res. 2004;27(3):193–202.
Martins LC, Conceição FL, Muxfeldt ES, Salles GF. Prevalence and associated factors of subclinical hypercortisolism in patients with resistant hypertension. J Hypertens. 2012;30(5):967–73. doi:10.1097/HJH.0b013e3283521484.
Trifanescu R, Carsote M, Caragheorgheopol A, Hortopan D, Dumitrascu A, Dobrescu M, Poiana C. Screening for secondary endocrine hypertension in young patients. Maedica (Buchar). 2013;8(2):108–15.
Thomas RM, Ruel E, Shantavasinkul PC, Corsino L. Endocrine hypertension: an overview on the current etiopathogenesis and management options. World J Hypertens. 2015;5(2):14–27.
Isidori AM, Graziadio C, Paragliola RM, Cozzolino A, Ambrogio AG, Colao A, Corsello SM, Pivonello R, ABC Study Group. The hypertension of Cushing’s syndrome: controversies in the pathophysiology and focus on cardiovascular complications. J Hypertens. 2015;33(1):44–60. doi:10.1097/HJH.0000000000000415.
Pecori Giraldi F, Ambrogio AG, De Martin M, et al. Specificity of first-line tests for the diagnosis of Cushing’s syndrome: assessment in a large series. J Clin Endocrinol Metab. 2007;92(11):4123–9.
Elamin MB, Murad MH, Mullan R, et al. Accuracy of diagnostic tests for Cushing’s syndrome: a systematic review and metaanalyses. J Clin Endocrinol Metab. 2008;93(5):1553–62. doi:10.1210/jc.2008-0139.
Elias PC, Martinez EZ, Barone BF, Mermejo LM, Castro M, Moreira AC. Late-night salivary cortisol has a better performance than urinary free cortisol in the diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab. 2014;99(6):2045–51. doi:10.1210/jc.2013-4262 Epub 2014 Mar 14.
Ceccato F, Barbot M, Zilio M, Frigo AC, Albiger N, Camozzi V, Antonelli G, Plebani M, Mantero F, Boscaro M, Scaroni C. Screening tests for Cushing’s syndrome: urinary free cortisol role measured by LC–MS/MS. J Clin Endocrinol Metab. 2015;100(10):3856–61. doi:10.1210/jc.2015-2507.
Valassi E, Swearingen B, Lee H, Nachtigall LB, Donoho DA, Klibanski A, Biller BM. Concomitant medication use can confound interpretation of the combined dexamethasone-corticotropin releasing hormone test in Cushing’s syndrome. J Clin Endocrinol Metab. 2009;94:4851–9. doi:10.1210/jc.2009-1500.
Alexandraki KI, Grossman AB. Is urinary free cortisol of value in the diagnosis of Cushing’s syndrome? Curr Opin Endocrinol Diabetes Obes. 2011;18(4):259–63. doi:10.1097/MED.0b013e3283487193. Review. PubMed PMID:21681089.
Ceccato F, Antonelli G, Barbot M, Zilio M, Mazzai L, Gatti R, Zaninotto M, Mantero F, Boscaro M, Plebani M, Scaroni C. The diagnostic performance of urinary free cortisol is better than the cortisol/cortisone ratio in detecting de novo Cushing’s syndrome: the use of a LC–MS/MS method in routine clinical practice. Eur J Endocrinol. 2014;171(1):1–7. doi:10.1530/EJE-14-0061.
Raff H. Cushing’s syndrome: diagnosis and surveillance using salivary cortisol. Pituitary. 2012;15:64–70.
Ceccato F, Barbot M, Zilio M, et al. Performance of salivary cortisol in the diagnosis of Cushing’s syndrome, adrenal incidentaloma, and adrenal insufficiency. Eur J Endocrinol. 2013;169(1):31–6. doi:10.1530/EJE-13-0159.
Deutschbein T, Broecker-Preuss M, Flitsch J, Jaeger A, Althoff R, Walz MK, Mann K, Petersenn S. Salivary cortisol as a diagnostic tool for Cushing’s syndrome and adrenal insufficiency: improved screening by an automatic immunoassay. Eur J Endocrinol. 2012;166(4):613–8. doi:10.1530/EJE-11-0945.
Erickson D, Singh RJ, Sathananthan A, Vella A, Bryant SC. Late-night salivary cortisol for diagnosis of Cushing’s syndrome by liquid chromatography/tandem mass spectrometry assay. Clin Endocrinol (Oxf). 2012;76(4):467–72. doi:10.1111/j.1365-2265.2011.04239.x.
Antonelli G, Ceccato F, Artusi C, Marinova M, Plebani M. Salivary cortisol and cortisone by LC–MS/MS: validation, reference intervals and diagnostic accuracy in Cushing’s syndrome. Clin Chim Acta. 2015;451(Pt B):247–51. doi:10.1016/j.cca.2015.10.004.
Graham UM, Hunter SJ, McDonnell M, Mullan KR, Atkinson AB. A comparison of the use of urinary cortisol to creatinine ratios and nocturnal salivary cortisol in the evaluation of cyclicity in patients with Cushing’s syndrome. J Clin Endocrinol Metab. 2013;98(1):E72–6. doi:10.1210/jc.2012-2925.
Handelsman DJ, Wartofsky L. Requirement for mass spectrometry sex steroid assays in the Journal of Clinical Endocrinology and Metabolism. J Clin Endocrinol Metab. 2013;98(10):3971–3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflicts of interest or financial interests to disclose that might be perceived as influencing the impartiality of the reported research.
Ethical approval
For authors’ patient population the study was performed in accordance with the guidelines in the Declaration of Helsinki, the Ethics Committee of the University-Hospital of Padova approved the study and data management.
Informed consent
All subjects gave informed consent.
Rights and permissions
About this article
Cite this article
Ceccato, F., Boscaro, M. Cushing’s Syndrome: Screening and Diagnosis. High Blood Press Cardiovasc Prev 23, 209–215 (2016). https://doi.org/10.1007/s40292-016-0153-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40292-016-0153-4