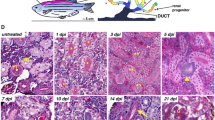
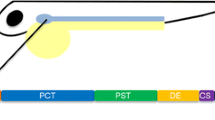
Abstract
The vertebrate kidney possesses the capacity to repair damaged nephrons, and this potential is conserved regardless of the complexity of species-specific kidneys. However, many aquatic vertebrates possess the ability to not only repair existing nephrons, but also generate new nephrons after injury. Adult zebrafish have the ability to recover from acute renal injury not only by replacing lost injured epithelial cells of endogenous nephrons, but by also generating de novo nephrons. This strong regeneration potential, along with other unique characteristics such as the high degree of genetic conservation with humans, the ease of harvesting externally fertilized, transparent embryos, the accessibility to larval and adult kidneys, and the ability to perform whole organism phenotypic small molecule screens, has positioned zebrafish as a unique vertebrate model to study kidney injury. In this review, we provide an overview of the contribution of zebrafish larvae/adult studies to the understanding of renal regeneration, diseases, and therapeutic discovery.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Thadhani R, Pascual M, Bonventre JV (1996) Acute renal failure. N Engl J Med 334(22):1448–1460
Chertow GM et al (2005) Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16(11):3365–3370
Lameire N, Van Biesen W, Vanholder R (2006) The changing epidemiology of acute renal failure. Nature clinical practice. Nephrology 2(7):364–377
Bellomo R, Kellum JA, Ronco C (2012) Acute kidney injury. Lancet 380(9843):756–766
Lameire NH et al (2013) Acute kidney injury: an increasing global concern. Lancet 382:170–179
Rewa O, Bagshaw SM (2014) Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol 10(4):193–207
Bucaloiu ID et al (2012) Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int 81(5):477–485
Chawla LS et al (2012) The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int 79(12):1361–1369
Coca SG, Singanamala S, Parikh CR (2012) Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 81(5):442–448
Murugan R, Kellum JA (2011) Acute kidney injury: what’s the prognosis? Nat Rev Nephrol 7(4):209–217
Wald R et al (2012) Risk of chronic dialysis and death following acute kidney injury. Am J Med 125(6):585–593
Wu VC et al (2011) Acute-on-chronic kidney injury at hospital discharge is associated with long-term dialysis and mortality. Kidney Int 80(11):1222–1230
Molitoris BA et al (2012) Designing clinical trials in acute kidney injury. Clin J Am Soc Nephrol 7(5):842–843
Davidson AJ (2011) Uncharted waters: nephrogenesis and renal regeneration in fish and mammals. Pediatr Nephrol 26(9):1435–1443
Bacallao R, Fine LG (1989) Molecular events in the organization of renal tubular epithelium: from nephrogenesis to regeneration. Am J Physiol 257(6 Pt 2):F913–F924
Sharfuddin AA, Molitoris BA (2011) Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol 7(4):189–200
Bonventre JV (2003) Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol 14(Suppl 1):S55–S61
Bussolati B et al (2005) Isolation of renal progenitor cells from adult human kidney. Am J Pathol 166(2):545–555
Humphreys BD et al (2008) Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2(3):284–291
Loverre A et al (2008) Increase of proliferating renal progenitor cells in acute tubular necrosis underlying delayed graft function. Transplantation 85(8):1112–1119
• Cianciolo Cosentino C et al (2013) Histone deacetylase inhibitor enhances recovery after AKI. J Am Soc Nephrol 24(6):943–953. This publication validates the strategy that discoveries made using a zebrafish embryonic screening model are directly translatable to mammalian models of AKI
• Diep CQ et al (2011) Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature 470(7332):95–100. This publication identifies a stem/progenitor cell in the adult zebrafish that is important for driving neo-nephrogenesis
Wingert RA et al (2007) The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS Genet 3(10):1922–1938
Drummond IA et al (1998) Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development 125(23):4655–4667
Zhou W et al (2010) Characterization of mesonephric development and regeneration using transgenic zebrafish. Am J Physiol 299(5):F1040–F1047
Reimschuessel R (2001) A fish model of renal regeneration and development. ILAR J 42(4):285–291
Karasawa T et al (2011) Calreticulin binds to gentamicin and reduces drug-induced ototoxicity. Toxicol Sci 124(2):378–387
Lopez-Novoa JM et al (2011) New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int 79(1):33–45
Cianciolo Cosentino C et al (2010) Intravenous microinjections of zebrafish larvae to study acute kidney injury. J Vis Exp. doi:10.3791/2079
Hentschel DM et al (2005) Acute renal failure in zebrafish: a novel system to study a complex disease. Am J Physiol Renal Physiol 288(5):F923–F929
Bonventre JV, Yang L (2011) Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121(11):4210–4221
Hartman HA, Lai HL, Patterson LT (2007) Cessation of renal morphogenesis in mice. Dev Biol 310(2):379–387
Johnson CS, Holzemer NF, Wingert RA (2011) Laser ablation of the zebrafish pronephros to study renal epithelial regeneration. J Vis Exp 54
Palmyre A et al (2014) Collective epithelial migration drives kidney repair after acute injury. PLoS One 9(7):e101304
Arlt VM, Stiborova M, Schmeiser HH (2002) Aristolochic acid as a probable human cancer hazard in herbal remedies: a review. Mutagenesis 17(4):265–277
Ding YJ, Chen YH (2012) Developmental nephrotoxicity of aristolochic acid in a zebrafish model. Toxicol Appl Pharmacol 261(1):59–65
Sato N et al (2004) Acute nephrotoxicity of aristolochic acids in mice. J Pharm Pharmacol 56(2):221–229
Vanherweghem JL et al (1993) Rapidly progressive interstitial renal fibrosis in young women: association with slimming regimen including Chinese herbs. Lancet 341(8842):387–391
Tryggvason K, Patrakka J, Wartiovaara J (2006) Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 354(13):1387–1401
Tryggvason K, Wartiovaara J (2005) How does the kidney filter plasma? Physiology (Bethesda) 20:96–101
Hentschel DM et al (2007) Rapid screening of glomerular slit diaphragm integrity in larval zebrafish. Am J Physiol Renal Physiol 293(5):F1746–F1750
Ryan GB, Rodewald R, Karnovsky MJ (1975) An ultrastructural study of the glomerular slit diaphragm in aminonucleoside nephrosis. Lab Invest 33(5):461–468
He B et al (2011) Podocin-green fluorescence protein allows visualization and functional analysis of podocytes. J Am Soc Nephrol 22(6):1019–1023
Huang J et al (2013) A zebrafish model of conditional targeted podocyte ablation and regeneration. Kidney Int 83(6):1193–1200
• Zhou W, Hildebrandt F (2012) Inducible podocyte injury and proteinuria in transgenic zebrafish. J Am Soc Nephrol 23(6):1039–1047. This publication describes the generation of a zebrafish transgenic model for the study of glomerular pathogenesis and podocyte regeneration
Curado S, Stainier DY, Anderson RM (2008) Nitroreductase-mediated cell/tissue ablation in zebrafish: a spatially and temporally controlled ablation method with applications in developmental and regeneration studies. Nat Protoc 3(6):948–954
Pisharath H, Parsons MJ (2009) Nitroreductase-mediated cell ablation in transgenic zebrafish embryos. Methods Mol Biol 546:133–143
Appel D et al (2009) Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol 20(2):333–343
Grouls S et al (2012) Lineage specification of parietal epithelial cells requires beta-catenin/Wnt signaling. J Am Soc Nephrol 23(1):63–72
Sander V et al (2015) The small molecule probe PT-Yellow labels the renal proximal tubules in zebrafish. Chem Commun (Camb) 51(2):395–398
Driever W et al (1996) A genetic screen for mutations affecting embryogenesis in zebrafish. Development 123:37–46
Bingham C et al (2001) Mutations in the hepatocyte nuclear factor-1beta gene are associated with familial hypoplastic glomerulocystic kidney disease. Am J Hum Genet 68(1):219–224
Mochizuki T et al (1996) PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272(5266):1339–1342
Sun Z et al (2004) A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development 131(16):4085–4093
Kishimoto N et al (2008) Cystic kidney gene seahorse regulates cilia-mediated processes and Wnt pathways. Dev Cell 14(6):954–961
Li J, Sun Z (2011) Qilin is essential for cilia assembly and normal kidney development in zebrafish. PLoS One 6(11):e27365
Pedersen LB, Rosenbaum JL (2008) Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol 85:23–61
Smith LA et al (2006) Development of polycystic kidney disease in juvenile cystic kidney mice: insights into pathogenesis, ciliary abnormalities, and common features with human disease. J Am Soc Nephrol 17(10):2821–2831
Cao Y et al (2009) Chemical modifier screen identifies HDAC inhibitors as suppressors of PKD models. Proc Natl Acad Sci USA 106(51):21819–21824
Hellman NE et al (2010) The zebrafish foxj1a transcription factor regulates cilia function in response to injury and epithelial stretch. Proc Natl Acad Sci USA 107(43):18499–18504
Huang L et al (2014) A possible zebrafish model of polycystic kidney disease: knockdown of wnt5a causes cysts in zebrafish kidneys. J Vis Exp 94:e52156–e52156
Hildebrandt F, Attanasio M, Otto E (2009) Nephronophthisis: disease mechanisms of a ciliopathy. J Am Soc Nephrol 20(1):23–35
Lin F et al (2003) Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci USA 100(9):5286–5291
Pazour GJ, Witman GB (2003) The vertebrate primary cilium is a sensory organelle. Curr Opin Cell Biol 15(1):105–110
Yoder BK (2007) Role of primary cilia in the pathogenesis of polycystic kidney disease. J Am Soc Nephrol 18(5):1381–1388
Yoder BK, Hou X, Guay-Woodford LM (2002) The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 13(10):2508–2516
Lansbury PT Jr (2004) Back to the future: the ‘old-fashioned’ way to new medications for neurodegeneration. Nat Med 10(Suppl):S51–S57
Lee JA et al (2012) Modern phenotypic drug discovery is a viable, neoclassic pharma strategy. J Med Chem 55(10):4527–4538
Murphey RD et al (2006) A chemical genetic screen for cell cycle inhibitors in zebrafish embryos. Chem Biol Drug Des 68(4):213–219
Sams-Dodd F (2005) Target-based drug discovery: is something wrong? Drug Discov Today 10(2):139–146
Lawrence S (2007) Drug output slows in 2006. Nat Biotechnol 25(10):1073
Kamb A (2005) Opinion: what’s wrong with our cancer models? Nat Rev Drug Discov 4(2):161–165
Prior M et al (2014) Back to the future with phenotypic screening. ACS Chem Neurosci 5(7):503–513
Deo RC, MacRae CA (2011) The zebrafish: scalable in vivo modeling for systems biology. Wiley Interdiscip Rev Syst Biol Med 3(3):335–346
Swinney DC, Anthony J (2011) How were new medicines discovered? Nat Rev Drug Discov 10(7):507–519
Peal DS et al (2011) Novel chemical suppressors of long QT syndrome identified by an in vivo functional screen. Circulation 123(1):23–30
Burns CG et al (2005) High-throughput assay for small molecules that modulate zebrafish embryonic heart rate. Nat Chem Biol 1(5):263–264
Peterson RT et al (2004) Chemical suppression of a genetic mutation in a zebrafish model of aortic coarctation. Nat Biotechnol 22(5):595–599
Milan DJ et al (2003) Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation 107(10):1355–1358
Hao J et al (2010) In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol 5(2):245–253
White RM et al (2011) DHODH modulates transcriptional elongation in the neural crest and melanoma. Nature 471(7339):518–522
Kokel D et al (2010) Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat Chem Biol 6(3):231–237
Rihel J et al (2010) Zebrafish behavioral profiling links drugs to biological targets and rest/wake regulation. Science 327(5963):348–351
North TE et al (2007) Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature 447(7147):1007–1011
de Groh ED et al (2010) Inhibition of histone deacetylase expands the renal progenitor cell population. J Am Soc Nephrol 21(5):794–802
Novitskaya T et al (2014) A PTBA small molecule enhances recovery and reduces postinjury fibrosis after aristolochic acid-induced kidney injury. Am J Physiol Renal Physiol 306(5):F496–F504
Acknowledgments
The laboratory of Neil Hukriede was supported by the National Institutes of Health Grants 2R01 DK069403, 1RC4 DK090770, 2R01 HD053287, and 1P30DK079307. The laboratory of Maria Cecilia Cirio was supported by the Agencia Nacional de Promoción Científica y Tecnológica de Argentina, FONCyT, PICT 2013. The laboratory of Mark de Caestecker was supported by National Institutes of Health Grants 1R01 HL093057 and 1RC4DK090770.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Zebrafish as a Model for Pathobiology.
Rights and permissions
About this article
Cite this article
Cirio, M.C., de Caestecker, M.P. & Hukriede, N.A. Zebrafish Models of Kidney Damage and Repair. Curr Pathobiol Rep 3, 163–170 (2015). https://doi.org/10.1007/s40139-015-0080-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40139-015-0080-4