Abstract
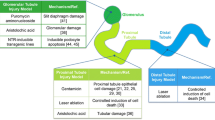
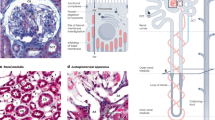
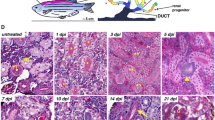
The major functions of the vertebrate kidney are the removal of metabolic waste and the balance of salt and water. These roles are fulfilled by nephrons, which generally comprise a blood filter (glomerulus) attached to an epithelial tubule. The number of nephrons in the mammalian kidney is set at the end of kidney organogenesis in the late fetal or neonatal period. Subsequent increases in nephron size and functionality then occur during postnatal growth to match increases in body mass/fluid. Because of this strategy of renal development, injuries or birth defects that reduce nephron number lead to a permanent nephron deficit and increase the risk of kidney disease. In contrast to mammals, fish kidneys continue to add nephrons throughout their lifespan. In response to renal injury, fish increase the rate of nephrogenesis, effectively replacing lost nephrons and maintaining their nephron endowment. A better understanding of the remarkable nephrogenic abilities of fish kidneys may lead to innovative ways to restore nephrogenesis in the adult mammalian kidney. This review examines our current understanding of nephrogenesis in mammals and fish and explores possible explanations for why fish, but not mammals, utilize a perpetual nephrogenesis strategy to grow and maintain their kidneys.



Similar content being viewed by others
References
Hentschel H, Elger M (1989) Structure and function of the kidney. In: Kinne RKH (ed) Comparative physiology, vol 1. Karger, Basel, pp 1–240
Dressler GR (2009) Advances in early kidney specification, development and patterning. Development 136:3863–3874
Reimschuessel R (2001) A fish model of renal regeneration and development. ILAR J 42:285–291
Kobayashi M, Valerius T, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP (2008) Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3:169–181
Bard JB, Gordon A, Sharp L, Sellers WI (2001) Early nephron formation in the developing mouse kidney. J Anat 199:385–392
Costantini F, Kopan R (2010) Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev Cell 18:698–712
Cebrian C, Borodo K, Charles N, Herzlinger DA (2004) Morphometric index of the developing murine kidney. Dev Dyn 231:601–608
Yuan HT, Tipping PG, Li XZ, Long DA, Woolf AS (2002) Angiopoietin correlates with glomerular capillary loss in anti-glomerular basement membrane glomerulonephritis. Kidney Int 61:2078–2089
Hartman HA, Lai HL, Patterson LT (2007) Cessation of renal morphogenesis in mice. Dev Biol 310:379–387
Gasser B, Mauss Y, Ghnassia JP, Favre R, Kohler M, Yu O, Vonesch JL (1993) A quantitative study of normal nephrogenesis in the human fetus: its implication in the natural history of kidney changes due to low obstructive uropathies. Fetal Diagn Ther 8:371–384
Haycock GB (1998) Development of glomerular filtration and tubular sodium reabsorption in the human fetus and newborn. Br J Urol 81 [Suppl 2]:33–38
Fonseca Ferraz ML, Dos Santos AM, Cavellani CL, Rossi RC, Correa RR, Dos Reis MA, de Paula Antunes Teixara V, da Cunha Castro EC (2008) Histochemical and immunohistochemical study of the glomerular development in human fetuses. Pediatr Nephrol 23:257–262
Gubhaju L, Black MJ (2005) The baboon as a good model for studies of human kidney development. Pediatr Res 58:505–509
Rytand DA (1938) The number and size of mammalian glomeruli as related to the kidney and body weight with method for their enumeration and measurement. Am J Anat 62:507–520
Maluf NS (1995) Kidney of elephants. Anat Rec 242:491–514
McCrory WW (1972) Renal function in the postnatal period. In: Developmental nephrology. Harvard University Press, Cambridge, pp 123–161
Spitzer A, Edelmann CM Jr (1971) Maturational changes in pressure gradients for glomerular filtration. Am J Physiol 221:1431–1435
Satlin LM, Woda CBZ, Schwartz GJ (2003) Development of function in the metanephric kidney. In: Vize PD, Woolf AS, Bard JB (eds) The kidney: from normal development to congenital disease. Academic Press, Amsterdam, pp 278–325
Brenner BM (1985) Nephron adaptation to renal injury or ablation. Am J Physiol 249:F324–F337
Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM (1981) Hyperfiltration in remnant nephrons. Am J Physiol 241:F85–F93
Brenner BM, Mackenzie HS (1997) Nephron mass as a risk factor for progression of renal disease. Kidney Int Suppl 63:S124–S127
Vehaskari VM, Woods LL (2005) Prenatal programming of hypertension: lessons from experimental models. J Am Soc Nephrol 16:2545–2556
Hayslett IP (1979) Functional adaptation to reduction in renal mass. Physiol Rev 59:137–164
Johnson G (1851) On some varieties of renal disease, with especial reference to diagnosis and prognosis. Lond J Med 26:109–122
Tseng SCG (1996) Regulation and clinical implications of corneal epithelial stem cells. Mol Biol Rep 23:47–58
Haegebarth A, Clevers H (2009) Wnt signaling, Lgr5, and stem cells in the intestine and skin. Am J Pathol 174:715–721
Hopkins C, Li J, Rae F, Little MH (2009) Stem cell options for kidney disease. J Pathol 217:265–281
Benigni A, Morigi M, Remuzzi G (2010) Kidney regeneration. Lancet 375:1310–1317
Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV (2008) Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2:284–291
Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q (2004) The renal papilla is a niche for adult kidney stem cells. J Clin Invest 114:795–804
Dekel B, Zangi L, Shezen E, Reich-Zeliger S, Eventov-Friedman S, Katchman H, Jacob-Hirsch J, Amariglio N, Rechavi G, Margalit R, Reisner Y (2006) Isolation and characterization of nontubular sca-1 + lin- multipotent stem/progenitor cells from adult mouse kidney. J Am Soc Nephrol 17:3300–3314
Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, Camussi G (2005) Isolation of renal progenitor cells from adult human kidney. Am J Pathol 166:545–555
Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, Jeffery R, Hunt T, Alison M, Cook T, Pusey C, Wright NA (2001) Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol 195:229–235
Vogetseder A, Palan T, Bacic D, Kaissling B, Le Hir M (2007) Proximal tubular epithelial cells are generated by division of differentiated cells in the healthy kidney. Am J Physiol Cell Physiol 292:C807–C813
Vogetseder A, Picard N, Gaspert A, Walch M, Kaissling B, Le Hir M (2008) Proliferation capacity of the renal proximal tubule involves the bulk of differentiated epithelial cells. Am J Physiol Cell Physiol 294:C22–C28
Fujigaki Y, Sakakima M, Sun Y, Fujikura T, Tsuji T, Yasuda H, Hishida A (2009) Cell division and phenotypic regression of proximal tubular cells in response to uranyl acetate insult in rats. Nephrol Dial Transplant 24:2686–2692
Prescott LF (1966) The normal urinary excretion rates of renal tubular cells, leucocytes and red blood cells. Clin Sci 31:425–435
Croft DN (1970) Body iron loss and cell loss from epithelia. Proc R Soc Med 63:1221–1224
Moghe MA (1945) Development of the mesonephros in a teleostean—Thynnichthys sandkhol. Q J Micr Sci 85:129–151
Fedorova S, Miyamoto R, Harada T, Isogai S, Hashimoto H, Ozato K, Wakamatsu Y (2008) Renal glomerulogenesis in medaka fish, Oryzias latipes. Dev Dyn 237:2342–2352
Nash J (1931) The number and size of glomeruli in kidneys of fishes, with observations on the morphology of the renal tubules of fishes. Am J Anat 47:425–445
Mourier JP (1979) Incorporation of 3 H-thymidine in the nephron of Gasterosteus aculeatus L. and its stimulation by methyltestosterone. A high-speed scintillation autoradiographic study. Cell Tissue Res 201:249–262
Reimschuessel R, Bennett RO, May EB, Lipsky MM (1990) Development of newly formed nephrons in the goldfish kidney following hexachlorobutadiene-induced nephrotoxicity. Toxicol Pathol 18:32–38
Reimschuessel R, Bennett RO, May EA, Lipsky MM (1990) Renal tubular cell regeneration, cell proliferation and chronic nephrotoxicity in the goldfish (Carassius auratus) following exposure to a single sublethal dose of hexachlorobutadiene. Dis Aquat Organ 8:211–224
Hentschel H, Elger M, Dawson M, Renfro JL (2000) Urinary tract. In: Ostrander GK (ed) The laboratory fish. Handbook of experimental animals. Academic Press, London
Wrobel KH, Jouma S (2004) Morphology, development and comparative anatomical evaluation of the testicular excretory pathway in Acipenser. Ann Anat 186:99–113
Hentschel H (1991) Developing nephrons in adolescent dogfish, Scyliorhinus caniculus (L.), with reference to ultrastructure of early stages, histogenesis of the renal countercurrent system, and nephron segmentation in marine elasmobranchs. Am J Anat 190:309–333
Elger M, Hentschel H, Litteral J, Wellner M, Kirsch T, Luft FC, Haller H (2003) Nephrogenesis is induced by partial nephrectomy in the elasmobranch Leucoraja erinacea. J Am Soc Nephrol 14:1506–1518
Watanabe N, Kato M, Suzuki N, Inoue C, Fedorova S, Hashimoto H, Maruyama S, Matsuo S, Wakamatsu Y (2009) Kidney regeneration through nephron neogenesis in medaka. Dev Growth Differ 51:135–143
Ford P (1958) Studies on the development of the kidney of the Pacific pink salmon (Oncorhynchus gorbuscha (Walbaum)). II. Variation in glomerular count of the kidney of the pacific pink salmon. Can J Zool 36:45–47
Carey JR, Judge DS (2000) Longevity records: life spans of mammals, birds, amphibians, reptiles, and fish. Odense University Press, Odense
Haynes G (1991) Mammoths, mastodons, and elephants. Cambridge University Press, Cambridge
Okuda N, Tayasu I, Yanagisawa Y (1998) Determinate growth in a paternal mouth-brooding fish whose reproductive success is limited by buccal capacity. Evol Ecol 12:681–699
Kramer-Zucker AG, Wiessner S, Jensen AM, Drummond IA (2005) Organization of the pronephric filtration apparatus in zebrafish requires Nephrin, Podocin and the FERM domain protein Mosaic eyes. Dev Biol 285:316–329
Brown JA, Jackson BA, Oliver JA, Henderson IW (1978) Single nephron filtration rates (SNGFR) in the trout. Salmo gairdneri. Validation of the use of ferrocyanide and the effects of environmental salinity. Pflugers Arch 377:101–108
Moriarty RJ, Logan AG, Rankin JC (1978) Measurement of single nephron filtration rate in the kidney of the river lamprey, Lampetra fluviatilis L. J Exp Biol 77:57–71
Marshall EK Jr (1934) Comparative physiology of the kidney in relation to theories of renal secretion. Physiol Rev 14:133–159
Gray P (1930) The development of the amphibian kidney. I. The development of the mesonephros of rana temporaria. Q J Micr Sci 73:507–545
Fox H (1977) The urinogenital system of reptiles. In: Gans C, Parsons TS (eds) Biology of the reptilia, vol 6. Academic Press, London, pp 1–157
Beuchat CA, Braun EJ (1988) Allometry of the kidney: implications for the ontogeny of osmoregulation. Am J Physiol 255:R760–R767
Solomon SE (1985) The morphology of the kidney of the green turtle (Chelonia mydas L.). J Anat 140:355–369
Burggren WW, Johansen K (1982) Ventricular hemodynamics in the monitor lizard Varanus exanthematicus: pulmonary and systemic pressure separation. J Exp Biol 96:343–354
Holmes B (1975) A reconsideration of the phylogeny of the tetrapod heart. J Morphol 147:209–228
Wideman RF Jr (1989) Maturation of glomerular size distribution profiles in domestic fowl (Gallus gallus). J Morphol 201:205–213
Koteja P (2004) The evolution of concepts on the evolution of endothermy in birds and mammals. Physiol Biochem Zool 77:1043–1050
Woolf AS, Palmer SJ, Snow ML, Fine LG (1990) Creation of a functioning chimeric mammalian kidney. Kidney Int 38:991–997
Armstrong JF, Kaufman MH, van Heyningen V, Bard JB (1993) Embryonic kidney rudiments grown in adult mice fail to mimic the Wilms’ phenotype, but show strain-specific morphogenesis. Exp Nephrol 1:168–174
Rogers SA, Lowell JA, Hammerman NA, Hammerman MR (1998) Transplantation of developing metanephroi into adult rats. Kidney Int 54:27–37
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davidson, A.J. Uncharted waters: nephrogenesis and renal regeneration in fish and mammals. Pediatr Nephrol 26, 1435–1443 (2011). https://doi.org/10.1007/s00467-011-1795-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-011-1795-z