Abstract
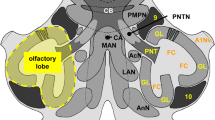
The cytoarchitectonic subdivisions and neuronal classes of the visual wulst were studied by cresyl violet, Golgi Colonnier and rapid Golgi technique. The wulst has been categorized into four laminae viz. the most superficial hyperpallium apicale (HA), intermediate interstitial nucleus of the hyperpallium apicale (IHA), hyperpallium intercalatum (HI), and innermost laminae hyperpallium densocellulare (HD). The wulst neurons have been classified into four main cell types: projection neurons having spinous dendrites and axon projecting widely within the same or different regions; local circuit neurons with aspinous dendrites and local axon arborization; stellate neurons being small with thin sparsely spinous dendrites and small sized granule cells with local axon arborization. The projection neurons are further sub classified into pyramidal (moderately spinous and sparsely spinous) and multipolar neurons (highly spinous, moderately spinous and sparsely spinous). Moderately spinous pyramidal neurons are present in the HA whereas sparsely spinous pyramidal neurons are present in the HD. The highly and moderately spinous multipolar neurons are encountered in the HA, HI and HD whereas moderately and sparsely spinous multipolar neurons are found in the IHA and HD respectively. The granule cells are of two types; spinous and aspinous, restricted only in the IHA. Local circuit neurons are present in all laminae except IHA. Stellate neurons are sparsely spinous found in all the four laminae. The dendrites have spines with small stalk and a knob like head. The morphology of dendritic spines (stalk length and head diameter) varies in different neurons and regions. The present findings have been compared with data reported in the wulst of other birds and also with the neuronal morphology of reptilian dorsal cortex and mammalian visual cortex.













Similar content being viewed by others
References
Karten HJ, Hodos W, Nauta WJH, Revzin AM (1973) Neural connections of the ‘visual wulst’ of the avian telencephalon. Experimental studies in the pigeon (Columba livia) and owl (Speotyto cunicularia). J Comp Neurol 150:253–277
Wilson P (1980) The organization of the visual hyperstriatum in the domestic chick II. Receptive field properties of single units. Brain Res 188(2):333–345
Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Gunturkun O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED (2004) Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol 473:377–414
Shimizu T, Karten HJ (1990) Immunohistochemical analysis of the visual wulst of the pigeon (Columba livia). J Comp Neurol 300:346–369
Sorenson EM, Chiappinelli VA (1992) Localization of 3H-nicotine, 125I-kappa-bungarotoxin, and 125I-alpha-bungarotoxin binding to nicotinic sites in the chicken forebrain and midbrain. J Comp Neurol 323:1–12
Watanabe M, Ito H, Masi H (1983) Cytoarchitecture and visual receptive neurons in the wulst of the Japanese quail (Coturnix coturnix japonica). J Comp Neurol 213:188–198
Montagnese CM, Krebs JR, Meyer G (1996) The dorsomedial and dorsolateral forebrain of the zebra finch, Taeniopygia guttata: a Golgi study. Cell Tissue Res 283:263–282
Tömböl T, Maglόczky Z (1990) Cytoarchitecture of chicken wulst: a Golgi study in cell types and their maturation after hatching. Acta Morphol Hung 38:35–53
Tömböl T (1995) Golgi structure of telencephalon of the chicken. Abaevo, Budapest
Bagnoli P, Francesconi W, Magni F (1982) Visual wulst–optic tectum relationships in birds: a comparison with the mammalian corticotectal system. Arch Ital Biol 120:212–235
Medina L, Reiner A (2000) Do birds possess homologues of mammalian primary visual, somatosensory and motor cortices? Trends Neurosci 23:1–12
Shimizu T, Cox K, Karten HJ (1995) Intratelencephalic projections of the visual wulst in pigeons (Columba livia). J Comp Neurol 359:551–572
Veenman CL, Wild JM, Reiner A (1995) Organization of the avian ‘corticostriatal’ projection system: a retrograde and anterograde pathway tracing study in pigeons. J Comp Neurol 354:87–126
Sakal ID, Maurya RC, Srivastava UC (2010) Quantitative neuronal diversity in the cerebral cortex of Calotes versicolor (Daudin, 1802). Natl Acad Sci Lett 33(5&6):171–176
Maurya RC, Srivastava UC (2012) Neuronal morphology of dorsal cerebral cortex of the Indian wall lizard, H. flaviviridis (Rüppell). Asian J Exp Sci 26(2):83–88
Reiner A (1993) Neurotransmitter organization and connections of turtle cortex: implications for the evolution of mammalian isocortex. Comp Biochem Physiol 104A:735–748
Veenman CL, Reiner A (1996) Ultrastructural study of the targets of cortical afferents in the avian striatum. Brain Res 707:1–12
Budzynski CA, Gagliardo A, Ioale P, Bingman VP (2002) Participation of the homing pigeon thalamofugal visual pathway in sun-compass associative learning. Eur J Neurosci 15(1):197–210
Maekawa F, Komine O, Sato K, Kanamatsu T, Uchimura M, Tanaka K, Ohki-Hamazaki H (2006) Imprinting modulates processing of visual information in the visual wulst of chicks. BMC Neurosci 7:75
Shimizu T, Hodos W (1989) Reversal learning in pigeons: effects of selective lesions of the wulst. Behav Neurosci 103(2):262–272
Blaesing B, Nossoll M, Teuchert Noodt G, Dawirs RR (2001) Postnatal maturation of prefrontal pyramidal neurons is sensitive to a single early dose of methamphetamine in gerbil (Meriones unguiculatus). J Neural Trans 103:101–103
Valverde F (1970) The Golgi method, a tool for comparative structural analysis. In: Nauta WJH, Ebbesson SOE (eds) Contemporary research methods in neuroanatomy. Springer, New York, pp 12–31
Feldman ML, Peters A (1979) A technique for estimating total spine numbers on Golgi-impregnated dendrites. J Comp Neurol 188:527–542
Srivastava UC, Chand P, Maurya RC (2007) Cytoarchitectonic organization and morphology of the cells of hippocampal complex in strawberry finch, Estrilda amandava. Cell Mol Biol 53:103–120
Srivastava UC, Chand P, Maurya RC (2009) Neuronal classes in the corticoid complex of the telencephalon of the strawberry finch, Estrilda amandava. Cell Tissue Res 336:393–409
Tömböl T, Davies DC, Németh A, Sebestény T, Alpár A (2000) A comparative Golgi study of chicken (Gallus domesticus) and homing pigeon (Columba livia) hippocampus. Anat Embryol 201:85–101
Garey LJ, Winkelmann E, Brauer K (1985) Golgi and Nissl studies of the visual cortex of the bottlenose dolphin. J Comp Neurol 240:305–321
Hassiotis M, Ashwell KW (2003) Neuronal classes in the isocortex of a monotreme, the Australian echidna (Tachyglossus aculeatus). Brain Behav Evol 61:6–27
Malach R (1994) Cortical columns as devices for maximizing neuronal diversity. Trends Neurosci 17:101–104
Poirazi P, Mel B (2000) Impact of active dendrites and structural plasticity on the storage capacity of neuronal tissue. Neuron 29:779–796
Holtmaat AJGD, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, Svoboda K (2005) Transient and persistent dendritic spines in the neocortex in vivo. Neuron 45:279–291
Peters A, Fairén A (1978) Smooth and sparsely spined stellate cells in the visual cortex of the rat: a study using a combined Golgi-electron microscope technique. J Comp Neurol 181:129–172
Simons DJ, Woolsey TA (1984) Morphology of Golgi-Cox-impregnated barrel neurons in rat SmI cortex. J Comp Neurol 230:119–132
Jones EG (1975) Varieties and distribution of non-pyramidal cells in the somatic sensory cortex of the squirrel monkey. J Comp Neurol 160:205–267
Tyler CJ, Dunlop SA, Lund RD, Harman AM, Dann JF, Beazley LD, Lund JS (1998) Anatomical comparison of the macaque and marsupial visual cortex: common features that may reflect retention of essential cortical elements. J Comp Neurol 400:449–468
Fairén A, Valverde F (1980) A specialized type of neuron in the visual cortex of cat: a Golgi and electron microscope study of chandelier cells. J Comp Neurol 194:761–779
Lund JS, Henry GH, MacQueen CL, Harvey AR (1979) Anatomical organization of the primary visual cortex (area 17) of the cat: a comparison with area 17 of macaque monkey. J Comp Neurol 184:599–618
Somogyi P, Freund TF, Cowey A (1982) The axo-axonic interneuron in the cerebral cortex of the rat, cat and monkey. Neuroscience 7:2577–2607
Fairén A, De Felipe AJ, Regidor J (1984) Non-pyramidal neurons, general account. In: Peters A, Jones EG (eds) Cerebral cortex, vol 1., Cellular components of the cerebral cortexPlatinum press, New York, pp 201–253
Peters A, Saint Marie RL (1984) Smooth and sparsely spinous nonpyramidal cells forming local axonal plexuses. In: Peters A, Jones EG (eds) Cerebral cortex. Plenum Press, New York, pp 419–445
Hall WC, Ebner FF (1970) Thalamotelencephalic projections in the turtle (Pseudemys scripta). J Comp Neurol 140:101–122
Kenigfest N, Martinez-Marcos A, Belekhova M, Font C, Lanuza E, Desfilis E, Martinez-Garcia F (1997) A lacertilian dorsal retinorecipient thalamus: a re-investigation in the old-world lizard Podarcis hispanica. Brain Behav Evol 50:313–334
Lohman AHM, Van Woerden-Verkley I (1978) Ascending connections to the forebrain in the Tegu lizard. J Comp Neurol 182:555–594
Garcia-Verdugo JM, Regidor Garcia J, Castellano Lopez B, Lopez Garcia C (1983) Ultrastructure of neuronal cell bodies in the dorsal cortex of Lacerta galloti. J Hirnforsch 24(5):485–494
Guirado S, Davila JC, De la Calle A, Marin-Giron F (1987) A Golgi study of the dorsal cortex in the lizard Psammodromus algirus. J Morphol 194:265–274
Connors BW, Kriegstein AR (1986) Cellular physiology of the turtle visual cortex: distinctive properties of pyramidal and stellate neurons. J Neurosci 6:164–177
Srivastava UC, Maurya RC, Shishodiya U (2007) Cyto-architecture and morphology of the different neuronal types of the cerebral cortex of the Indian lizard, Mabouia carinata (Schneider). Proc Natl Acad Sci India B 77(4):331–347
Sakal ID, Maurya RC, Srivastava UC (2010) Quantitative neuronal diversity in the cerebral cortex of Calotes versicolor (Daudin, 1802). Nat Acad Sci Lett 33(5&6):171–176
Maurya RC, Srivastava UC (2006) Morphological diversity of the medial cortex neurons in the common indian wall lizard, Hemidactylus flaviviridis. Nat Acad Sci Lett 29(9&10):375–383
Srivastava UC, Maurya RC, Chand P (2009) Cyto-architecture and neuronal types of the dorsomedial cerebral cortex of the common Indian wall lizard, Hemidactylus flaviviridis. Arch Ital Biol 147:21–35
Srivastava UC, Singh S, Singh D (2012) Seasonal fluctuation in the neuronal classes of Parahippocampal area of P. krameri (Scopoli, 1769) and E. scolopaceus (Linnaeus, 1758). Cell Mol Biol 58:OL1768–OL1779. doi:10.1170/208
Srivastava UC, Gaur P (2013) Naturally occurring neuronal plasticity in visual wulst of Baya weaver, Ploceus philippinus (Linnaeus, 1766). Cell Tissue Res. doi:10.1007/s00441-013-1579-9
Alvarez AV, Sabatini BL (2007) Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci 30:79–97
Rall W (1978) Dendritic spines and synaptic potency. In: Porter R (ed) Studies in neurophysiology. Cambridge University Press, Cambridge, pp 203–209
Livezey BC, Zusi RL (2007) Higher-order phylogeny of modern birds (Theropoda, Aves: Neornithes) based on comparative anatomy. II. Analysis and discussion. Zool J Linn Soc 149:1–95
Hackett SJ et al (2008) A phylogenomic study of birds reveals their evolutionary history. Science 320:1763
Acknowledgments
This work is supported by the D. Phil. Fellowship under University Grant Commission scheme to P. Chand. The authors thank the Head of the Department of Zoology, University of Allahabad, Allahabad for providing essential facilities for the present investigation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chand, P., Maurya, R.C. & Srivastava, U.C. Neuronal Morphology and Spine Density of the Visual Wulst of the Strawberry Finch, Estrilda amandava . Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 83, 627–642 (2013). https://doi.org/10.1007/s40011-013-0188-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-013-0188-4