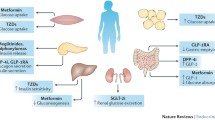
Abstract
Type 2 diabetes mellitus (T2DM) is one of the most prevalent diseases in the world. An important difference in effectiveness and toxicity of hypoglycemic agents has been associated with the presence of genetic variants in people with T2DM. We conducted a literature review up November 2015 by combining keywords type 2 diabetes mellitus, hypoglycemic agents and pharmacogenetics (PKG). Metformin, sulfonylureas, and meglitinide drugs are widely used for the T2DM treatment, although new drugs in combination with metformin are administered. Genetic variants in proteins that function as carriers, channels, or metabolizing enzymes affect both the pharmacokinetics and pharmacodynamics of these agents. Significant progress in T2DM’s pharmacogenetics has been made; however, more studies involving a larger number of patients from different ethnic groups must be done. Furthermore, patients with T2DM generally are complex patients receiving hypolipidemic and hypotensive medications. Drug-drug interaction studies between these drugs must be done to really know the contribution of each polymorphism in drug effectiveness and/or toxicity.
Similar content being viewed by others
References
Panten U, Schwanstecher M, Schwanstecher C. Sulfonylurea receptors and mechanism of sulfonylurea action. Exp Clin Endocrinol Diabetes. 1996;104:1–9. doi:10.1055/s-0029-1211414.
Sesti G, Laratta E, Cardellini M, Andreozzi F, Del Guerra S, Irace C, et al. The E23K variant of KCNJ11 encoding the pancreatic beta-cell adenosine 5′-triphosphate-sensitive potassium channel subunit Kir6.2 is associated with an increased risk of secondary failure to sulfonylurea in patients with type 2 diabetes. J Clin Endocrinol Metab. 2006;91:2334–9. doi:10.1210/jc.2005-2323.
Javorsky M, Klimcakova L, Schroner Z, Zidzik J, Babjakova E, Fabianova M, et al. KCNJ11 gene E23K variant and therapeutic response to sulfonylureas. Eur J Intern Med. 2012;23:245–9. doi:10.1016/j.ejim.2011.10.018.
Holstein A, Hahn M, Stumvoll M, Kovacs P. The E23K variant of KCNJ11 and the risk for severe sulfonylurea-induced hypoglycemia in patients with type 2 diabetes. Horm Metab Res. 2009;41:387–90. doi:10.1055/s-0029-1192019.
Zhang H, Liu X, Kuang H, Yi R, Xing H. Association of sulfonylurea receptor 1 genotype with therapeutic response to gliclazide in type 2 diabetes. Diabetes Res Clin Pract. 2007;77:58–61. doi:10.1016/j.diabres.2006.10.021.
Xu H, Murray M, McLachlan AJ. Influence of genetic polymorphisms on the pharmacokinetics and pharmaco-dynamics of sulfonylurea drugs. Curr Drug Metab. 2009;10:643–58.
Wang J, Hu F, Feng T, Zhao J, Yin L, Li L, et al. Meta-analysis of associations between TCF7L2 polymorphisms and risk of type 2 diabetes mellitus in the Chinese population. BMC Med Genet. 2013;14:8. doi:10.1186/1471-2350-14-8.
Javorský M, Schroner Z. Association between TCF7L2 genotype and glycemic control in diabetic patients treated with gliclazide. Int J Endocrinol. 2013;2013:1–5. doi:10.1155/2013/374858.
Schroner Z, Dobrikova M, Klimcakova L, Javorsky M, Zidzik J, Kozarova M, et al. Variation in KCNQ1 is associated with therapeutic response to sulphonylureas. Med Sci Monit. 2011;17:CR392–6.
Sesti G, Marini MA, Cardellini M, Sciacqua A, Frontoni S, Andreozzi F, et al. The Arg972 variant in insulin receptor substrate-1 is associated with an increased risk of secondary failure to sulfonylurea in patients with type 2 diabetes. Diabetes Care. 2004;27:1394–8.
Kirchheiner J, Brockmöller J, Meineke I, Bauer S, Rohde W, Meisel C, et al. Impact of CYP2C9 amino acid polymorphisms on glyburide kinetics and on the insulin and glucose response in healthy volunteers. Clin Pharmacol Ther. 2002;71:286–96. doi:10.1067/mcp.2002.122476.
Becker M, Visser L, Trienekens P, Hofman A, van Schaik R, Bhc S. Cytochrome P450 2C9 *2 and *3 polymorphisms and the dose and effect of sulfonylurea in type II diabetes mellitus. Clin Pharmacol Ther. 2008;83:288–92. doi:10.1038/sj.clpt.6100273.
Shon J, Yoon Y, Kim M-J, Kim K, Lim Y, Liu K, et al. Chlorpropamide 2-hydroxylation is catalysed by CYP2C9 and CYP2C19 in vitro: chlorpropamide disposition is influenced by CYP2C9, but not by CYP2C19 genetic polymorphism. Br J Clin Pharmacol. 2005;59:552–63. doi:10.1111/j.1365-2125.2005.02364.x.
Niemi M, Cascorbi I, Timm R, Kroemer HK, Neuvonen PJ, Kivistö KT. Glyburide and glimepiride pharmacokinetics in subjects with different CYP2C9 genotypes. Clin Pharmacol Ther. 2002;72:326–32. doi:10.1067/mcp.2002.127495.
Suzuki K, Yanagawa T, Shibasaki T, Kaniwa N, Hasegawa R, Tohkin M. Effect of CYP2C9 genetic polymorphisms on the efficacy and pharmacokinetics of glimepiride in subjects with type 2 diabetes. Diabetes Res Clin Pract. 2006;72:148–54. doi:10.1016/j.diabres.2005.09.019.
Tan B, Zhang Y, Chen X, Zhao X-H, Li G-X, Zhong D-F. The effects of CYP2C9 and CYP2C19 genetic polymorphisms on the pharmacokinetics and pharmacodynamics of glipizide in Chinese subjects. Eur J Clin Pharmacol. 2010;66:145–51. doi:10.1007/s00228-009-0736-2.
Lee CR, Pieper JA, Hinderliter AL, Blaisdell JA, Goldstein JA. Evaluation of cytochrome P4502C9 metabolic activity with tolbutamide in CYP2C91 heterozygotes. Clin Pharmacol Ther. 2002;72:562–71. doi:10.1067/mcp.2002.127913.
Malaisse WJ. Mechanism of action of a new class of insulin secretagogues. Exp Clin Endocrinol Diabetes. 1999;107(Suppl :S140–3). doi:10.1055/s-0029-1212170.
Dornhorst A. Insulinotropic meglitinide analogues. Lancet. 2001;358:1709–16. doi:10.1016/S0140-6736(01)06715-0.
Kirchheiner J, Meineke I, Müller G, Bauer S, Rohde W, Meisel C, et al. Influence of CYP2C9 and CYP2D6 polymorphisms on the pharmacokinetics of nateglinide in genotyped healthy volunteers. Clin Pharmacokinet. 2004;43:267–78. doi:10.2165/00003088-200443040-00005.
Cheng Y, Wang G, Zhang W, Fan L, Chen Y, Zhou H-H. Effect of CYP2C9 and SLCO1B1 polymorphisms on the pharmacokinetics and pharmacodynamics of nateglinide in healthy Chinese male volunteers. Eur J Clin Pharmacol. 2013;69:407–13. doi:10.1007/s00228-012-1364-9.
Niemi M, Backman JT, Kajosaari LI, Leathart JB, Neuvonen M, Daly AK, et al. Polymorphic organic anion transporting polypeptide 1B1 is a major determinant of repaglinide pharmacokinetics. Clin Pharmacol Ther. 2005;77:468–78. doi:10.1016/j.clpt.2005.01.018.
Kalliokoski A, Neuvonen M, Neuvonen PJ, Niemi M. Different effects of SLCO1B1 polymorphism on the pharmacokinetics and pharmacodynamics of repaglinide and nateglinide. J Clin Pharmacol. 2008;48:311–21. doi:10.1177/0091270007311569.
Ruzilawati AB, Gan SH. CYP3A4 genetic polymorphism influences repaglinide’s pharmacokinetics. Pharmacology. 2010;85:357–64. doi:10.1159/000302731.
Huang Q, Yin J-Y, Dai X-P, Wu J, Chen X, Deng C-S, et al. Association analysis of SLC30A8 rs13266634 and rs16889462 polymorphisms with type 2 diabetes mellitus and repaglinide response in Chinese patients. Eur J Clin Pharmacol. 2010;66:1207–15. doi:10.1007/s00228-010-0882-6.
Xiang Q, Cui YM, Zhao X, Yan L, Zhou Y. The influence of MDR1 G2677T/a genetic polymorphisms on the pharmacokinetics of repaglinide in healthy Chinese volunteers. Pharmacology. 2012;89:105–10. doi:10.1159/000336345.
Yu M, Xu X-J, Yin J-Y, Wu J, Chen X, Gong Z-C, et al. KCNJ11 Lys23Glu and TCF7L2 rs290487(C/T) polymorphisms affect therapeutic efficacy of repaglinide in Chinese patients with type 2 diabetes. Clin Pharmacol Ther. 2010;87:330–5. doi:10.1038/clpt.2009.242.
Yu W, Hu C, Zhang R, Wang C, Qin W, Lu J, et al. Effects of KCNQ1 polymorphisms on the therapeutic efficacy of oral antidiabetic drugs in Chinese patients with type 2 diabetes. Clin Pharmacol Ther. 2011;89:437–42. doi:10.1038/clpt.2010.351.
Huang Q, Yin J, Dai X, Pei Q, Dong M, Zhou Z, et al. IGF2BP2 variations influence repaglinide response and risk of type 2 diabetes in Chinese population. Acta Pharmacol Sin. 2010;31:709–17. doi:10.1038/aps.2010.47.
Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med. 2002;137:25–33.
Strack T. Metformin: a review. Drugs Today (Barc). 2008;44:303–14.
Lipska KJ, Bailey CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes Care. 2011;34:1431–7. doi:10.2337/dc10-2361.
Gong L, Goswami S, Giacomini KM, Altman RB, Klein TE. Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2012;22:820–7. doi:10.1097/FPC.0b013e3283559b22.
Wang L, Weinshilboum R. Metformin pharmacogenomics: biomarkers to mechanisms. Diabetes. 2014;63:2609–10. doi:10.2337/db14-0609.
Tarasova L, Kalnina I, Geldnere K, Bumbure A, Ritenberga R, Nikitina-Zake L, et al. Association of genetic variation in the organic cation transporters OCT1, OCT2 and multidrug and toxin extrusion 1 transporter protein genes with the gastrointestinal side effects and lower BMI in metformin-treated type 2 diabetes patients. Pharmacogenet Genomics. 2012;22:659–66. doi:10.1097/FPC.0b013e3283561666.
Shu Y, Brown C, Castro RA, Shi RJ, Lin ET, Owen RP, et al. Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clin Pharmacol Ther. 2008;83:273–80. doi:10.1038/sj.clpt.6100275.
Christensen MMH, Brasch-Andersen C, Green H, Nielsen F, Damkier P, Beck-Nielsen H, et al. The pharmacogenetics of metformin and its impact on plasma metformin steady-state levels and glycosylated hemoglobin A1c. Pharmacogenet Genomics. 2011;21:837–50. doi:10.1097/FPC.0b013e32834c0010.
Yang P, Nicolás JC, Galván CA, Vélez P, Da Ronco L, Díaz GT, et al. Effectiveness of metformin in patients with type II diabetes related to variants in the SLC22A1 gene | Eficácia de Metformina em doentes com diabetes tipo II, relacionado com variantes do gene SLC22A1. Acta Bioquim Clin Latinoam. 2014;48:229–35.
Song IS, Shin HJ, Shim EJ, Jung IS, Kim WY, Shon JH, et al. Genetic variants of the organic cation transporter 2 influence the disposition of metformin. Clin Pharmacol Ther. 2008;84:559–62. doi:10.1038/clpt.2008.61.
Becker ML, Visser LE, van Schaik RHN, Hofman A, Uitterlinden AG, Stricker BHC. Genetic variation in the multidrug and toxin extrusion 1 transporter protein influences the glucose-lowering effect of metformin in patients with diabetes: a preliminary study. Diabetes. 2009;58:745–9. doi:10.2337/db08-1028.
Stocker SL, Morrissey KM, Yee SW, Castro RA, Xu L, Dahlin A, et al. The effect of novel promoter variants in MATE1 and MATE2 on the pharmacokinetics and pharmacodynamics of metformin. Clin Pharmacol Ther. 2013;93:186–94. doi:10.1038/clpt.2012.210.
Choi JH, Yee SW, Ramirez AH, Morrissey KM, Jang GH, Joski PJ, et al. A common 5′-UTR variant in MATE2-K is associated with poor response to metformin. Clin Pharmacol Ther. 2011;90:674–84. doi:10.1038/clpt.2011.165.
Zhou K, Bellenguez C, Spencer CCA, Bennett AJ, Coleman RL, Tavendale R, et al. Common variants near ATM are associated with glycemic response to metformin in type 2 diabetes. Nat Genet. 2011;43:117–20. doi:10.1038/ng.735.
Hauner H. The mode of action of thiazolidinediones. Diabetes Metab Res Rev. 2012;18(Suppl 2):S10–5.
Kung J, Henry RR. Thiazolidinedione safety. Expert Opin Drug Saf. 2012;11:565–79. doi:10.1517/14740338.2012.691963.
Wang L, Teng Z, Cai S, Wang D, Zhao X, Yu K. The association between the PPARγ2 Pro12Ala polymorphism and nephropathy susceptibility in type 2 diabetes: a meta-analysis based on 9,176 subjects. Diagn Pathol. 2013;8:118. doi:10.1186/1746-1596-8-118.
Knouff C, Auwerx J. Peroxisome proliferator-activated receptor-gamma calls for activation in moderation: lessons from genetics and pharmacology. Endocr Rev. 2004;25:899–918. doi:10.1210/er.2003-0036.
Ramírez-Salazar M, Pérez-Luque E, Fajardo-Araujo M, Garza SM, Malacara JM. Effect of the Pro12Ala polymorphism of the PPAR gamma 2 gene on response to pioglitazone treatment in menopausal women. Menopause. 2008;15:1151–6. doi:10.1097/gme.0b013e31816d5b2d.
Kang ES, Park SY, Kim HJ, Kim CS, Ahn CW, Cha BS, et al. Effects of Pro12Ala polymorphism of peroxisome proliferator-activated receptor gamma2 gene on rosiglitazone response in type 2 diabetes. Clin Pharmacol Ther. 2005;78:202–8. doi:10.1016/j.clpt.2005.04.013.
Hsieh M-C, Lin K-D, Tien K-J, Tu S-T, Hsiao J-Y, Chang S-J, et al. Common polymorphisms of the peroxisome proliferator-activated receptor-gamma (Pro12Ala) and peroxisome proliferator-activated receptor-gamma coactivator-1 (Gly482Ser) and the response to pioglitazone in Chinese patients with type 2 diabetes mellitus. Metabolism. 2010;59:1139–44. doi:10.1016/j.metabol.2009.10.030.
Pei Q, Huang Q, Yang G, Zhao Y, Yin J, Song M, et al. PPAR-γ2 and PTPRD gene polymorphisms influence type 2 diabetes patients’ response to pioglitazone in China. Acta Pharmacol Sin. 2013;34:255–61. doi:10.1038/aps.2012.144.
Makino H, Shimizu I, Murao S, Kondo S, Tabara Y, Fujiyama M, et al. A pilot study suggests that the G/G genotype of resistin single nucleotide polymorphism at −420 may be an independent predictor of a reduction in fasting plasma glucose and insulin resistance by pioglitazone in type 2 diabetes. Endocr J. 2009;56:1049–58.
Wang J, Bao Y, Hu C, Zhang R, Wang C, Lu J, et al. Effects of ABCA1 variants on rosiglitazone monotherapy in newly diagnosed type 2 diabetes patients. Acta Pharmacol Sin. 2008;29:252–8. doi:10.1111/j.1745-7254.2008.00744.x.
Daily EB, Aquilante CL. Cytochrome P450 2C8 pharmacogenetics: a review of clinical studies. Pharmacogenomics. 2009;10:1489–510. doi:10.2217/pgs.09.82.
Stage TB, Christensen MMH, Feddersen S, Beck-Nielsen H, Brøsen K. The role of genetic variants in CYP2C8, LPIN1, PPARGC1A and PPARγ on the trough steady-state plasma concentrations of rosiglitazone and on glycosylated haemoglobin A1c in type 2 diabetes. Pharmacogenet Genomics. 2013;23:219–27. doi:10.1097/FPC.0b013e32835f91fc.
Zhou F, Huang Q, Dai X, Yin J, Wu J, Zhou H, et al. Impact of retinol binding protein 4 polymorphism on rosiglitazone response in Chinese type 2 diabetic patients. Zhong Nan Da Xue Xue Bao Yi Xue Ban2011;36:949–57. doi:10.3969/j.issn.1672-7347.2011.10.004.
Kang ES, Park SY, Kim HJ, Ahn CW, Nam M, Cha BS, et al. The influence of adiponectin gene polymorphism on the rosiglitazone response in patients with type 2 diabetes. Diabetes Care. 2005;28:1139–44. doi:10.2337/diacare.28.5.1139.
van de Laar FA. Alpha-glucosidase inhibitors in the early treatment of type 2 diabetes. Vasc Health Risk Manag. 2008;4:1189–95.
Andrulionyte L, Kuulasmaa T, Chiasson J-L, Laakso M. Single nucleotide polymorphisms of the peroxisome proliferator-activated receptor-alpha gene (PPARA) influence the conversion from impaired glucose tolerance to type 2 diabetes: the STOP-NIDDM trial. Diabetes. 2007;56:1181–6. doi:10.2337/db06-1110.
Andrulionytè L, Zacharova J, Chiasson J-L, Laakso M. Common polymorphisms of the PPAR-gamma2 (Pro12Ala) and PGC-1alpha (Gly482Ser) genes are associated with the conversion from impaired glucose tolerance to type 2 diabetes in the STOP-NIDDM trial. Diabetologia. 2004;47:2176–84. doi:10.1007/s00125-004-1577-2.
Scheen AJ. A review of gliptins for 2014. Expert Opin Pharmacother. 2015;16:43–62. doi:10.1517/14656566.2015.978289.
Filippatos TD, Athyros VG, Elisaf MS. The pharmacokinetic considerations and adverse effects of DPP-4 inhibitors [corrected]. Expert Opin Drug Metab Toxicol. 2014;10:787–812. doi:10.1517/17425255.2014.907274.
Zimdahl H, Ittrich C, Graefe-Mody U, Boehm BO, Mark M, Woerle H-J, et al. Influence of TCF7L2 gene variants on the therapeutic response to the dipeptidylpeptidase-4 inhibitor linagliptin. Diabetologia. 2014;57:1869–75. doi:10.1007/s00125-014-3276-y.
Asakura M, Fujii H, Atsuda K, Itoh T, Fujiwara R. Dipeptidyl peptidase-4 greatly contributes to the hydrolysis of vildagliptin in human liver. Drug Metab Dispos. 2015;43:477–84. doi:10.1124/dmd.114.062331.
Beinborn M, Worrall CI, McBride EW, Kopin AS. A human glucagon-like peptide-1 receptor polymorphism results in reduced agonist responsiveness. Regul Pept. 2005;130:1–6. doi:10.1016/j.regpep.2005.05.001.
Christensen M, Knop FK. Once-weekly GLP-1 agonists: how do they differ from exenatide and liraglutide? Curr Diab Rep. 2010;10:124–32. doi:10.1007/s11892-010-0102-x.
de Luis DA, Ovalle HF, Soto GD, Izaola O, de la Fuente B, Romero E. Role of genetic variation in the cannabinoid receptor gene (CNR1) (G1359A polymorphism) on weight loss and cardiovascular risk factors after liraglutide treatment in obese patients with diabetes mellitus type 2. J Investig Med. 2014;62:324–7. doi:10.231/JIM.0000000000000032.
Jabbour SA, Goldstein BJ. Sodium glucose co-transporter 2 inhibitors: blocking renal tubular reabsorption of glucose to improve glycaemic control in patients with diabetes. Int J Clin Pract. 2008;62:1279–84. doi:10.1111/j.1742-1241.2008.01829.x.
Geerlings S, Fonseca V, Castro-Diaz D, List J, Parikh S. Genital and urinary tract infections in diabetes: impact of pharmacologically-induced glucosuria. Diabetes Res Clin Pract. 2014;103:373–81. doi:10.1016/j.diabres.2013.12.052.
Yu L, Lv J-C, Zhou X, Zhu L, Hou P, Zhang H. Abnormal expression and dysfunction of novel SGLT2 mutations identified in familial renal glucosuria patients. Hum Genet. 2011;129:335–44. doi:10.1007/s00439-010-0927-z.
Enigk U, Breitfeld J, Schleinitz D, Dietrich K, Halbritter J, Fischer-Rosinsky A, et al. Role of genetic variation in the human sodium-glucose cotransporter 2 gene (SGLT2) in glucose homeostasis. Pharmacogenomics. 2011;12:1119–26. doi:10.2217/pgs.11.69.
Kasichayanula S, Liu X, Griffen SC, Lacreta FP, Boulton DW. Effects of rifampin and mefenamic acid on the pharmacokinetics and pharmacodynamics of dapagliflozin. Diabetes Obes Metab. 2013;15:280–3. doi:10.1111/dom.12024.
Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2016 executive summary. Endocr Pract. 2016;22:84–113. doi:10.4158/EP151126.CS.
Acknowledgments
PY was supported by the Training Program from UCC-CONICET. This work was supported by UCC grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical responsibilities of authors
The manuscript has not been submitted to any other journals, has not been published previously, and has not been fabricated or manipulated.
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
The informed consent of each patient was collected in each of the cited articles.
Rights and permissions
About this article
Cite this article
Yang, P., Heredia, V.O., Beltramo, D.M. et al. Pharmacogenetics and personalized treatment of type 2 diabetes mellitus. Int J Diabetes Dev Ctries 36, 508–518 (2016). https://doi.org/10.1007/s13410-016-0517-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-016-0517-2