Abstract
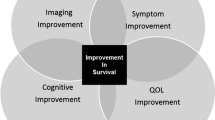
Primary brain tumors and their treatment are associated with a significant impact on function and quality of life (QOL). Patient-reported outcomes (PROs) are measures that allow report of the impact directly from the patient. Instruments to measure both QOL and symptom burden have been developed for use in the primary brain tumor patient population. Use of these instruments coupled with tumor response assessment and other objective measures will allow for evaluation of the net clinical benefit for the patient.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Armstrong TS. Head's up on the treatment of malignant glioma patients. Oncol Nurs Forum. 2009;36:E232–40.
Cahill JE, Armstrong TS. Caring for an adult with a malignant primary brain tumor. Nursing. 2011;41:28–33. quiz 33-4.
Armstrong TS, Vera-Bolanos E, Gilbert MR. Clinical course of adult patients with ependymoma: results of the Adult Ependymoma Outcomes Project. Cancer. 2011;117:5133–41.
Bradley SE, Sherwood PR, Kuo J, et al. Perceptions of economic hardship and emotional health in a pilot sample of family caregivers. J Neurooncol. 2009;93:333–42.
Mackworth N, Fobair P, Prados MD. Quality of life self-reports from 200 brain tumor patients: comparisons with Karnofsky performance scores. J Neurooncol. 1992;14:243–53.
Salander P, Bergenheim AT, Henriksson R. How was life after treatment of a malignant brain tumour? Soc Sci Med. 2000;51:589–98.
Strang S, Strang P. Spiritual thoughts, coping and 'sense of coherence' in brain tumour patients and their spouses. Palliat Med. 2001;15:127–34.
Strang S, Strang P, Ternestedt BM. Existential support in brain tumour patients and their spouses. Support Care Cancer. 2001;9:625–33.
Bradley S, Sherwood PR, Donovan HS, et al. I could lose everything: understanding the cost of a brain tumor. J Neurooncol. 2007;85:329–38.
• Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. Important paper establishing level 1 evidence of the impact of chemo-radiation on outcome in GBM.
Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66.
Mauer ME, Bottomley A, Taphoorn MJ. Evaluating health-related quality of life and symptom burden in brain tumour patients: instruments for use in experimental trials and clinical practice. Curr Opin Neurol. 2008;21:745–53.
Lamborn KR, Yung WK, Chang SM, et al. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10:162–70.
Polley MY, Lamborn KR, Chang SM, et al. Six-month progression-free survival as an alternative primary efficacy endpoint to overall survival in newly diagnosed glioblastoma patients receiving temozolomide. Neuro Oncol. 2010;12:274–82.
Quant EC, Wen PY. Response assessment in neuro-oncology. Curr Oncol Rep. 2011;13:50–6.
Meyers CA, Rock EP, Fine HA. Refining endpoints in brain tumor clinical trials. J Neurooncol. 2012;108:227–30.
Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–72.
Chamberlain MC. Pseudoprogression in glioblastoma. J Clin Oncol. 2008;26:4359. author reply 4359-60.
Fink J, Born D, Chamberlain MC. Pseudoprogression: relevance with respect to treatment of high-grade gliomas. Curr Treat Options Oncol. 2011;12:240–52.
Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–87.
Chamberlain MC. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006;67:2089. author reply 2089.
Lucas J, Zada G. Radiology: criteria for determining response to treatment and recurrence of high-grade gliomas. Neurosurg Clin N Am. 2012;23:269–76. viii.
Lassman AB, Holland EC. Incorporating molecular tools into clinical trials and treatment for gliomas? Curr Opin Neurol. 2007;20:708–11.
Nelson SJ. Assessment of therapeutic response and treatment planning for brain tumors using metabolic and physiological MRI. NMR Biomed. 2011;24:734–49.
•• Arpinelli F, Bamfi F. The FDA guidance for industry on PROs: the point of view of a pharmaceutical company. Health Qual Life Outcomes. 2006;4:85. Paper outlining FDA position on the use of PRO instruments.
DeMuro C, Clark M, Mordin M, et al. Reasons for rejection of patient-reported outcome label claims: a compilation based on a review of patient-reported outcome use among new molecular entities and biologic license applications, 2006-2010. Value Health. 2012;15:443–8.
Gnanasakthy A, Mordin M, Clark M, et al. A review of patient-reported outcome labels in the United States: 2006 to 2010. Value Health. 2012;15:437–42.
Rock EP, Kennedy DL, Furness MH, et al. Patient-reported outcomes supporting anticancer product approvals. J Clin Oncol. 2007;25:5094–9.
Patrick DL, Burke LB, Powers JH, et al. Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health. 2007;10 Suppl 2:S125–37.
Galanis E, Wu W, Cloughesy T, et al. Phase 2 trial design in neuro-oncology revisited: a report from the RANO group. Lancet Oncol. 2012;13:e196–204.
• Armstrong TS, Mendoza T, Gning I, et al. Validation of the M.D. Anderson Symptom Inventory Brain Tumor Module (MDASI-BT). J Neurooncol. 2006;80:27–35. PRO instrument developed for report of symtpom burden in brain tumor patients.
Armstrong TS, Cohen MZ, Eriksen L, et al. Content validity of self-report measurement instruments: an illustration from the development of the Brain Tumor Module of the M.D. Anderson Symptom Inventory. Oncol Nurs Forum. 2005;32:669–76.
Armstrong TS, Gning I, Mendoza TR, et al. Clinical utility of the MDASI-BT in patients with brain metastases. J Pain Symptom Manag. 2009;37:331–40.
Armstrong TS, Vera-Bolanos E, Gning I, et al. The impact of symptom interference using the MD Anderson Symptom Inventory-Brain Tumor Module (MDASI-BT) on prediction of recurrence in primary brain tumor patients. Cancer. 2011;117:3222–8.
Lien K, Zeng L, Nguyen J, et al. FACT-Br for assessment of quality of life in patients receiving treatment for brain metastases: a literature review. Expert Rev Pharmacoecon Outcomes Res. 2011;11:701–8.
• Weitzner MA, Meyers CA, Gelke CK, et al. The Functional Assessment of Cancer Therapy (FACT) scale. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer. 1995;75:1151–61. Validation of Quality of life instrument for primary brain tumor patients.
• Taphoorn MJ, Claassens L, Aaronson NK, et al. An international validation study of the EORTC brain cancer module (EORTC QLQ-BN20) for assessing health-related quality of life and symptoms in brain cancer patients. Eur J Cancer. 2010;46:1033–40. Validation of Quality of life instrument for primary brain tumor patients.
Mauer M, Stupp R, Taphoorn MJ, et al. The prognostic value of health-related quality-of-life data in predicting survival in glioblastoma cancer patients: results from an international randomised phase III EORTC Brain Tumour and Radiation Oncology Groups, and NCIC Clinical Trials Group study. Br J Cancer. 2007;97:302–7.
Mauer ME, Taphoorn MJ, Bottomley A, et al. Prognostic value of health-related quality-of-life data in predicting survival in patients with anaplastic oligodendrogliomas, from a phase III EORTC brain cancer group study. J Clin Oncol. 2007;25:5731–7.
• Cleeland CS, Sloan JA. Assessing the Symptoms of Cancer Using Patient-Reported Outcomes (ASCPRO): searching for standards. J Pain Symptom Manag. 2010;39:1077–85. Paper reviewing position of ASCPRO committee on issues related to standards for use of PROs.
Reyes-Gibby CC, Aday L, Cleeland C. Impact of pain on self-rated health in the community-dwelling older adults. Pain. 2002;95:75–82.
Schwartz CE, Bode R, Repucci N, et al. The clinical significance of adaptation to changing health: a meta-analysis of response shift. Qual Life Res. 2006;15:1533–50.
Tierney DK, Facione N, Padilla G, et al. Response shift: a theoretical exploration of quality of life following hematopoietic cell transplantation. Cancer Nurs. 2007;30:125–38.
Armstrong TS, Cron SG, Bolanos EV, et al. Risk factors for fatigue severity in primary brain tumor patients. Cancer. 2010;116:2707–15.
Fox SW, Lyon D, Farace E. Symptom clusters in patients with high-grade glioma. J Nurs Scholarsh. 2007;39:61–7.
Gleason Jr JF, Case D, Rapp SR, et al. Symptom clusters in patients with newly-diagnosed brain tumors. J Support Oncol. 2007;5:427–33. 436.
Cleeland CS: Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr:16-21, 2007
Armstrong TS, Wefel JS, Won M, et al: Abstract 2016: Clinical utility of neurocognitive function (NCF), quality of life (QOL), and symptom assessment as prognostic factors for survival and measures of treatment effects on RTOG 0525. Journal of Clinical Oncology 29 2011
Witgert ME, Meyers CA. Neurocognitive and quality of life measures in patients with metastatic brain disease. Neurosurg Clin N Am. 2011;22:79–85. vii.
Nekolaichuk CL, Maguire TO, Suarez-Almazor M, et al. Assessing the reliability of patient, nurse, and family caregiver symptom ratings in hospitalized advanced cancer patients. J Clin Oncol. 1999;17:3621–30.
Nekolaichuk CL, Bruera E, Spachynski K, et al. A comparison of patient and proxy symptom assessments in advanced cancer patients. Palliat Med. 1999;13:311–23.
Teske K, Daut RL, Cleeland CS. Relationships between nurses' observations and patients' self-reports of pain. Pain. 1983;16:289–96.
Parsaie FA, Golchin M, Asvadi I. A comparison of nurse and patient perceptions of chemotherapy treatment stressors. Cancer Nurs. 2000;23:371–4.
Puntillo K, Neighbor M, O'Neil N, et al. Accuracy of emergency nurses in assessment of patients' pain. Pain Manag Nurs. 2003;4:171–5.
Barsevick AM, Cleeland CS, Manning DC, et al. ASCPRO recommendations for the assessment of fatigue as an outcome in clinical trials. J Pain Symptom Manag. 2010;39:1086–99.
Cella D, Bullinger M, Scott C, et al. Group vs individual approaches to understanding the clinical significance of differences or changes in quality of life. Mayo Clin Proc. 2002;77:384–92.
Tait RC, Chibnall JT, Kalauokalani D. Provider judgments of patients in pain: seeking symptom certainty. Pain Med. 2009;10:11–34.
Sprangers MA, Aaronson NK. The role of health care providers and significant others in evaluating the quality of life of patients with chronic disease: a review. J Clin Epidemiol. 1992;45:743–60.
Davies E, Clarke C. Views of bereaved relatives about quality of survival after radiotherapy for malignant cerebral glioma. J Neurol Neurosurg Psychiatry. 2005;76:555–61.
Armstrong TS, Wefel JS, Gning I, et al: Congruence of primary brain tumor patient and caregiver symptom report. Cancer, 2012
Brown PD, Decker PA, Rummans TA, et al. A prospective study of quality of life in adults with newly diagnosed high-grade gliomas: comparison of patient and caregiver ratings of quality of life. Am J Clin Oncol. 2008;31:163–8.
Cleeland CS. Cancer-related symptoms. Semin Radiat Oncol. 2000;10:175–90.
Corkrey R, Parkinson L. Interactive voice response: review of studies 1989-2000. Behav Res Methods Instrum Comput. 2002;34:342–53.
Corkrey R, Parkinson L. A comparison of four computer-based telephone interviewing methods: getting answers to sensitive questions. Behav Res Methods Instrum Comput. 2002;34:354–63.
Lustria ML, Cortese J, Noar SM, et al. Computer-tailored health interventions delivered over the Web: review and analysis of key components. Patient Educ Couns. 2009;74:156–73.
Dumrongpakapakorn P, Hopkins K, Sherwood P, et al. Computer-mediated patient education: opportunities and challenges for supporting women with ovarian cancer. Nurs Clin North Am. 2009;44:339–54.
Alvina AA, Gilbert MR, Armstrong TS: Feasibility of Use of Electronic Data Capture in Primary Brain Tumor Patients Neuro-Oncology, In Press
Snyder CF, Watson ME, Jackson JD, et al. Patient-reported outcome instrument selection: designing a measurement strategy. Value Health. 2007;10 Suppl 2:S76–85.
Burris H, Storniolo AM. Assessing clinical benefit in the treatment of pancreas cancer: gemcitabine compared to 5-fluorouracil. Eur J Cancer. 1997;33 Suppl 1:S18–22.
Burris 3rd HA, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Armstrong, T.S. Measuring Clinical Benefit: Use of Patient-Reported Outcomes (PRO) in Primary Brain Tumor Clinical Trials. Curr Oncol Rep 15, 27–32 (2013). https://doi.org/10.1007/s11912-012-0276-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-012-0276-2