Abstract
Objectives
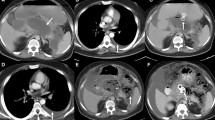
Low-dose ionizing radiation from medical imaging has been indirectly linked with subsequent cancer. Computed tomography (CT) is the gold standard for defining pancreatic necrosis. The primary goal was to identify the frequency and effective radiation dose of CT imaging for patients with necrotizing pancreatitis.
Methods
All patients with necrotizing pancreatitis (2003–2007) were retrospectively analyzed for CT-related radiation exposure.
Results
Necrosis was identified in 18% (238/1290) of patients with acute pancreatitis (mean age = 53 years; hospital/ICU length of stay = 23/7 days; mortality = 9%). A median of five CTs/patient [interquartile range (IQR) = 4] were performed during a median 2.6-month interval. The average effective dose was 40 mSv per patient (equivalent to 2,000 chest X-rays; 13.2 years of background radiation; one out of 250 increased risk of fatal cancer). The actual effective dose was 63 mSv considering various scanner technologies. CTs were infrequently (20%) followed by direct intervention (199 interventional radiology, 118 operative, 12 endoscopic) (median = 1; IQR = 2). Magnetic resonance imaging did not have a CT-sparing effect. Mean direct hospital costs increased linearly with CT number (R = 0.7).
Conclusions
The effective radiation dose received by patients with necrotizing pancreatitis is significant. Management changes infrequently follow CT imaging. The ubiquitous use of CT in necrotizing pancreatitis raises substantial public health concerns and mandates a careful reassessment of its utility.
Similar content being viewed by others
References
Neoptolemos JP, Raraty M, Finch M, Sutton R. Acute pancreatitis: the substantial human and financial costs. Gut 1998;42:886–891.
Uhl W, Warshaw A, Imrie C, Bassi C, McKay CJ, Lankisch PG, Carter R, Di Magno E, Banks PA, Whitcomb DC, Dervenis C, Ulrich CD, Satake K, Ghaneh P, Hartwig W, Werner J, McEntee G, Neoptolemos JP, Buchler MW. IAP guidelines for the surgical management of acute pancreatitis. Pancreatology 2002;2:565–573.
Frey CF, Zhou H, Harvey DJ, White RH. The incidence and case-fatality rates of acute biliary, alcoholic, and idiopathic pancreatitis in California, 1994–2001. Pancreas 2006;33:336–344.
Bradley EL 3rd, Howard TJ, van Sonnenberg E, Fotoohi M. Intervention in necrotizing pancreatitis: an evidence-based review of surgical and percutaneous alternatives. J Gastrointest Surg 2008;12:634–639.
Howard TJ, Patel JB, Zyromski NJ, Sandrasegaran K, Yu J, Nakeeb A, Pitt HA, Lillemoe KD. Declining morbidity and mortality rates in the surgical management of pancreatic necrosis. J Gastrointest Surg 2007;11:43–49.
Parikh PY, Pitt HA, Kilbane M, Howard TJ, Nakeeb A, Schmidt CM, Lillemoe KD, Zyromski NJ. Pancreatic necrosectomy: North American mortality is much lower than expected. J Am Coll Surg 2009;209:712–719.
Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11–13, 1992. Arch Surg 1993;128:586–590.
Karimgani I, Porter KA, Langevin RE, Banks PA. Prognostic factors in sterile pancreatic necrosis. Gastroenterology 1992;103:1636–1640.
Charbonney E, Nathens AB. Severe acute pancreatitis: A review. Surg Infect 2008;9:573–578.
Balthazar EJ, Ranson JH, Naidich DP, Megibow AJ, Caccavale R, Cooper MM. Acute pancreatitis: Prognostic value of CT. Radiology 1985;156:767–772.
De Waele JJ, Delrue L, Hoste EA, De Vos M, Duyck P, Colardyn FA. Extrapancreatic inflammation on abdominal computed tomography as an early predictor of disease severity in acute pancreatitis: evaluation of a new scoring system. Pancreas 2007;34:185–190.
Hui CM, MacGregor JH, Tien HC, Kortbeek JB. Radiation dose from initial trauma assessment and resuscitation: review of the literature. Can J Surg 2009;52:147–152.
Fazel R, Krumholz HM, Wang Y, Ross JS, Chen J, Ting HH, Shah ND, Nasir K, Einstein AJ, Nallamothu BK. Exposure from low-dose ionizing radiation from medical imaging procedures. NEJM 2009;361:849–857.
Brenner DJ, Hall EJ. Computed tomography—An increasing source of radiation exposure. NEJM 2009;357:2277–2284.
Brix G, Nissen-Meyer S, Lechel U, Nissen-Meyer J, Griebel J, Nekolaa AE, Becker C, Reiser M. Radiation exposures of cancer patients from medical x-rays: How relevant are they for individual patients and population exposure? Eur J Rad 2009; 72(2):342–347
Sources and effects of ionizing radiation: United Nations Scientific Committee on the effects of atomic radiation: UNESCEAR 2000 report to the General Assembly. New York: United Nations, 2000.
IMV 2006 CT Market Summary Report. Des Plains, IL: IMV Medical Information Division, 2006.
Amis Jr ES, Butler PF, Applegate KE, Birnbaum SB, Brateman LF, Hevezi JM, Mettler FA, Morin RL, Pentecost MJ, Smith GC, Strauss KJ, Zeman RK. American College of Radiology. American College of Radiology White paper on radiation dose in medicine. J Am Coll Radiol 2007;4:272–284.
Nekolla E, Veit R, Griebel J, Brix G. Frequency and effective dose of diagnostic x-ray procedures in Germany. Biomed Tech 2005;5-:1334–1335.
Mettler FA. Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: A catalog. Radiology 2008;248:254–263.
Nishizawa K, Matsumoto M, Iwai K, Maruyama T. Survey of CT practice in Japan and collective effective dose estimation. Nippon Acta Radiol 2004;64:151–158.
Goldacer MJ, Roberts SE. Hospital admission for acute pancreatitis in an English population, 1963–98: database study of incidence and mortality. BMJ 2004;328:1466–1469.
Eland IA, Sturkenboom MJ, Wilson JH, Sticker BH. Incidence and mortality of acute pancreatitis between 1985 and 1995. Scand J Gastroenterol 2000;35:1110–1116.
Floyd A, Pederson L, Nielsen GL, Thorladcius-Ussing O, Sorensen HT. Secular trends in incidence and 30-day case fatality of acute pancreatitis in North Jutland County, Denmark: a register-based study from 1981–2000. Scand J Gastroeterol 2002;37:1461–1465.
Appelros S, Borgstrom A. Incidence, etiology and mortality rate of acute pancreatitis over 10 years in a defined urban population in Sweden. Br J Surg 1999;86:465–470.
National Research Council. Health risks from exposure to low levels of ionizing radiation: BEIR VII phase 2. Washington, DC: National Academic Press, 2006.
Ambrose J, Hounsfield G. Computerized transverse axial tomography. Br J Radiol 1973;46:148–149.
Frush DP. Review of radiation issues for computed tomography. Semin Ultrasound CT MR 2004;25:17–24.
Mayo JR, Aldrich J, Muller NL. Radiation exposure at chest CT: a statement of the Fleischner Society. Radiology 2003;228:15–21.
Hamilton DR, Murray JD, Ball CG. Cardiac health for astronauts: coronary calcification scores and CRP as criteria for selection and retention. Aviat Space Environ Med 2006;77:377–387.
Ball CG, Kirkpatrick AW, Feliciano DV. Can J Surg. The occult pneumothorax: what have we learned? Can J Surg 2009;52:E173–E179.
Heiken JP, Peterson CM, Menias CO. Virtual colonoscopy for colorectal cancer screening: current status. Cancer Imaging 2005;5:S133–S139.
Brenner DJ, Georgsson MA. Mass screening with CT colonography: should the radiation exposure be of concern? Gastroenterology 2005;129:328–337.
Henschke CL, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS. Survival of patients with stage I lung cancer detected on CT screening. NEJM 2006;355:1763–1771.
Bach PB, Jett JR, Pastorino U, Tockman MS, Swensen SJ, Begg CB. Computed tomography screening and lung cancer outcomes. JAMA 2007;297:953–961.
Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associates with radiation exposure from 64-slice computed tomography coronary angiography. JAMA 2007;18:317–323.
Brenner DJ, Elliston CD. Estimated radiation risks potentially associated with full-body CT screening. Radiology 2004;232:735–738.
Beinfeld MT, Wittenberg E, Gazella GS. Cost-effectiveness of whole-body CT screening. Radiology 2005;234: 415–422.
UNSCEAR 2000. The United Nations Scientific Committee on the effects of atomic radiation. Health Phys 2000;79:314.
Kim PK, Gracias VH, Maidment AD, O’Shea M, Reilly PM, Schwab CW. Cumulative radiation dose caused by radiologic studies in critically ill trauma patients. J Trauma 2004;57:510–514.
What’s NEXT? Nationwide Evaluation of X-ray Trends: 2000 computed tomography. Conference of Radiation Control Program Directors, Department of Health and Human Services. 2006. http://www.crcpd.org/Pubs/NexTrifolds/NEXT2000CT_T.pdf. Accessed on November 2, 2009.
Ron E. Ionizing radiation and cancer risk: evidence from epidemiology. Pediatr Radiol 2002;32:232–237.
Tubiana M. Computed tomography and radiation exposure. NEJM 2009;358:850–853.
Breckow J. Linear-no-threshold is a radiation-protection standard rather than a mechanistic effect model. Radiat Environ Biophys 2006;44:257–260.
Tubiana M, Aurengo A, Averbeck D, Masse R. The debate on the use of linear no threshold for assessing the effects of low doses. J Radiol Prot 2006;26:317–324.
Tubiana M, Aurengo A, Averbeck D, Masse R. Low-dose risk assessment: comments on the summary of the International Workshop. Radiat Res 2007;167:742–744.
Giles J. Study warns of ‘avoidable’ risks of CT scans. Nature 2004;431:391.
Casarett G, Bair WJ, Meinhold CB. NCRP Report No. 91. Recommendations on limited exposure to ionizing radiation. Bethesda (MD) National Council on Radiation Protection and Measurements. 1980; p. 3–10.
International Commission on Radiation Protection. Development of the Draft 2005 recommendations of the ICRP: a collection of papers. A report of ICRP supporting guidance 4. Ann ICRP 2004;34 Suppl:1–44.
American College of Surgeons: Committee on Trauma. National Trauma Data Bank 2009 Annual Report. http://www.facs.org/trauma/ntdb/ntdbannualreport2009.pdf. Accessed on November 17, 2009.
Garfinkel L. Probability of developing or dying of cancer. United States, 1991. Sta Bull Metrop Insur Co 1995;76:1–201.
Slovis T. CT and computed radiography: the pictures are great, but is the radiation dose greater than required? AJR Am J Roentgenol 2002;179:39–41.
Tien HC, Tremblay LN, Rizoli SB, Gelberg J, Spencer F, Caldwell C, Brenneman FD. Radiation exposure from diagnostic imaging in severely injured trauma patients. J Trauma 2007;176:289–296.
Strate T, Yekebas E, Knoefel WT, Bloechle C, Izbicki JR. Pathogenesis and the natural course of chronic pancreatitis. Eur J Gastroenterol Hepatol 2002;14:929–934.
Yamada Y, Mori H, Matsumoto S, Kiyosue H, Hori Y, Hongo N. Pancreatic adenocarcinoma versus pancreatitis: differentiation with triple-phase helical CT. Abdom Imaging 2010. 35(2):163–171.
Morgan DE, Ragheb CM, Lockhart ME, Cary B, Fineberg NS, Berland LL. Acute pancreatitis: Computed tomography utilization and radiation exposure are related to severity but not patient age. Clin Gastroenterol Hepatol 2010;8:303–308.
United States Nuclear Regulatory Commission. Regulation (10 CFR), subpart B. Washington, DC: Nuclear Regulatory Commission. http://www.nrc.gov/reading-rm/doc-collections/cfr/part02/part020-1101.html. Accessed on November 2, 2009.
Prasad KN, Cole WC, Haase GM. Radiation protection in humans: extending the concept of low as reasonably achievable (ALARA) from dose to biological damage. Br J Radiol 2003;327:371–372.
Plurad D, Green D, Demetriades D, Rhee P. The increasing use of chest computed tomography for trauma: is it being overutilized? J Trauma 2007;62:631–5.
Lee CI, Haims AH, Monico EP, Forman HP. Diagnostic CT scans: assessment of patient, physician, and radiologist awareness of radiation dose and possible risk. Radiology 2004; 231:393–398.
Goske MJ, Applegate KE, Boylan J, Butler PR, Callahan MJ, Coley BD, Farley S, Frush DP, Hernanz-Schulman M, Jaramillo D, Johnson ND, Kaste SC, Morrison G, Strauss KJ. Image Gently(SM): a national education and communication campaign in radiology using the science of social marketing. J Am Coll Radiol 2008;5:1200–1205.
Gerber TC, Carr JJ, Arai AE, Dixon RL, Ferrari VA, Gomes AS, Heller GV, McCollough CH, McNitt-Gray MF, Mettler FA, Mieres JH, Morin RL, Yester MV. Ionizing radiation in cardiac imaging: a science advisory from the American Heart Association Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention 2009;119:1056–1065.
McCollough CH. CT dose: How to measure, how to reduce. Health Phys 2008;95:508–517.
Lee CH, Goo JM, Ye HJ, Ye SJ, Park CM, Chun EJ, Im JG. Radiation dose modulation techniques in the multidetector CT era: from basics to practice. Radiographics 2008;28:1451–1459.
Acknowledgements
We would like to acknowledge Karl Mockler for the technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Discussant
Dr. Jeffrey B. Matthews (Chicago, IL): You have highlighted a very important issue that transcends this disease, which is the issue of reducing radiation exposure among hospitalized patients who are undergoing treatment for various disorders. It is obviously particularly a problem for patients with necrotizing pancreatitis because of the frequent interest in getting follow-up imaging studies. Your study poses the larger question of diagnostic restraint, trying to optimize that ratio between the number of times we get an imaging study and the times we are going to intervene on the basis of the findings. When we are dealing with necrotizing pancreatitis, I think sometimes we have to remind ourselves that this is not a disease that responds to radiation therapy, but it is the nature of the disease that repeat imaging is going to be needed because so many of these people are going to require repeat intervention.
In many institutions, including our own, we image pancreatic disease with a tri-phasic CT scan. You have used bi-phasic in 35% of your patients, and while these thin-slice studies are very useful as an initial way to define the extent of disease in a variety of pancreatic conditions, it may or may not be necessary for the follow-up studies (and one can question whether it is really needed even at the initial presentation of acute pancreatitis). So I think there is certainly an opportunity to reduce the exposure. In our institution, our radiologists push back very hard on our almost reflexive ordering of pancreatic protocol CTs. Have you started to put in place tighter protocols in your institution to reduce the use of multiphase studies as the frequency of these studies?
Secondly, you made the point that, in your retrospective study, the use of MR did not alter the number of total CT images. I think that may also reflect the fact that we, as surgeons, simply find it easier to read CTs rather than MR images. While it is difficult to obtain MR imaging in critically ill ICU patients, there is probably also an opportunity to substitute MR for CT to follow the progression of collections during convalescence. Going forward, are you doing anything to increase the use of MR as your routine follow-up studies to reducing the number of times that you would be using multiphasic CT studies?
Closing Discussant
Dr. Chad G. Ball: Whether you are talking about young trauma patients and injury screening technologies, or about surveillance in necrotizing pancreatitis, I think many of these issues are fundamentally the same. As a result, I divide this topic into three separate areas.
First, is the test going to change your management? This is clearly a physician-driven factor. It is also different for everybody within their individual practice. As a result, it relies on personal vigilance.
The second component is hardware. With recent improvements in scanner hardware, the effective dose is going down almost exponentially. This refers to the number of channels or the number of slices. So a 128 scanner is not just twice as good as a 64-slice scanner; it is substantially more than that in terms of reducing the effective dose.
The third concept that is important to this issue is the wizards who write the scanner software. Techniques such as progressive modulation and automatic exposure control are but two examples. There is a whole host of very neat trickery. With every iteration, a new version of their software is substantially better. Although some of these tricks are specific to trauma patient screening, most are still relevant to all patients. You also want a radiology group that is going to be active and be willing to absorb the financial cost of updating software and hardware because outside of the individual ordering physician, that is the only way to limit the effective dose.
Shielding non-scanned body parts is also a helpful tool. Unfortunately, it is something we tend to ignore and therefore the practical reality is it does not happen very often.
The MR question is a very intriguing one. Indiana University is one of the most aggressive MR pancreas institutions in the world. They have done somewhere between 3,500 and 4,000 pancreas-specific MRs. The radiologists are particularly proud of this practice. The truth, however, is that as surgeons, we use it most commonly in a clinical setting to evaluate ductal integrity and therefore to avoid getting into unplanned skullduggery within the operating room in scenarios such as disconnected left pancreatic remnants.
In terms of the CT-sparing effect, sure, if you were going to get five CTs plus an MR, then theoretically it spared a CT in that given patient. When we looked at it retrospectively, however, those patients, at the end of the day, were getting the same number of CT scans as the non-MRI folks. Do I think that is something that has to be a significant focus moving forward at our institution, as well as elsewhere? Absolutely. All non-radiation technologies must be explored as potential options. The last point I will make is that MR imaging is limited somewhat if you have a large fluid collection associated with necrotizing pancreatitis. It makes it tough to delineate some of the typical markers that we all look for.
Discussant
Dr. Charles Vollmer, Jr. (Boston, MA): This is a fantastic job. Great work.
Two quick questions: Were you able to break down the proportion of these scans that were done in the diagnostic mode before the definitive intervention and then those obtained thereafter, after a definitive intervention? I know that could be a little hard to ascertain because there are multiple interventions in some of these cases, but I am just wondering how much of this is because we are worried about when to act, how to act and when to pull the trigger, versus thereafter; Did we do a good job? Are we surveilling and following up the effect of the intervention?
The second question is along the lines of this MRI discussion by Dr Matthews. Could we even help and simplify this even better and save costs by using ultrasounds, since by and large most of us are worried about fluid collection development and management?
Closing Discussant
Dr. Chad G. Ball: The simple answer to your first question is that about one fifth of the radiation exposure is up-front. The reality is that at IU, there are arguably eight very busy pancreatic surgeons, and the individual practice variance is substantial. I suspect that if you looked at other institutions across the country, the way people use CT, MRI, and ERCP in their clinical management of this disease probably varies dramatically. As a result, it is a tough question to answer by just saying 20%. I think it is more complicated.
Your second question is a very interesting idea. I use a significant amount of ultrasonography in the care of critically injured patients. Although I do not necessarily know too much data about using it in the context you mention, it seems like a great thought.
Rights and permissions
About this article
Cite this article
Ball, C.G., Correa-Gallego, C., Howard, T.J. et al. Radiation Dose from Computed Tomography in Patients with Necrotizing Pancreatitis: How Much Is Too Much?. J Gastrointest Surg 14, 1529–1535 (2010). https://doi.org/10.1007/s11605-010-1314-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-010-1314-8