Abstract
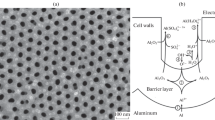
Aluminium anodization behavior in ammonium sebacate solution (w = 4%) in ethylene glycol, and in several H3PO4-containing electrolytes, has been investigated. A new mechanism is proposed for the formation of porous anodic films. The model emphasizes the close relationship between pore generation and oxygen evolution. PO4 3− ions incorporated in the anodic films behave as the primary source of avalanche electrons. It is the avalanche electronic current through the barrier film that causes oxygen evolution during anodization. When growth of anodic oxide and oxygen evolution occur simultaneously at the aluminium anode, cavities or pores are formed in the resulting films. Accordingly, the mechanisms of growth of barrier and porous films are not very different in nature. These findings are a decisive new step towards full understanding of the nature of anodic alumina films.
Graphical abstract







Similar content being viewed by others
References
Masuda H, Fukuda K (1995) Science 268:1466
Yanagishita T, Sasaki M, Nishio K, Masuda H (2004) Adv Mater 16:429
Whitney TM, Searson PC, Jiang JS, Chien CL (1993) Science 261:1316
Taberna PL, Mitra S, Poizot P, Simon P, Tarascon J-M (2006) Nat Mater 5:567
Chu SZ, Wada K, Inoue S, Todoroki S (2002) Chem Mater 14:266
Matsumoto F, Nishio K, Masuda H (2004) Adv Mater 16:2105
Keller F, Hunter MS, Robinson DL (1953) J Electrochem Soc 100:411
Spooner RC, Forsyth WJ (1963) Nature 200:1002
Thompson GE, Wood GC (1981) Nature 290:230
Parkhutik VP, Shershulsky VI (1992) J Phys D Appl Phys 25:1258
Jessensky O, Muller F, Gösele U (1998) J Electrochem Soc 145:3735
Thompson GE (1997) Thin Solid Films 297:192
Lee W, Ji R, Gösele U, Nielsch K (2006) Nat Mater 5:741
Li F, Zhang L, Metzger RM (1998) Chem Mater 10:2470
Masuda H, Hasegwa F, Ono S (1997) J Electrochem Soc 144:L127
Macdonald DD (1993) J Electrochem Soc 140:L27
Crevecoeur C, de Wit HJ (1987) J Electrochem Soc 134:808
Zhuravlyova E, Iglesias-Rubianes L, Pakes A, Skeldon P, Thompson GE, Zhou X, Quance T, Graham MJ, Habazaki H, Shimizu K (2002) Corros Sci 44:2153
Li Y, Shimada H, Sakairi M, Shigyo K, Takahashi H, Seo M (1997) J Electrochem Soc 144:866
Zhu XF, Li DD, Song Y, Xiao YH (2005) Mater Lett 59:3160
Liu Y, Alwitt RS, Shimizu K (2000) J Electrochem Soc 147:1382
Zhu XF, Liu L, Song Y, Jia HB, Yu HD, Xiao XM, Yang XL (2008) Monatsh Chem 139:999
Albella JM, Montero I, Martinez-Duart JM (1987) Electrochim Acta 32:255
Patermarakis G, Masavetas K (2006) J Electroanal Chem 588:179
Song Y, Zhu XF, Wang X, Che J, Du Y (2001) J Appl Electrochem 31:1273
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, X., Zhu, X., Jia, H. et al. Oxygen evolution: the mechanism of formation of porous anodic alumina. Monatsh Chem 140, 595–600 (2009). https://doi.org/10.1007/s00706-008-0098-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-008-0098-y