Abstract
Background
Vasospasm of both large and small parenchymal arteries may contribute to the occurrence of delayed ischemic neurological deficits, and nitric oxide(NO) is an important mediators in the development of cerebral vasospasm after subarachnoid hemorrhage (SAH). We used a rabbit two-hemorrhage model to investigate changes in plasma NO after SAH, and the relationship between NO and brain microcirculation.
Methods
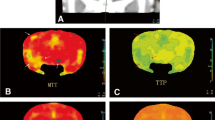
SAH was induced in rabbits and a control group was sham operated. There were 32 rabbits in each group that survived the second operation, and they were randomly assigned to four groups of eight rabbits each for follow-up assessments on Days 1, 4, 7, or 14, respectively. Cerebral blood flow (CBF), cerebral blood volume (CBV), and mean transit time (MTT) were calculated at six regions of interest (ROIs): symmetrical areas of the frontal, parietal-occipital, and temporal lobes. Before the contrast CT scan, blood was drawn from the central artery of the ear for measurement of plasma NO.
Results
In the control group, there was no difference in CBV, CBF, and MTT in the six ROIs, and plasma NO was unchanged. Compared to controls, in the SAH group, CBV decreased slightly in the six ROIs (P > 0.05), frontal lobe CBF decreased, MTT increased (P < 0.05, for both), and NO plasma levels were significantly lower (P < 0.01).
Conclusions
There was a significant correlation between the increase in MTT and the decrease in plasma NO (P < 0.05), We hypothesized that normalization of NO might have a positive influence on brain microcirculation following SAH.






Similar content being viewed by others
References
Alaraj A, Charbel FT, Amin-Hanjani S (2009) Peri-operative measures for treatment and prevention of cerebral vasospasm following subarachnoid hemorrhage. Neurol Res 31:651–659
Alexander S, Poloyac S, Hoffman L, Gallek M, Ren D, Balzer J, Kassam A, Conley Y (2009) Endothelial Nitric Oxide Synthase Tagging Single Nucleotide Polymorphisms and Recovery from Aneurysmal Subarachnoid Hemorrhage. Biol Res Nurs 11:42–52
Dorsch NW, King MT (2009) A review of cerebral vasospasm in aneurysmal subarachnoid hemorrhage. Part I. Incidence and effects. J Clin Neurosci 1:19–26
Frontera JA, Fernandez A, Schmidt JM, Claassen J, Wartenberg KE, Badjatia N, Connolly ES, Mayer SA (2009) Defining vasospasm after subarachnoid hemorrhage: what is the most clinically relevant definition? Stroke 40(6):1963–1968
Güresir E, Raabe A, Jaiimsin A, Dias S, Raab P, Seifert V, Vatter H (2010) Histological evidence of delayed ischemic brain tissue damage in the rat double-hemorrhage model. J Neurol Sci 293:18–22
Jeon H, Ai J, Sabri M, Tariq A, Shang X, Chen G, Macdonald RL (2009) Neurological and neurobehavioral assessment of experimental subarachnoid hemorrhage. BMC Neurosci 10:103
Kanazawa R, Kato M, Ishikawa K, Eguchi T, Teramoto A (2007) Convenience of the computed tomography perfusion method for cerebral vasospasm detection after subarachnoid hemorrhage. Surg Neurol 67:604–611
Kudo K, Terae S, Katoh C, Oka M, Shiga T, Tamaki N, Miyasaka K (2003) Quantitative cerebral blood flow measurement with dynamic perfusion CT using the vascular-pixel elimination method: comparison with H2(15)O positron emission tomography. AJNR Am J Neuroradiol 24:419–426
Kunze E, Pham M, Raslan F, Stetter C, Lee JY, Solymosi L, Ernestus RI, Vince GH, Westermaier T (2012) Value of perfusion CT,Transcranial Doppler Sonlgraphy, and Neurological Examination to Detect delayed vasospasm after aneurysaml subarachnoid hemorrhage. Radiol Res Pract 2012:231206
Leenders KL, Perani D, Lammertsma AA, Heather JD, Buckingham P, Healy MJ, Gibbs JM, Wise RJ, Hatazawa J, Herold S (1990) Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain 113:27–47
Neuschmelting V, Marbacher S, Fathi AR, Jakob SM, Fandino J (2009) Elevated level of endothelin-1 in cerebrospinal fluid and lack of nitric oxide in basilar arterial plasma associated with cerebral vasospasm after subarachnoid haemorrhage in rabbits. Acta Neurochir (Wien) 151:795–801
Park S, Yamaguchi M, Zhou C, Calvert JW, Tang J, Zhang JH (2004) Neurovascular Protection Reduces Early Brain Injury After Subarachnoid Hemorrhage. Stroke 35:2412–2417
Pennings FA, Bouma GJ, Ince C (2004) Direct observation of the human cerebral microcirculation during aneurysm surgery reveals increased arteriolar contractility. Stroke 35:1284–1288
Pluta RM (2006) Dysfunction of nitric oxide synthases as a cause and therapeutic target in delayed cerebral vasospasm after SAH. Neurol Res 28:730–737
Pluta PM, Oldfield EH (2007) Analysis of Nitric Oxide (NO) in Cerebral Vasospasm After aneursmal bleeding. Rev Recent Clin Trials 2:59–67
Sabri M, Ai J, Macdonald RL (2011) Dissociation of Vasospasm and Secondary Effects of Experimental Subarachnoid Hemorrhage by Clazosentan. Stroke 42:1454–1460
Sabri M, Ai J, Knight B, Tariq A, Jeon H, Shang X, Marsden PA, Loch Macdonald R (2011) Uncoupling of endothelial nitric oxide synthase after experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab 31:190–199
Sanelli PC, Anumula N, Johnson CE, Comunale JP, Tsiouris AJ, Riina H, Segal AZ, Stieg PE, Zimmerman RD, Mushlin AI (2013) Evaluating CT perfusion using outcome measures of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. AJNR Am J Neuroradiol 34(2):292–298
Stein SC, Browne KD, Chen XH, Smith DH, Graham DI (2006) Thromboembolism and delayed cerebral ischemia after subarachnoid hemorrhage: an autopsy study. Neurosurgery 59:781–788
Strong MJ, Wolff AV, Wakayama I, Garruto RM (1991) Aluminum-induced chronic myelopathy in rabbits (2010). Neurotoxicology 12:9–21
Vellimana AK, Milner E, Azad TD, Harries MD, Zhou ML, Gidday JM, Han BH, Zipfel GJ (2011) Endothelial Nitric Oxide Synthase Mediates Endogenous Protection Against Subarachnoid Hemorrhage-Induced Cerebral Vasospasm. Stroke 42:776–782
Wang L, Shi JX, Yin HX, Ma CY, Zhang QR (2005) The Influence of Subarachnoid Hemorrhage on Neurons: An Animal Model. Ann Clin Lab Sci 35:79–85
Weidauer S, Lanfermann H, Raabe A, Zanella F, Seifert V, Beck J (2007) Impairment of Cerebral Perfusion and Infarct Patterns Attributable to Vasospasm After Aneurysmal Subarachnoid Hemorrhage:A Prospective MRI and DSA Study. Stroke 38:1831–1836
Wintermark M, Fischbein NJ, Smith WS, Ko NU, Quist M, Dillon WP (2005) Accuracy of dynamic perfusion CT with deconvolution in detecting acute hemishperic stoke. AJNR Am J Neuroradiol 26:104–112
Zhou ML, Shi JX, Zhu JQ, Hang CH, Mao L, Chen KF, Yin HX (2007) Comparison between one- and two-hemorrhage models of cerebral vasospasm in rabbits. J Neurosci Methods 159:318–324
Acknowledgement
This work was supported by grant 81071151 from the National Natural Science Foundation of China.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
The present manuscript demonstrated the correlation of cerebral perfusion and a biomarker after subarachnoid hemorrhage in animals.
This study supports the importance of CT-perfusion in aneurysmal SAH.
Daniel Haenggi
Duesseldorf, Germany
Rights and permissions
About this article
Cite this article
Zheng, R., Qin, L., Li, S. et al. CT perfusion-derived mean transit time of cortical brain has a negative correlation with the plasma level of Nitric Oxide after subarachnoid hemorrhage. Acta Neurochir 156, 527–533 (2014). https://doi.org/10.1007/s00701-013-1968-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-013-1968-6