Abstract
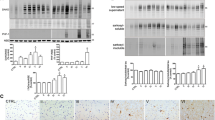
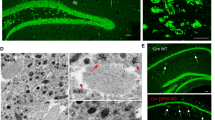
The reasons for the selective vulnerability of distinct neuronal populations in neurodegenerative disorders are unknown. The cholinergic neurons of the basal forebrain are vulnerable to pathology and loss early in Alzheimer’s disease and in a number of other neurodegenerative disorders of the elderly. In the primate, including man, these neurons are rich in the calcium buffer calbindin-D28K. Here, we confirm that these neurons undergo a substantial loss of calbindin in the course of normal aging and report a further loss of calbindin in Alzheimer’s disease both at the level of RNA and protein. Significantly, cholinergic neurons that had lost their calbindin in the course of normal aging were those that selectively degenerated in Alzheimer’s disease. Furthermore, calbindin-containing neurons were virtually resistant to the process of tangle formation, a hallmark of the disease. We conclude that the loss of calcium buffering capacity in these neurons and the resultant pathological increase in intracellular calcium are permissive to tangle formation and degeneration.





Similar content being viewed by others
References
Arendt T, Bigl V, Arendt A, Tennstdet A (1983) Loss of neurons in the nucleus basalis of Meynert in Alzheimer’s disease, paralysis agitans and Korsakoffs disease. Acta Neuropathol 61:101–108
Arikuni T, Kubota K (1984) Substantia innominata projection to caudate nucleus in macaque monkeys. Brain Res 302:184–189
Armstrong DM, Ikonomovic MD, Sheffield R, Wenthold RJ (1994) AMPA-selective glutamate receptor subtype immunoreactivity in the entorhinal cortex of non-demented elderly and patients with Alzheimer’s disease. Brain Res 639:207–216
Baudier J, Cole RD (1987) Phosphorylation of tau proteins to a state like that in Alzheimer’s brain is catalyzed by a calcium/calmodulin-dependent kinase and modulated by phospholipids. J Biol Chem 262:17577–17583
Biernat J, Gustke N, Drewes G, Mandelkow EM, Mandelkow E (1993) Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron 11:153–163
Braak H, Braak E (1991) Neuropathological staging of Alzheimer’s disease. Acta Neuropathol 82:239–259
Bu J, Sathyendra V, Nagykery N, Geula C (2003) Age-related changes in calbindin-D28k, calretinin, and parvalbumin-immunoreactive neurons in the human cerebral cortex. Exp Neurol 182:220–231
Bullock R, Bergman H, Touchon J et al (2006) Effect of age on response to rivastigmine or donepezil in patients with Alzheimer’s disease. Curr Med Res Opin 22:483–494
Buritica E, Villamil L, Guzman F et al (2009) Changes in calcium-binding protein expression in human cortical contusion tissue. J Neurotrauma 26:2145–2155
D’Orlando C, Fellay B, Schwaller B et al (2001) Calretinin and calbindin D-28k delay the onset of cell death after excitotoxic stimulation in transfected P19 cells. Brain Res 909:145–158
de Talamoni N, Smith CA, Wasserman RH et al (1993) Immunocytochemical localization of the plasma membrane calcium pump, calbindin-D28k, and parvalbumin in Purkinje cells of avian and mammalian cerebellum. Proc Natl Acad Sci USA 90:11949–11953
Demuro A, Mina E, Kayed R et al (2005) Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem 280:17294–17300
Fine A, Hoyle C, Maclean CJ, Levatte TL, Baker HF, Ridley RM (1997) Learning impairments following injection of a selective cholinergic immunotoxin, ME20.4 IgG-saporin, into the basal nucleus of Meynert in monkeys. Neurosci 81:331–343
Geula C, Bu J, Nagykery N (2003) Loss of calbindin-D28k from aging human cholinergic basal forebrain: relation to neuronal loss. J Comp Neurol 455:249–259
Geula C, Mesulam M–M (1999) Cholinergic systems in Alzheimer disease. In: Terry RD, Katzman R, Bick KL, Sisodia SS (eds) Alzheimer disease, 2nd edn. Lippincott Williams and Wilkins, Philadelphia, pp 269–292
Geula C, Nagykery N, Nicholas A, Wu CK (2008) Cholinergic neuronal and axonal abnormalities are present early in aging and in Alzheimer disease. J Neuropathol Exp Neurol 67:309–318
Geula C, Schatz CR, Mesulam MM (1993) Differential localization of NADPH-diaphorase and calbindin-D28k within the cholinergic neurons of the basal forebrain, striatum and brainstem in the rat, monkey, baboon and human. Neuroscience 54:461–476
Gomez-Isla T, Price JL, McKeel DWJ, Morris JC, Growdon JH, Hyman BT (1996) Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci 16:4491–4500
Hartigan JA, Johnson GV (1999) Transient increases in intracellular calcium result in prolonged site-selective increases in tau phosphorylation through a glycogen synthase kinase 3beta-dependent pathway. J Biol Chem 274:21395–21401
Iacopino AM, Quintero EM, Miller EK (1994) Calbindin-D28K: a potential neuroprotective protein. Neurodegeneration 3:1–20
Ikonomovic MD, Nocera R, Mizukami K, Armstrong DM (2000) Age-related loss of the AMPA receptor subunits GluR2/3 in the human nucleus basalis of Meynert. Exp Neurol 166:363–375
Khachaturian ZS (1994) Calcium hypothesis of Alzheimer’s disease and brain aging. Ann N Y Acad Sci 747:1–11
Kojetin DJ, Venters RA, Kordys DR, Thompson RJ, Kumar R, Cavanagh J (2006) Structure, binding interface and hydrophobic transitions of Ca2+-loaded calbindin-D(28K). Nat Struct Mol Biol 13:641–647
Lasagna-Reeves CA, Castillo-Carranza DL, Guerrero-Muoz MJ, Jackson GR, Kayed R (2010) Preparation and characterization of neurotoxic tau oligomers. Biochemistry 49:10039–10041
Lehericy S, Hirsch EC, Cervera-Pierot P et al (1993) Heterogeneity and selectivity of the degeneration of cholinergic neurons in the basal forebrain of patients with Alzheimer’s disease. J Comp Neurol 330:15–31
Levey AI, Bolam JP, Rye DB (1986) A light and electron microscopic procedure for sequential double antigen localization using diaminobenzidine and benzidine dihydrochloride. J Histochem Cytochem 34:1449–1457
Lowenstein DH, Gwinn RP, Seren MS, Simon RP, McIntosh TK (1994) Increased expression of mRNA encoding calbindin-D28K, the glucose-regulated proteins, or the 72 kDa heat-shock protein in three models of acute CNS injury. Mol Brain Res 22:299–308
Mattson MP, Rydel RE, Lieberburg I, Smith-Swintosky VL (1993) Altered calcium signaling and neuronal injury: stroke and Alzheimer’s disease as examples. Review. Ann New York Acad Sci 679:1–21
Mesulam M, Shaw P, Mash D, Weintraub S (2004) Cholinergic nucleus basalis tauopathy emerges early in the aging-MCI-AD continuum. Ann Neurol 55:815–828
Mesulam M–M, Mufson EJ, Levey AI, Wainer BH (1983) Cholinergic innervation of cortex by the basal forebrain: Cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol 214:170–197
Mesulam M–M, Mufson EJ, Wainer BH (1986) Three-dimensional representation and cortical projection topography of the nucleus basalis (Ch4) in the macaque: concurrent demonstration of choline acetyltransferase and retrograde transport with a stabilized tetramethylbenzidine method for horseradish peroxidase. Brain Res 367:301–308
Miller RJ (1991) The control of neuronal Ca2 + homeostasis. Prog Neurobiol 37:255–285
Mirra SS, Heyman A, McKeel D et al (1991) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–486
Morris JC, Heyman A, Mohs RC et al (1989) CERAD Investigators. The Consortium to establish a Registry for Alzheimer’s Disease. Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 39:1159–1165
Mufson EJ, Ma SY, Cochran EJ et al (2000) Loss of nucleus basalis neurons containing trkA immunoreactivity in individuals with mild cognitive impairment and early Alzheimer’s disease. J Comp Neurol 427:19–30
Perry EK, Irving D, Kerwin JM et al (1993) Cholinergic transmitter and neurotrophic activities in Lewy body dementia: similarity to Parkinson’s and distinction from Alzheimer disease. Alzheimer Dis Assoc Disord 7(2):69–79
Rintoul GL, Raymond LA, Baimbridge KG (2001) Calcium buffering and protection from excitotoxic cell death by exogenous calbindin-D28k in HEK 293 cells. Cell Calcium 29:277–287
Samuel WA, Henderson VW, Miller CA (1991) Severity of dementia in Alzheimer disease and neurofibrillary tangles in multiple brain regions. Alzhemier Dis Assoc Disor 5:1–11
Scharfman HE, Schwartzkroin PA (1989) Protection of dentate hilar cells from prolonged stimulation by intracellular calcium chelation. Science 246:249–262
Schliebs R, Arendt T (2011) The cholinergic system in aging and neuronal degeneration. Behav Brain Res (in press)
Stoehr JD, Mobley SL, Roice D et al (1997) The effects of selective cholinergic basal forebrain lesions and aging upon expectancy in the rat. Neurobiol Lear Mem 67:214–227
Wagner U, Utton M, Gallo JM, Miller CC (1996) Cellular phosphorylation of tau by GSK-3 beta influences tau binding to microtubules and microtubule organisation. J Cell Sci 109:1537–1543
Wu CK, Nagykery N, Hersh LB, Scinto LF, Geula C (2003) Selective age-related loss of calbindin-D28k from basal forebrain cholinergic neurons in the common marmoset (Callithrix jacchus). Neuroscience 120:249–259
Wu C-K, Hersh LB, Geula C (2000) Cyto- and chemoarchitecture of basal forebrain cholinergic neurons in the common marmoset (Callithirx jacchus). Exp Neurol 165:306–326
Wu C-K, Mesulam M–M, Geula C (1997) Age-related loss of calbindin from human basal forebrain cholinergic neurons. Neuroreport 8:2209–2213
Acknowledgments
We are grateful to Girgis Girgis and Katherine Gasho for expert technical assistance. This work was supported in part by a Zenith Fellows Award (C.G.) from the Alzheimer’s Association; and by grants from the National Institute on Aging (AG014706 and AG027141). A portion of the tissue used in these studies was received from the Northwestern University (AG013854) and Massachusetts General Hospital (AG005134) Alzheimer’s Disease Centers.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
D. Riascos, D. de Leon and A. Baker-Nigh contributed equally to the work reported here.
Rights and permissions
About this article
Cite this article
Riascos, D., de Leon, D., Baker-Nigh, A. et al. Age-related loss of calcium buffering and selective neuronal vulnerability in Alzheimer’s disease. Acta Neuropathol 122, 565–576 (2011). https://doi.org/10.1007/s00401-011-0865-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-011-0865-4