Abstract
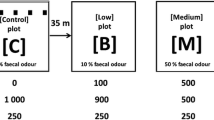
Predation influences the ecology and behaviour of prey species and it is well known that the risk of predation affects prey’s decision making. We investigated whether predation risk through moon phase and exposure to the faecal odour of a natural predator, the red fox Vulpes vulpes, affect feeding behaviour and physiological response in wood mice (Apodemus sylvaticus). Antipredatory response was studied by live trapping under new and full moon in odourless control areas and areas experimentally manipulated with red fox fresh faeces. Food intake by individuals was determined as the amount of bait remaining in each trap and the physiological response was measured non-invasively analysing faecal corticosterone metabolites (FCM). Traps treated with faeces of red fox were the most avoided, and this avoidance was more significant during full moon. Food intake by wood mice varied according to the moon phase being significantly lower under full moon nights. We found sex, breeding condition and weight of individuals explaining the variation found in FCM concentrations, but no changes in FCM levels due to moon phase or exposure to red fox faeces were detected. These results indicate that wood mice avoid red fox faecal odour and this antipredatory response as well as feeding behaviour are significantly influenced by moon phase. However, no physiological response was found due to predation risk suggesting that wood mice do not take these predation cues enough reliable to experience physiological changes.



Similar content being viewed by others
References
Abrahams MV, Dill LM (1989) A determination of the energetic equivalence of the risk of predation. Ecology 70:999–1007
Abrams PA (1986) Is predator–prey coevolution an arms race? Trends Ecol Evol 1:108–110
Andreolini F, Jemiolo B, Novotny M (1987) Dynamics of excretion of urinary chemosignals in the house mouse (Mus musculus) during the natural estrous cycle. Experientia 43:998–1002
Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS (2005) The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav R 29:1123–1144
Barja I (2009) Decision making in plant selection during the faecal-marking behaviour of wild wolves. Anim Behav 77:489–493
Barja I, de Miguel FJ, Bárcena F (2005) Faecal marking behaviour of Iberian wolf in different zones of their territory. Folia Zool 54:21–29
Barja I, Silván G, Rosellini S, Piñeiro A, González-Gil A, Camacho L, Illera JC (2007) Stress physiological responses to tourist pressure in a wild population of European pine marten. J Steroid Biochem 104:136–142
Barja I, Escribano G, Lara C, Virgós E, Benito J, Rafart E (2012) Non-invasive monitoring of adrenocortical activity in European badgers (Meles meles) and effects of sample collection and storage on faecal cortisol metabolite concentrations. Anim Biol 62:419–432
Barreto GR, Macdonald DW (1999) The response of water voles, Arvicola terrestris, to the odours of predators. Anim Behav 57:1107–1112
Bauman DE (2000) Regulation of nutrient partitioning during lactation: homeostasis and homeorhesis revisited. In: Cronjé PB (ed) Ruminant physiology: digestion, metabolism, growth and reproduction. CAB, New York, pp 311–327
Bednekoff PA, Lima SL (2004) Risk allocation and competition in foraging groups: reversed effects of competition if group size varies under risk of predation. Proc R Soc Lond B 271:1491–1496
Boonstra R, Hik D, Singleton GR, Tinnikov A (1998) The impact of predator-induced stress on the snowshoe hare cycle. Ecol Monogr 79:371–394
Brown JS, Kotler BP (2004) Hazardous duty pay and the foraging cost of predation. Ecol Lett 7:999–1014
Brown JS, Laundré JW, Gurung M (1999) The ecology of fear: optimal foraging, game theory, and trophic interactions. J Mammal 80:385–399
Calder CJ, Gorman ML (1991) The effects of red fox Vulpes vulpes faecal odours on the feeding behaviour of Orkney voles Microtus arvalis. J Zool 224:599–606
Daly M, Behrends PR, Wilson MI, Jacobs LF (1992) Behavioural modulation of predation risk: moonlight avoidance and crepuscular compensation in a nocturnal desert rodent, Dipodomys merriami. Anim Behav 44:1–9
Dantzer B, McAdam AG, Palme R, Fletcher QE, Boutin S, Humphries MM, Boonstra R (2010) Fecal cortisol metabolite levels in free-ranging North American red squirrels: assay validation and the effects of reproductive condition. Gen Comp Endocrinol 167:279–286
Díaz M (1992) Rodent seed predation in cereal crop areas of central Spain: effects of physiognomy, food availability, and predation risk. Ecography 15:77–85
Díaz M, Torre I, Peris A, Tena L (2005) Foraging behavior of wood mice as related to presence and activity of genets. J Mammal 86:1178–1185
Dickman CR, Doncaster CP (1984) Responses of small mammals to red fox (Vulpes vulpes) odour. J Zool 204:521–531
Eilam D (2004) Locomotor activity in common spiny mice (Acomys cahirinuse): the effect of light and environmental complexity. BMC Ecology 4:16
Eilam D, Dayan T, Ben-Eliyahu S, Schulman I, Shefer G, Hendrie CA (1999) Differential behavioural and hormonal responses of voles and spiny mice to owl calls. Anim Behav 58:1085–1093
Epple G, Belcher AM, Kuderling I, Zeller U, Scolnick L, Greenfield KL, Smith ABI (1993) Making sense out of scents: species differences in scent glands, scent-marking behaviour, and scent-mark composition in the Callitrichidae. In: Rylands AB (ed) Marmosets and tamarins: systematics, behaviour, and ecology. Oxford University Press, New York, pp 123–151
Fletcher QE, Boonstra R (2006) Do captive male meadow voles experience acute stress in response to weasel odour? Can J Zool 84:583–588
Gurnell J, Flowerdew JR (1994) Live trapping small mammals. A practical guide. The Mammal Society, London
Hauger R, Thrivikraman K, Plotsky P (1994) Age-related alterations of hypothalamic–pituitary–adrenal axis function in male Fischer 344 rats. Endocrinology 134:1528–1536
Hayes RA, Morelli TL, Wright PC (2006) Volatile components of lemur scent secretions vary throughout the year. Am J Primatol 68:1202–1207
Hirschenhauser K, Möstl E, Wallner B, Dittami J, Kotrschal K (2000) Endocrine and behavioural responses of male greylag geese (Anser anser) to pairbond challenges during the reproductive season. Ethology 106:63–77
Hutchings MR, White PCL (2000) Mustelid scent–marking in managed ecosystems: implications for population management. Mamm Rev 30:157–169
Jedrzejewski W, Rychlik L, Jedrzejewska B (1993) Responses of bank voles to odours of seven species of predators: experimental data and their relevance to natural predator–vole relationships. Oikos 68:251–257
Jemiolo B, Xie TM, Andreolini F, Baker AEM, Novotny M (1991) The t complex of the mouse: chemical characterization by urinary volatile profiles. J Chem Ecol 17:353–367
Kats LB, Dill LM (1998) The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience 5:361–394
Kaufman DW, Kaufman GA (1982) Effect of moonlight on activity and microhabitat use by Ord's kangaroo rat (Dipodomys ordii). J Mammal 63:309–312
Korte SM (2001) Corticosteroids in relation to fear, anxiety and psychopathology. Neurosci Biobehav R 25:117–142
Kotler BP, Brown JS, Hasson O (1991) Owl predation on gerbils: the role of body size, illumination, and habitat structure on rates of predation. Ecology 72:2249–2260
Kotler BP, Brown J, Mukherjee S, Berger-Tal O, Bouskila A (2010) Moonlight avoidance in gerbils reveals a sophisticated interplay among time allocation, vigilance and state-dependent foraging. Proc R Soc Lond B 277:1469–1474
Lima SL (1998) Stress and decision making under the risk of predation: recent developments from behavioral, reproductive, and ecological perspectives. Adv Stud Behav 27:215–290
Lima SL, Bednekoff PA (1999) Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am Nat 153:649–659
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Lima SL, Valone TJ (1986) Influence of predation risk on diet selection: a simple example in the grey squirrel. Anim Behav 34:536–544
Liu J, Chen Y, Guo L, Gu B, Liu H, Hou A, Liu X, Sun L, Liu D (2006) Stereotypic behavior and fecal cortisol level in captive giant pandas in relation to environmental enrichment. Zoo Biol 25:445–459
Longland WS, Price MV (1991) Direct observations of owls and heteromyid rodents: can predation risk explain microhabitat use? Ecology 72:2261–2273
Martín J, Barja I, López P (2010) Chemical scent constituents in feces of wild Iberian wolves (Canis lupus signatus). Biochem Syst Ecol 38:1096–1102
Millspaugh JJ, Washburn BE (2003) Within-sample variation of fecal glucocorticoid measurements. Gen Comp Endocrinol 132:21–26
Monclús R, Rödel HG, Palme R, Von Holst D, de Miguel J (2006) Non-invasive measurement of the physiological stress response of wild rabbits to the odour of a predator. Chemoecology 16:25–29
Monclús R, Palomares F, Tablado Z, Martínez-Fontúrbel A, Palme R (2009) Testing the threat-sensitive predator avoidance hypothesis: physiological responses and predator pressure in wild rabbits. Oecologia 158:615–623
Möstl E, Palme R (2002) Hormones as indicators of stress. Domest Anim Endocrin 23:67–74
Möstl E, Rettenbacher S, Palme R (2005) Measurement of corticosterone metabolites in birds' droppings: an analytical approach. Ann NY Acad Sci 1046:17–34
Navarro–Castilla Á, Barja I (2014) Antipredatory response and food intake in wood mice (Apodemus sylvaticus) under simulated predation risk by resident and novel carnivorous predators. Ethology 120:90–98
Navarro-Castilla Á, Barja I, Olea PP, Piñeiro A, Mateo-Tomás P, Silván G, Illera JC (2014) Are degraded habitats from agricultural crops associated with elevated faecal glucocorticoids in a wild population of common vole (Microtus arvalis)? Mamm Biol 79:36–43
Navarro–Castilla Á, Mata C, Ruiz-Capillas P, Palme R, Malo JE, Barja I (2014) Are motorways potential stressors of roadside wood mice (Apodemus sylvaticus) populations? PLoS One 9:e91942
Newman JA, Recer GM, Zwicker SM, Caraco T (1988) Effects of predation hazard on foraging "constraints": patch-use strategies in grey squirrels. Oikos 53:93–97
Orrock JL, Danielson BJ, Brinkerhoff RJ (2004) Rodent foraging is affected by indirect, but not by direct, cues of predation risk. Behav Ecol 15:433–437
Padial JM, Ávila E, Sanchez JM (2002) Feeding habits and overlap among red fox (Vulpes vulpes) and stone marten (Martes foina) in two Mediterranean mountain habitats. Mamm Biol 67:137–146
Palme R, Touma C, Arias N, Dominchin MF, Lepschy M (2013) Steroid extraction: get the best out of faecal samples. Wien Tierärztl Monat 100:238–246
Penteriani V, Kuparinen A, del Mar Delgado M, Palomares F, López-Bao JV, Fedriani JM, Calzada J, Moreno S, Villafuerte R, Campioni L (2013) Responses of a top and a meso predator and their prey to moon phases. Oecologia 173:753–766
Piñeiro A, Barja I, Silván G, Illera JC (2012) Effects of tourist pressure and reproduction on physiological stress response in wildcats: management implications for species conservation. Wildlife Res 39:532–539
Raymer J, Wiesler D, Novotny M, Asa C, Seal US, Mech LD (1984) Volatile constituents of wolf (Canis lupus) urine as related to gender and season. Experientia 40:707–709
Reeder DM, Kramer KM (2005) Stress in free-ranging mammals: integrating physiology, ecology, and natural history. J Mammal 86:225–235
Scordato ES, Dubay G, Drea CM (2007) Chemical composition of scent marks in the ringtailed lemur (Lemur catta): glandular differences, seasonal variation, and individual signatures. Chem Senses 32:493–504
Sheriff MJ, Dantzer B, Delehanty B, Palme R, Boonstra R (2011) Measuring stress in wildlife: techniques for quantifying glucocorticoids. Oecologia 166:869–887
Stoddart DM (1982) Demonstration of olfactory discrimination by the short-tailed vole, Microtus agrestis L. Anim Behav 30:293–294
Strier KB, Lynch JW, Ziegler TE (2003) Hormonal changes during the mating and conception seasons of wild northern muriquis (Brachyteles arachnoides hypoxanthus). Am J Primatol 61:85–99
Sundell J, Dudek D, Klemme I, Koivisto E, Pusenius J, Ylönen H (2004) Temporal variation of fear and vole behaviour: an experimental field test of the predation risk allocation hypothesis. Oecologia 139:157–162
Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, Ravussin E (1996) Effects of glucocorticoids on energy metabolism and food intake in humans. Am J Physiol-Endoc M 271:317–325
Touma C, Palme R (2005) Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of validation. Ann NY Acad Sci 1046:54–74
Touma C, Sachser N, Möstl E, Palme R (2003) Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen Comp Endocrinol 130:267–278
Touma C, Palme R, Sachser N (2004) Analyzing corticosterone metabolites in fecal samples of mice: a noninvasive technique to monitor stress hormones. Horm Behav 45:10–22
Wingfield JC, Hunt K, Breuner C, Dunlap K, Fowler GS, Freed L, Lepson J (1997) Environmental stress, field endocrinology, and conservation biology. In: Clemmons JR, Buchholds R (eds) Behavioral approaches to conservation in the wild. Cambridge University Press, Cambridge, UK, pp 95–131
Wolfe JL, Summerlin CT (1989) The influence of lunar light on nocturnal activity of the old-field mouse. Anim Behav 37:410–414
Ylönen H, Eccard JA, Jokinen I, Sundell J (2006) Is the antipredatory response in behaviour reflected in stress measured in faecal corticosteroids in a small rodent? Behav Ecol Sociobiol 60:350–358
Acknowledgments
The authors wish to thank the Comunidad Autónoma of Madrid (Spain) for providing the permits required to conduct this study. We are grateful to Itziar Cabrero for her help in the field, to Cañada Real Open Center, especially to José España, for supplying the red fox faecal material needed to carry out the experiments. We thank Rupert Palme for performing the EIA in the Biochemistry lab at the Vetmeduni Vienna and for his generous revision of this manuscript and making useful comments to improve it. We would also like to thank Edith Klobetz-Rassam and Nino Arias for excellent help with the laboratory analysis. In addition, we would like to thank Dr. Vincenzo Penteriani and an anonymous referee for their useful comments and suggestions to improve this manuscript. Á. Navarro-Castilla was supported by a FPU scholarship from the Ministerio de Educación y Ciencia of Spain.
Ethical standards
In this research, we fulfilled all the regulations concerning to handling and treatment of animals in accordance with the European Communities Council Directives of 24 November 1986 (86/609/EEC) for animal experiments. Manipulations of animals were done under the permit of the Comunidad Autónoma de Madrid (Spain) reference number 10/422505.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E. Korpimäki
Rights and permissions
About this article
Cite this article
Navarro-Castilla, Á., Barja, I. Does predation risk, through moon phase and predator cues, modulate food intake, antipredatory and physiological responses in wood mice (Apodemus sylvaticus)?. Behav Ecol Sociobiol 68, 1505–1512 (2014). https://doi.org/10.1007/s00265-014-1759-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-014-1759-y