Abstract
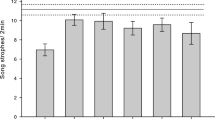
We evaluated the effect of conspecific abundance and habitat quality of leks on the territorial behaviour of males in an exploded lekking species, the Little Bustard (Tetrax tetrax). The hypothesis that males more intensely defend territories with higher conspecific abundance and better habitat quality was evaluated experimentally analysing the agonistic response of experimental males to male decoys placed on their displaying areas. Decoy experiments showed that the intensity of display territory defence by little bustard males is density dependent. The time experimental males took to return to their display sites after decoy placement decreased with abundance of both males and females. The strength of their final response was positively associated to local male and female abundance in the vicinity of their display sites. Habitat quality also influenced males’ display territory defence since the intensity of male response increased with the degree of natural vegetation cover. Habitat quality was particularly relevant in explaining variation of experimental males’ snort call rate, which decreased with the degree in plough cover and increased with the number of fields in the lekking area. Snort call rate decreased with the level of male aggregation and was lowest in males exhibiting the strongest aggressive response to decoys. These results add new evidence for the density dependence of species’ breeding territorial behaviour, supporting density-dependent models of lek formation and reinforcing the role of resources defence in exploded lek mating systems.


Similar content being viewed by others
References
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Appolonio M, Festa-Bianchet M, Mari F (1989) Effects of removal of successful males in a fallow deer lek. Ethology 83:320–325
Arroyo B, Bretagnolle V (1999) Field identification of individual Little bustard Tetrax tetrax males using plumage patterns. Ardeola 46:53–60
Beehler BM, Foster MS (1988) Hotshots, hotspots and female preferences in the organization of lek mating systems. Am Nat 131:203–219
Belfrage K, Björklund J, Salomonsson L (2005) The effects of farm size and organic farming on diversity of birds, pollinators, and plants in a Swedish landscape. Ambio 34:582–588
Bessa-Gomes C, Legendre S, Clobert J (2004) Alle effects, mating systems and the extinction risk in populations with two sexes. Ecol Lett 7:802–812
Bradbury JW (1981) The evolution of leks. In: Alexander RD, Tinkle DW (eds) Natural selection and social behaviour. Chiron Press, New York, pp 138–169
Bretagnolle V, Mougeot F, Thibault JC (2008) Density dependence in a recovering osprey population: demographic and behavioural processes. J Anim Ecol 77:998–1007
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practice information-theoretic approach. Springer, New York
Clutton-Brock TH, Parker GA (1992) Potential reproductive rates and the operation of sexual selection. Q Rev Biol 67:437–456
Clutton-Brock TH, Guiness FE, Albon SD (1982) Red deer: the behaviour and ecology of two sexes. University of Chicago Press, Chicago
Cramp S, Simmons KEL (1980) The birds of the Western Palearctic, vol II. Oxford University Press, Oxford
Del Hoyo J, Elliot A, Sargatal J (1996) Handbook of the birds of the world, vol III. Lynx Edicions, Barcelona
Delgado MP, Morales MB, Traba J, García de la Morena EL (2009) Determining the effects of habitat management and climate on the population trends of a declining steppe birds. Ibis 151:440–451
Delgado MP, Traba J, García de la Morena EL, Morales MB (2010) Habitat selection and density-dependent relationships in spatial occupancy by male little bustards Tetrax tetrax. Ardea 98:185–194
Emlen ST, Oring LW (1977) Ecology, sexual selection and the evolution of mating systems. Science 197:215–223
Faria N, Rabaça J, Morales MB (2012a) The importance of grazing regime in the provision of breeding habitat for grassland birds: the case of the endangered little bustard (Tetrax tetrax). J Nat Conserv 20:211–218
Faria N, Rabaça J, Morales MB (2012b) Linking plant composition and arthropod abundance to establish little bustard breeding requirements in pastureland dominated landscapes. Biodivers Conserv 21:2109–2125
Fasce P, Fasce L, Villers A, Bergese F, Bretagnolle V (2011) Long-term breeding demography and density dependence in an increasing population of Golden Eagles Aquila chrysaetos. Ibis 153:581–591
Festa-Bianchet M, Apollonio M, Mari F, Rasola G (1990) Aggression among lekking male fallow deer (Dama dama): territory effects and relationship with copulatory success. Ethology 85:236–246
Gabriel D, Thies C, Tscharntke T (2005) Local diversity of arable weeds increases with landscape complexity. Perspect Plant Ecol 7:85–93
García de la Morena EL, Bota G, Ponjoan A, Morales MB (2006) El sisón común en España. I Censo Nacional (2005). SEO⁄BirdLife, Madrid
Gibson RM, Taylor CE, Jefferson DR (1990) Lek formation by female choice: a simulation study. Behav Ecol 1:36–42
Gilliard ET (1969) Birds of paradise and bowerbirds. Weidenfeld and Nicholson, London
Gosling LM, Petrie M, Rainey ME (1987) Lekking in topi: a high cost specialist strategy. Anim Behav 35:616–618
Grant JA (1997) Territoriality. In: Godin JGJ (ed) Behavioural ecology of teleost fishes. Oxford University Press, Oxford, Oxford, pp 81–103
Gray TNE, Borey R, Hout SK, Chamman H, Collar NJ, Dolman PM (2009) Generality of models that predict the distribution of species: conservation activity and reduction of model transferability for a threatened bustard. Conserv Biol 23:433–439
Hernandez ML, Houston AI, McNamara JM (1999) Male rank and optimal lek size. Behav Ecol 10:73–79
Höglund J, Alatalo RV (1995) Leks. Princeton University Press, New York
Isvaran K, St. Mary CM (2003) When should males lek? Insights from a dynamic state variable model. Behav Ecol 14:876–886
Jiguet F, Bretagnolle V (2001) Courtship behaviour in a lekking species: individual variations and settlement tactics in male little bustard. Behav Process 55:107–118
Jiguet F, Bretagnolle V (2006) Manipulating lek size and composition using decoys: an experimental investigation of lek evolution models. Am Nat 168:758–768
Jiguet F, Bretagnolle V (2014) Sexy males and choosy females on exploded leks: correlates of male attractiveness in the Little Bustard. Behav Process 103:246–255
Jiguet F, Ollivier D (2002) Male phenotypic repeatability in the threatened Little Bustard Tetrax tetrax: a tool to estimate turnover and dispersal. Ardea 90:43–50
Jiguet F, Arroyo B, Bretagnolle V (2000) Lek mating systems: a case study in the Little Bustard Tetrax tetrax. Behav Process 51:63–82
Jiguet F, Jaulin S, Arroyo B (2002) Resource defence on exploded leks: do male little bustards, Tetrax tetrax, control resources for females? Anim Behav 63:899–905
Kokko H, Rankin DJ (2006) Lonely hearts or sex in the city? Density-dependent effects in mating systems. Philos Trans R Soc B 361:319–334
Kokko H, Sutherland WJ, Lindström J, Reynolds JD, MacKenzie A (1998) Individual mating success, lek stability, and the neglected limitations of statistical power. Anim Behav 56:755–762
Kokko H, Harris MP, Wanless S (2004) Competition for breeding sites and site-dependent population regulation in a highly colonial seabird, the common guillemot Uria aalge. J Anim Ecol 73:367–376
Kotrschal A, Taborsky B (2010) Resource defense or exploded Lek? A question of perspective. Ethology 116:1189–1198
Lapiedra O, Ponjoan A, Gamero A, Bota G, Mañosa S (2011) Brood ranging behaviour and breeding success of the threatened little bustard in an intensified cereal farmland area. Biol Conserv 144:2882–2890
Ligon JD (1999) The evolution of avian breeding systems. Oxford University Press, Oxford
López-Sepulcre A, Kokko H (2005) Territorial defense, territory size, and population regulation. Am Nat 166:317–329
Magaña M, Alonso JC, Palacín C (2011) Age-related dominance helps reduce male aggressiveness in great bustard leks. Anim Behav 82:203–221
Martinez C (1994) Habitat selection by the Little Bustard Tetrax tetrax in cultivated areas of central Spain. Biol Conserv 67:125–128
Morales MB, Jiguet F, Arroyo B (2001) Exploded leks: what bustards can teach us. Ardeola 48:85–98
Morales MB, García JT, Arroyo B (2005) Can landscape composition changes predict spatial and annual variation of little bustard male abundance? Anim Conserv 8:167–174
Morales MB, Traba J, Carriles E, Delgado MP, la García D, de la Morena EL (2008) Sexual differences in microhabitat selection of breeding little bustards Tetrax tetrax: ecological segregation based on vegetation structure. Acta Oecol 34:345–353
Morales MB, Traba J, Delgado MP, García de la Morena EL (2013) The use of fallows by nesting little bustard Tetrax tetrax females: implications for conservation in mosaic cereal farmland. Ardeola 60:85–97
Mysterud A, Solberg EJ, Yoccoz NG (2005) Ageing and reproductive effort in male moose under variable levels of intra-sexual competition. J Anim Ecol 74:742–754
Olea PP, Casas F, Redpath S, Viñuela J (2010) Bottoms up: great bustards use the sun to maximise signal efficacy. Behav Ecol Sociobiol 64:927–937
Orians GH (1969) On the evolution of mating systems in birds and mammals. Am Nat 103:589–603
Ponjoan A, Bota G, Mañosa S (2012) Ranging behaviour of little bustard males, Tetrax tetrax, in the lekking grounds. Behav Process 91:35–40
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Romero A, Chamorro L, Sans FX (2008) Weed diversity in crop edges and inner fields of organic and conventional dryland winter cereal crops in NE Spain. Agr Ecosyst Environ 124:97–104
Sanza MA, Traba J, Morales MB, Rivera D, Delgado MP (2012) Effects of landscape, conspecifics and heterospecifics on habitat selection by breeding farmland birds: the case of Calandra Lark (Melanocorypha calandra) and Corn Bunting (Emberiza calandra). J Ornithol 153:525–533
Schulz H (1986) Agonistic behaviour, territorial behaviour and courtship display of the Little Bustard (Tetrax tetrax). J Ornithol 127:125–204
Silva JP (2010) Factors affecting the abundance of the Little Bustard Tetrax tetrax: implications for conservation. PhD Thesis, University of Lisbon, Lisbon
Silva JP, Estanque B, Moreira F, Palmeirim JM (2014) Population density and use of grasslands by female Little Bustards during lek attendance, nesting and brood-rearing. J Ornithol 155:53–63
Soutullo A, Limiñana R, Urios V, Surroca M, Gill JA (2006) Density dependant regulation of population size in colonial breeders: allee and buffer effects in the migratory Montagu’s harrier. Oecologia 149:543–552
StatSoft Inc (2005) STATISTICA 7.1. www.statsoft.com
Sutherland WJ (1996) From individual behaviour to population ecology. Oxford University Press, Oxford
Tarjuelo R, Delgado MP, Bota G, Morales MB, Traba J, Ponjoan A, Hervás I, Mañosa S (2013) Not only habitat but also sex: factors affecting spatial distribution of Little Bustard Tetrax tetrax families. Acta Ornithol 48:119–128
Temeles EJ (1994) The role of neighbours in territorial systems: when are they ‘dear enemies’? Anim Behav 47:339–350
Traba J, Morales MB, García de la Morena EL, Delgado MP, Krištín A (2008) Selection of breeding territory by little bustard (Tetrax tetrax) males in Central Spain: the role of arthropod availability. Ecol Res 23:615–622
Villers A (2010) Écologie spatiale, processus comportementaux et dynamiques des populations d'une espèce menacée, l'outarde canepetière. PhD Thesis, Université de Paris VI, Paris
Whittingham MJ, Swetnam RD, Wilson JD, Chamberlain DE, Freckleton RP (2005) Habitat selection by yellowhammers (Emberiza citrinella) on lowland farmland at two spatial scales: implications for conservation management. J Appl Ecol 42:270–280
Widemo F, Owens YPF (1995) Lek size, male mating skew and the evolution of lekking. Nature 373:148–151
Wittenberger JF (1979) The evolution of mating systems in birds and mammals. In: Marler P, Vandenbergh JG (eds) Social behavior and communication. Springer, New York, pp 271–349
Wolff A, Paul JP, Martin JL, Bretagnolle V (2002) The benefits of extensive agriculture to birds: the case of the little bustard. J Appl Ecol 38:963–975
Acknowledgments
Particular thanks are due to Vincent Bretagnolle and Frédéric Jiguet for lending their little bustard decoys for experiments. We thank all farmers, hunting managers and gamekeepers of our study areas for allowing us to work on their properties. Salvador Luna, Laura Iglesias, Juan Bécares, Núria Pocino, Sergi Ricart, Pau Ferrer and María Castañeda collaborated during some stages of the work. FC and JM-P were supported by a JAE-Doc contract funded by Spanish Research Council and the European Social Fund (ESF), and ELG was funded by a FPU grant from the Spanish Ministry of Education. The comments of two anonymous reviewers sensibly improved the first version of the manuscript. This paper contributes to projects CGL2004-06147-C02-01, CGL2004-06147-C02-02 and CGL2009-13029/BOS of the Spanish Ministry of Science, as well as to the REMEDINAL2 network of the Community of Madrid (S-2009/AMB/1783). MBM, FC and GB contributed equally to the final outcome of the present study.
Ethical standards
The experiments here described complied with the laws of Spain and the regions (Catalonia, Castilla-La Mancha and Madrid) where they were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. A. Graves
Manuel B. Morales, Fabián Casas, and Gerard Bota contributed equally to this work.
Rights and permissions
About this article
Cite this article
Morales, M.B., Casas, F., García de la Morena, E. et al. Density dependence and habitat quality modulate the intensity of display territory defence in an exploded lekking species. Behav Ecol Sociobiol 68, 1493–1504 (2014). https://doi.org/10.1007/s00265-014-1758-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-014-1758-z