Abstract:
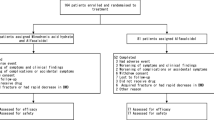
One hundred and forty-five patients suffering from diseases requiring long-term treatment with high doses of corticosteroids (30 mg/day or greater of prednisolone) were recruited to the study. Patients had to be steroid naive on entry to the study (not more than 15 days of treatment with a corticosteroid within the previous 24 months). Patients were randomized to receive either 1 mg/day alfacalcidol or placebo capsules for 12 months. Bone mineral density (BMD) of the lumbar spine was assessed by dual-photon absorptiometry on entry and after 3, 6 and 12 months’ treatment. Safety was monitored by the recording of all adverse events reported by patients and the regular screening of blood samples for hematology and serum biochemistry. Of the 145 patients, 74 were randomized to alfacalcidol and 71 to placebo. The treatment groups were well matched at baseline with no significant differences in demographic, clinical or biochemical parameters. The mean equivalent dose of prednisolone at baseline was 46.6 mg/day and 46.3 mg/day for the alfacalcidol and placebo group respectively. From the 145 patients randomized to treatment, 71 (38 who received alfacalcidol and 33 who received placebo) provided BMD data both at baseline and at 3, 6 and 12 months. The percentage change in BMD after 6 months’ treatment was –2.11% in the alfacalcidol group and –4.00% in the placebo group (p= 0.39). After 12 months the percentage change in BMD was +0.39% (CI: –4.28 to 4.81) in the alfacalcidol group and –5.67% (CI: –8.13 to –3.21) in the placebo group, this difference (6.06%, CI: 0.88 to 11.24) being statistically significant (p= 0.02). An intention to treat analysis also showed a significant difference between the two treatment groups in alfacalcidol’s favor (3.81%, p= 0.01; CI: 0.92 to 6.70). There was no significant difference between the two treatment groups in the corticosteroid dose at any time point during the study. Serum calcium was measured throughout and there were no significant differences between the two treatment groups at any visit. This study suggests that alfacalcidol can prevent corticosteroid-induced bone loss from the lumbar spine. Long-term use of alfacalcidol was not associated with any significant adverse effects in this diverse group of patients.
Similar content being viewed by others
Author information
Authors and Affiliations
Consortia
Additional information
Received: 22 May 1997 / Accepted: 27 April 1998
Rights and permissions
About this article
Cite this article
Reginster, J., Kuntz, D., Verdickt, W. et al. Prophylactic Use of Alfacalcidol in Corticosteroid-Induced Osteoporosis . Osteoporos Int 9, 75–81 (1999). https://doi.org/10.1007/s001980050118
Issue Date:
DOI: https://doi.org/10.1007/s001980050118