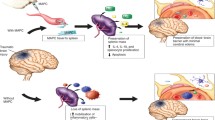
Abstract
Stroke, spinal cord injury, and traumatic brain injury are the major three causes of central nervous system injury. After the acute phase, most patients are left with significant motor, cognitive, and social impairments. Few treatments exist and there are no current therapeutic interventions altering their underlying pathological processes via tissue salvage, support, repair, or replacement at the cellular or subcellular level. Recent evidence suggests that the cell-based therapy exerts therapeutic benefits in relevant preclinical animal models. Furthermore, some cell types, like MSCs, have advanced into clinical trials. Here, we present the current status of MSCs in stroke, SCI, and TBI therapy from preclinical studies to clinical trials, with an emphasis on dosage, timing, and routes of delivery. We also discuss possible cellular and molecular mechanisms of action that mediate the effects of MSCs in these different disease models. Finally, we end with a discussion of important issues that require future study.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM et al (2011) Heart disease and stroke statistics – 2011 update: a report from the American Heart Association. Circulation 123(4):e18–e209, Epub 2010/12/17
The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group (1995) Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 333(24):1581–1587, Epub 1995/12/14
Katzan IL, Hammer MD, Hixson ED, Furlan AJ, Abou-Chebl A, Nadzam DM (2004) Utilization of intravenous tissue plasminogen activator for acute ischemic stroke. Arch Neurol 61(3):346–350, Epub 2004/03/17
Savitz SI, Dinsmore JH, Wechsler LR, Rosenbaum DM, Caplan LR (2004) Cell therapy for stroke. NeuroRx 1(4):406–414, Epub 2005/02/18
Kraus JF, Fife D, Conroy C (1987) Pediatric brain injuries: the nature, clinical course, and early outcomes in a defined United States’ population. Pediatrics 79(4):501–507, Epub 1987/04/01
Langlois JA, Rutland-Brown W, Wald MM (2006) The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil 21(5):375–378, Epub 2006/09/20
McGarry LJ, Thompson D, Millham FH, Cowell L, Snyder PJ, Lenderking WR et al (2002) Outcomes and costs of acute treatment of traumatic brain injury. J Trauma 53(6):1152–1159, Epub 2002/12/13
Westgren N, Levi R (1998) Quality of life and traumatic spinal cord injury. Arch Phys Med Rehabil 79(11):1433–1439, Epub 1998/11/20
Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS et al (1990) A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the second national acute spinal cord injury study. N Engl J Med 322(20):1405–1411, Epub 1990/05/17
Cengiz SL, Kalkan E, Bayir A, Ilik K, Basefer A (2008) Timing of thoracolomber spine stabilization in trauma patients; impact on neurological outcome and clinical course. A real prospective (rct) randomized controlled study. Arch Orthop Trauma Surg 128(9):959–966, Epub 2007/11/28
Vaccaro AR, Daugherty RJ, Sheehan TP, Dante SJ, Cotler JM, Balderston RA et al (1997) Neurologic outcome of early versus late surgery for cervical spinal cord injury. Spine (Phila Pa 1976) 22(22):2609–2613, Epub 1997/12/17
In ’t Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE et al (2004) Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 22(7):1338–1345
Erices A, Conget P, Minguell JJ (2000) Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol 109(1):235–242, Epub 2000/06/10
Panepucci RA, Siufi JL, Silva WA Jr, Proto-Siquiera R, Neder L, Orellana M et al (2004) Comparison of gene expression of umbilical cord vein and bone marrow-derived mesenchymal stem cells. Stem Cells 22(7):1263–1278, Epub 2004/12/08
Troyer DL, Weiss ML (2008) Wharton’s Jelly-derived cells are a primitive stromal cell population. Stem Cells 26(3):591–599, Epub 2007/12/11
Krampera M, Sartoris S, Liotta F, Pasini A, Angeli R, Cosmi L et al (2007) Immune regulation by mesenchymal stem cells derived from adult spleen and thymus. Stem Cells Dev 16(5):797–810, Epub 2007/11/15
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H et al (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13(12):4279–4295, Epub 2002/12/12
Trubiani O, Orsini G, Caputi S, Piatelli A (2006) Adult mesenchymal stem cells in dental research: a new approach for tissue engineering. Int J Immunopathol Pharmacol 19(3):451–460, Epub 2006/10/10
Shih DT, Lee DC, Chen SC, Tsai RY, Huang CT, Tsai CC et al (2005) Isolation and characterization of neurogenic mesenchymal stem cells in human scalp tissue. Stem Cells 23(7):1012–1020, Epub 2005/06/09
Hida N, Nishiyama N, Miyoshi S, Kira S, Segawa K, Uyama T et al (2008) Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem Cells 26(7):1695–1704, Epub 2008/04/19
Roufosse CA, Direkze NC, Otto WR, Wright NA (2004) Circulating mesenchymal stem cells. Int J Biochem Cell Biol 36(4):585–597, Epub 2004/03/11
Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE et al (2001) Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 32(11):2682–2688, Epub 2001/11/03
Pavlichenko N, Sokolova I, Vijde S, Shvedova E, Alexandrov G, Krouglyakov P et al (2008) Mesenchymal stem cells transplantation could be beneficial for treatment of experimental ischemic stroke in rats. Brain Res 1233:203–213, Epub 2008/08/05
Li Y, Chen J, Wang L, Lu M, Chopp M (2001) Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology 56(12):1666–1672, Epub 2001/06/27
Harting MT, Jimenez F, Xue H, Fischer UM, Baumgartner J, Dash PK et al (2009) Intravenous mesenchymal stem cell therapy for traumatic brain injury. J Neurosurg 110(6):1189–1197, Epub 2009/03/24
Osaka M, Honmou O, Murakami T, Nonaka T, Houkin K, Hamada H et al (2010) Intravenous administration of mesenchymal stem cells derived from bone marrow after contusive spinal cord injury improves functional outcome. Brain Res 1343:226–235, Epub 2010/05/18
Ohta M, Suzuki Y, Noda T, Ejiri Y, Dezawa M, Kataoka K et al (2004) Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp Neurol 187(2):266–278, Epub 2004/05/18
Chung DJ, Choi CB, Lee SH, Kang EH, Lee JH, Hwang SH et al (2009) Intraarterially delivered human umbilical cord blood-derived mesenchymal stem cells in canine cerebral ischemia. J Neurosci Res 87(16):3554–3567, Epub 2009/07/31
Lee JH, Chang HS, Kang EH, Chung DJ, Choi CB, Hwang SH et al (2009) Percutaneous transplantation of human umbilical cord blood-derived multipotent stem cells in a canine model of spinal cord injury. J Neurosurg Spine 11(6):749–757, Epub 2009/12/03
Li J, Zhu H, Liu Y, Li Q, Lu S, Feng M et al (2010) Human mesenchymal stem cell transplantation protects against cerebral ischemic injury and upregulates interleukin-10 expression in Macaca fascicularis. Brain Res 1334:65–72, Epub 2010/04/01
Li Y, Chopp M, Chen J, Wang L, Gautam SC, Xu YX et al (2000) Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab 20(9):1311–1319, Epub 2000/09/20
Liu H, Honmou O, Harada K, Nakamura K, Houkin K, Hamada H et al (2006) Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain 129(Pt 10):2734–2745, Epub 2006/08/12
Walczak P, Zhang J, Gilad AA, Kedziorek DA, Ruiz-Cabello J, Young RG et al (2008) Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke 39(5):1569–1574, Epub 2008/03/08
Bakshi A, Barshinger AL, Swanger SA, Madhavani V, Shumsky JS, Neuhuber B et al (2006) Lumbar puncture delivery of bone marrow stromal cells in spinal cord contusion: a novel method for minimally invasive cell transplantation. J Neurotrauma 23(1):55–65, Epub 2006/01/25
Coyne TM, Marcus AJ, Woodbury D, Black IB (2006) Marrow stromal cells transplanted to the adult brain are rejected by an inflammatory response and transfer donor labels to host neurons and glia. Stem Cells 24(11):2483–2492, Epub 2006/07/29
Coyne TM, Marcus AJ, Reynolds K, Black IB, Woodbury D (2007) Disparate host response and donor survival after the transplantation of mesenchymal or neuroectodermal cells to the intact rodent brain. Transplantation 84(11):1507–1516, Epub 2007/12/20
Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y et al (2002) Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 416(6880):542–545, Epub 2002/04/05
Ying QL, Nichols J, Evans EP, Smith AG (2002) Changing potency by spontaneous fusion. Nature 416(6880):545–548, Epub 2002/04/05
Wakabayashi K, Nagai A, Sheikh AM, Shiota Y, Narantuya D, Watanabe T et al (2010) Transplantation of human mesenchymal stem cells promotes functional improvement and increased expression of neurotrophic factors in a rat focal cerebral ischemia model. J Neurosci Res 88(5):1017–1025, Epub 2009/11/04
Shen LH, Li Y, Chen J, Zacharek A, Gao Q, Kapke A et al (2007) Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab 27(1):6–13, Epub 2006/04/06
Komatsu K, Honmou O, Suzuki J, Houkin K, Hamada H, Kocsis JD (2010) Therapeutic time window of mesenchymal stem cells derived from bone marrow after cerebral ischemia. Brain Res 1334:84–92, Epub 2010/04/13
de Vasconcelos Dos Santos A, da Costa Reis J, Diaz Paredes B, Moraes L, Jasmin, Giraldi-Guimaraes A et al (2010) Therapeutic window for treatment of cortical ischemia with bone marrow-derived cells in rats. Brain Res 1306:149–158, Epub 2009/10/06
Lu M, Chen J, Lu D, Yi L, Mahmood A, Chopp M (2003) Global test statistics for treatment effect of stroke and traumatic brain injury in rats with administration of bone marrow stromal cells. J Neurosci Methods 128(1–2):183–190, Epub 2003/09/02
Mahmood A, Lu D, Qu C, Goussev A, Chopp M (2006) Long-term recovery after bone marrow stromal cell treatment of traumatic brain injury in rats. J Neurosurg 104(2):272–277, Epub 2006/03/03
Mahmood A, Lu D, Lu M, Chopp M (2003) Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery 53(3):697–702; discussion 702–703. Epub 2003/08/29
Omori Y, Honmou O, Harada K, Suzuki J, Houkin K, Kocsis JD (2008) Optimization of a therapeutic protocol for intravenous injection of human mesenchymal stem cells after cerebral ischemia in adult rats. Brain Res 1236:30–38, Epub 2008/08/30
Kranz A, Wagner DC, Kamprad M, Scholz M, Schmidt UR, Nitzsche F et al (2010) Transplantation of placenta-derived mesenchymal stromal cells upon experimental stroke in rats. Brain Res 1315:128–136, Epub 2009/12/17
Mahmood A, Lu D, Yi L, Chen JL, Chopp M (2001) Intracranial bone marrow transplantation after traumatic brain injury improving functional outcome in adult rats. J Neurosurg 94(4):589–595, Epub 2001/04/17
Parr AM, Tator CH, Keating A (2007) Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant 40(7):609–619
Cui X, Chen J, Zacharek A, Li Y, Roberts C, Kapke A et al (2007) Nitric oxide donor upregulation of stromal cell-derived factor-1/chemokine (CXC motif) receptor 4 enhances bone marrow stromal cell migration into ischemic brain after stroke. Stem Cells 25(11):2777–2785, Epub 2007/07/21
Chen X, Katakowski M, Li Y, Lu D, Wang L, Zhang L et al (2002) Human bone marrow stromal cell cultures conditioned by traumatic brain tissue extracts: growth factor production. J Neurosci Res 69(5):687–691, Epub 2002/09/05
England T, Martin P, Bath PM (2009) Stem cells for enhancing recovery after stroke: a review. Int J Stroke 4(2):101–110, Epub 2009/04/23
Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J (1999) Identification of a neural stem cell in the adult mammalian central nervous system. Cell 96(1):25–34, Epub 1999/02/16
Meletis K, Barnabe-Heider F, Carlen M, Evergren E, Tomilin N, Shupliakov O et al (2008) Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol 6(7):e182, Epub 2008/07/25
Reier PJ (2004) Cellular transplantation strategies for spinal cord injury and translational neurobiology. NeuroRx 1(4):424–451, Epub 2005/02/18
Sykova E, Homola A, Mazanec R, Lachmann H, Konradova SL, Kobylka P et al (2006) Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant 15(8–9):675–687, Epub 2007/02/03
Barzilay R, Melamed E, Offen D (2009) Introducing transcription factors to multipotent mesenchymal stem cells: making transdifferentiation possible. Stem Cells 27(10):2509–2515, Epub 2009/07/11
Li Y, Chopp M (2009) Marrow stromal cell transplantation in stroke and traumatic brain injury. Neurosci Lett 456(3):120–123, Epub 2009/05/12
Qu R, Li Y, Gao Q, Shen L, Zhang J, Liu Z et al (2007) Neurotrophic and growth factor gene expression profiling of mouse bone marrow stromal cells induced by ischemic brain extracts. Neuropathology 27(4):355–363, Epub 2007/09/29
Song S, Kamath S, Mosquera D, Zigova T, Sanberg P, Vesely DL et al (2004) Expression of brain natriuretic peptide by human bone marrow stromal cells. Exp Neurol 185(1):191–197, Epub 2003/12/31
Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P et al (2002) Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99(10):3838–3843
Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S et al (2002) Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 30(1):42–48
Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD et al (2005) Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 105(10):4120–4126
Ohtaki H, Ylostalo JH, Foraker JE, Robinson AP, Reger RL, Shioda S et al (2008) Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci USA 105(38):14638–14643, Epub 2008/09/17
Vendrame M, Gemma C, de Mesquita D, Collier L, Bickford PC, Sanberg CD et al (2005) Anti-inflammatory effects of human cord blood cells in a rat model of stroke. Stem Cells Dev 14(5):595–604, Epub 2005/11/25
Jiang L, Womble T, Saporta S, Chen N, Sanberg CD, Sanberg PR et al (2010) Human umbilical cord blood cells decrease microglial survival in vitro. Stem Cells Dev 19(2):221–228, Epub 2009/10/01
Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M et al (2005) Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia 49(3):407–417, Epub 2004/11/13
Busch SA, Horn KP, Cuascut FX, Hawthorne AL, Bai L, Miller RH et al (2010) Adult NG2+ cells are permissive to neurite outgrowth and stabilize sensory axons during macrophage-induced axonal dieback after spinal cord injury. J Neurosci 30(1):255–265, Epub 2010/01/08
Andrews EM, Tsai SY, Johnson SC, Farrer JR, Wagner JP, Kopen GC et al (2008) Human adult bone marrow-derived somatic cell therapy results in functional recovery and axonal plasticity following stroke in the rat. Exp Neurol 211(2):588–592, Epub 2008/04/29
Chen J, Li Y, Zhang R, Katakowski M, Gautam SC, Xu Y et al (2004) Combination therapy of stroke in rats with a nitric oxide donor and human bone marrow stromal cells enhances angiogenesis and neurogenesis. Brain Res 1005(1–2):21–28, Epub 2004/03/27
Ding DC, Shyu WC, Chiang MF, Lin SZ, Chang YC, Wang HJ et al (2007) Enhancement of neuroplasticity through upregulation of beta1-integrin in human umbilical cord-derived stromal cell implanted stroke model. Neurobiol Dis 27(3):339–353, Epub 2007/07/27
Honma T, Honmou O, Iihoshi S, Harada K, Houkin K, Hamada H et al (2006) Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Exp Neurol 199(1):56–66, Epub 2005/06/22
Horita Y, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD (2006) Intravenous administration of glial cell line-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in the adult rat. J Neurosci Res 84(7):1495–1504, Epub 2006/09/26
Kang SK, Lee DH, Bae YC, Kim HK, Baik SY, Jung JS (2003) Improvement of neurological deficits by intracerebral transplantation of human adipose tissue-derived stromal cells after cerebral ischemia in rats. Exp Neurol 183(2):355–366, Epub 2003/10/14
Kim SS, Yoo SW, Park TS, Ahn SC, Jeong HS, Kim JW et al (2008) Neural induction with neurogenin1 increases the therapeutic effects of mesenchymal stem cells in the ischemic brain. Stem Cells 26(9):2217–2228, Epub 2008/07/12
Koh SH, Kim KS, Choi MR, Jung KH, Park KS, Chai YG et al (2008) Implantation of human umbilical cord-derived mesenchymal stem cells as a neuroprotective therapy for ischemic stroke in rats. Brain Res 1229:233–248, Epub 2008/07/19
Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Kobune M, Hirai S et al (2004) BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol Ther 9(2):189–197, Epub 2004/02/05
Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Ishii K, Kobune M et al (2005) Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol Ther 11(1):96–104, Epub 2004/12/09
Lee TH, Yoon JG (2008) Intracerebral transplantation of human adipose tissue stromal cells after middle cerebral artery occlusion in rats. J Clin Neurosci 15(8):907–912, Epub 2008/05/20
Li WY, Choi YJ, Lee PH, Huh K, Kang YM, Kim HS et al (2008) Mesenchymal stem cells for ischemic stroke: changes in effects after ex vivo culturing. Cell Transplant 17(9):1045–1059, Epub 2009/01/31
Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX et al (2002) Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology 59(4):514–523, Epub 2002/08/28
Liao W, Zhong J, Yu J, Xie J, Liu Y, Du L et al (2009) Therapeutic benefit of human umbilical cord derived mesenchymal stromal cells in intracerebral hemorrhage rat: implications of anti-inflammation and angiogenesis. Cell Physiol Biochem 24(3–4):307–316, Epub 2009/08/28
Liao W, Xie J, Zhong J, Liu Y, Du L, Zhou B et al (2009) Therapeutic effect of human umbilical cord multipotent mesenchymal stromal cells in a rat model of stroke. Transplantation 87(3):350–359, Epub 2009/02/10
Liu YP, Seckin H, Izci Y, Du ZW, Yan YP, Baskaya MK (2009) Neuroprotective effects of mesenchymal stem cells derived from human embryonic stem cells in transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab 29(4):780–791, Epub 2009/02/12
Nomura T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD (2005) I.V. infusion of brain-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Neuroscience 136(1):161–169, Epub 2005/10/19
Onda T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD (2008) Therapeutic benefits by human mesenchymal stem cells (hMSCs) and Ang-1 gene-modified hMSCs after cerebral ischemia. J Cereb Blood Flow Metab 28(2):329–340, Epub 2007/07/20
Skvortsova VI, Gubskiy LV, Tairova RT, Povarova OV, Cheglakov IB, Holodenko RV et al (2008) Use of bone marrow mesenchymal (stromal) stem cells in experimental ischemic stroke in rats. Bull Exp Biol Med 145(1):122–128, Epub 2008/11/26
Toyama K, Honmou O, Harada K, Suzuki J, Houkin K, Hamada H et al (2009) Therapeutic benefits of angiogenetic gene-modified human mesenchymal stem cells after cerebral ischemia. Exp Neurol 216(1):47–55, Epub 2008/12/20
Xiao J, Nan Z, Motooka Y, Low WC (2005) Transplantation of a novel cell line population of umbilical cord blood stem cells ameliorates neurological deficits associated with ischemic brain injury. Stem Cells Dev 14(6):722–733, Epub 2006/01/26
Yoo SW, Kim SS, Lee SY, Lee HS, Kim HS, Lee YD et al (2008) Mesenchymal stem cells promote proliferation of endogenous neural stem cells and survival of newborn cells in a rat stroke model. Exp Mol Med 40(4):387–397, Epub 2008/09/10
Zhang J, Li Y, Chen J, Yang M, Katakowski M, Lu M et al (2004) Expression of insulin-like growth factor 1 and receptor in ischemic rats treated with human marrow stromal cells. Brain Res 1030(1):19–27, Epub 2004/11/30
Mahmood A, Lu D, Qu C, Goussev A, Chopp M (2005) Human marrow stromal cell treatment provides long-lasting benefit after traumatic brain injury in rats. Neurosurgery 57(5):1026–1031; discussion 1026–1031. Epub 2005/11/15
Paul C, Samdani AF, Betz RR, Fischer I, Neuhuber B (2009) Grafting of human bone marrow stromal cells into spinal cord injury: a comparison of delivery methods. Spine (Phila Pa 1976) 34(4):328–334, Epub 2009/02/03
Fang KM, Chen JK, Hung SC, Chen MC, Wu YT, Wu TJ et al (2010) Effects of combinatorial treatment with pituitary adenylate cyclase activating peptide and human mesenchymal stem cells on spinal cord tissue repair. PLoS One 5(12):e15299, Epub 2010/12/29
Hu SL, Luo HS, Li JT, Xia YZ, Li L, Zhang LJ et al (2010) Functional recovery in acute traumatic spinal cord injury after transplantation of human umbilical cord mesenchymal stem cells. Crit Care Med 38(11):2181–2189, Epub 2010/08/17
Samdani AF, Paul C, Betz RR, Fischer I, Neuhuber B (2009) Transplantation of human marrow stromal cells and mono-nuclear bone marrow cells into the injured spinal cord: a comparative study. Spine (Phila Pa 1976) 34(24):2605–2612, Epub 2009/11/03
Himes BT, Neuhuber B, Coleman C, Kushner R, Swanger SA, Kopen GC et al (2006) Recovery of function following grafting of human bone marrow-derived stromal cells into the injured spinal cord. Neurorehabil Neural Repair 20(2):278–296, Epub 2006/05/09
Cizkova D, Rosocha J, Vanicky I, Jergova S, Cizek M (2006) Transplants of human mesenchymal stem cells improve functional recovery after spinal cord injury in the rat. Cell Mol Neurobiol 26(7–8):1167–1180, Epub 2006/08/10
Bang OY, Lee JS, Lee PH, Lee G (2005) Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol 57(6):874–882
Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY (2010) A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 28(6):1099–1106, Epub 2010/05/28
Ra JC, Shin IS, Kim SH, Kang SK, Kang BC, Lee HY et al (2011) Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev 20(8):1297–1308
Kishk NA, Gabr H, Hamdy S, Afifi L, Abokresha N, Mahmoud H et al (2010) Case control series of intrathecal autologous bone marrow mesenchymal stem cell therapy for chronic spinal cord injury. Neurorehabil Neural Repair 24(8):702–708, Epub 2010/07/28
Beyer Nardi N, da Silva Meirelles L (2006) Mesenchymal stem cells: isolation, in vitro expansion and characterization. Handb Exp Pharmacol 174:249–282, Epub 2005/12/24
Nöel D, Caton D, Roche S (2008) Cell specific differences between human adipose-derived and mesenchymal-stromal cells despite similar differentiation potentials. Exp Cell Res 314(7):1575–1584
Bernardo ME, Locatelli F, Fibbe WE (2009) Mesenchymal stromal cells. Ann N Y Acad Sci 1176:101–117, Epub 2009/10/03
Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A et al (2006) Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 24(2):376–385, Epub 2005/12/03
Kern S, Eichler H, Stoeve J, Kluter H, Bieback K (2006) Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24(5):1294–1301
Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R (2000) Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: implications for their use in cell therapy. Exp Hematol 28(6):707–715
Kadereit S, Deeds LS, Haynesworth SE, Koc ON, Kozik MM, Szekely E et al (2002) Expansion of LTC-ICs and maintenance of p21 and BCL-2 expression in cord blood CD34(+)/CD38(−) early progenitors cultured over human MSCs as a feeder layer. Stem Cells 20(6):573–582, Epub 2002/11/29
Tarte K, Gaillard J, Lataillade JJ, Fouillard L, Becker M, Mossafa H et al (2010) Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood 115(8):1549–1553, Epub 2009/12/25
Yamaguchi M, Hirayama F, Wakamoto S, Fujihara M, Murahashi H, Sato N et al (2002) Bone marrow stromal cells prepared using AB serum and bFGF for hematopoietic stem cells expansion. Transfusion 42(7):921–927, Epub 2002/10/12
Schallmoser K, Bartmann C, Rohde E, Reinisch A, Kashofer K, Stadelmeyer E et al (2007) Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion 47(8):1436–1446, Epub 2007/07/28
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Yang, B., El Khoury, R., Savitz, S.I. (2013). MSCs for the Treatment of Stroke, Spinal Cord Injury, and Traumatic Brain Injury: From Bench Work to Clinical Trials. In: Hematti, P., Keating, A. (eds) Mesenchymal Stromal Cells. Stem Cell Biology and Regenerative Medicine. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4614-5711-4_35
Download citation
DOI: https://doi.org/10.1007/978-1-4614-5711-4_35
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4614-5710-7
Online ISBN: 978-1-4614-5711-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)