Abstract
Sexual dimorphism in wing coloration is pervasive in butterflies and has been attributed to the process of sexual selection. However, this view has rarely been tested, partly owing to difficulties in estimating the mating success of males in the field. In the present study, we describe a method for assessing the mating success of male pipevine swallowtail (Battus philenor) butterflies, based on the appearance of their reproductive tracts. Laboratory experiments indicated that, in response to mating, components of the males’ reproductive tracts become shorter, decrease in mass, and change in appearance, irrespective of age; and these changes persist for at least 2 days. Using these indicators of recent mating, we examined the reproductive tracts of 68 field-caught males and found that the color of the dorsal hindwing, a feature that females use in mate choice, was significantly greener in males that had recently mated than in males that had not.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
There is a long history of interest in the diversity of butterfly wing pattern and coloration. Starting with work of Darwin (1874) and Wallace (1889), researchers have observed and discussed various issues related to wing pattern, such as interspecific similarity and variation (Nijhout 1990), ecological relevance (Rutowski 1997), color production mechanisms (Koch et al. 1998), evolutionary and developmental plasticity (Beldade et al. 2002), and genetics (Carroll et al. 1994), as well as intersexual differences. Because male butterflies typically exhibit brighter wing coloration and sometimes exhibit pattern elements that are not found in females, many researchers, including Darwin (1874), have speculated that the coloration of male wings results from female mating preferences associated with exaggerated visual signals (Kemp and Rutowski 2011; but see Allen et al. 2011).
A considerable amount of research has also been motivated by the intersexual variation of butterfly wing coloration; however, relative to other groups of colorful animals, such as birds, fish, and lizards (Blount et al. 2003; Grether et al. 2005; Hill and Montgomerie 1994; Keyser and Hill 1999), relatively little is known about the selective factors that promote the sexual dimorphism of wing color in butterflies (Kemp 2007). This deficit has partly stemmed from the difficulty of setting up the necessary assays of female preference. In addition, since sexual selection ultimately results from biased reproductive success, it is necessary to elucidate the relationship between male traits and reproductive success. However, the highly dispersed, cryptic, and ephemeral nature of butterfly copulation hinders the estimation of male mating success in the field (e.g., Rutowski 1997; Takeuchi 2016).
Here we report a new technique to assess the recent mating success of males in Lepidoptera that relies on changes that occur in the appearance of internal reproductive organs during mating. In some lepidopteran species, males transfer an ejaculate to females that can account for as much as 15% of the male body mass (e.g., Rutowski et al. 1983; Svärd and Wiklund 1989), and in many species, males can produce more than one spermatophore; however, it takes time for males to produce an ejaculate that is comparable in size to the one transferred during the previous mating (Bissoondath and Wiklund 1996; Watanabe and Hirota 1999). Therefore, the internal reproductive organs of mated males might differ in size, contents, or appearance from those of unmated males, at least for a few days after mating.
The typical arrangement of internal reproductive organs in male butterflies includes two fused testes that give rise to a pair of vas deferens, which are secretory ducts, that lead to the duplex, which is a pair of sperm storage organs. Two duplex ducts unite caudally to form the simplex, which is a single duct that leads to the intromittent organ, or aedeagus. Sperm move from the testes into the duplex via the vas deferens (Riemann et al. 1974; LaChance et al. 1977). Due to the arrangement of the reproductive organs in the male body (c.f. Fig. 14.1), the spermatophore materials and accessory substances in the simplex are transferred to the female body during mating before the sperm are transferred (Watanabe and Sato 1993). Thus, males might not be able to reserve spermatophore materials or accessory substance in the simplex and so the quantity or nature of these materials might be a good indicator of recent mating activity.
A schematic representation of the internal reproductive organs of a B. philenor male (After Sasaki et al. 2015)
The aims of the present study were (1) to document any changes that occur in the appearance of reproductive structures in male pipevine swallowtail (Battus philenor) butterflies as a result of mating, as well as the persistence of such changes after mating, in order to develop criteria for identifying males that had recently mated and (2) to examine the reproductive tracts of field-caught B. philenor males, assess the variation in their recent mating history, and determine whether their recent mating success was related to their phenotypes. Rutowski and Rajyaguru (2013) have reported that, in a captivity B. philenor, females use the dorsal hindwing coloration of males in mate choice.
This issue is mainly reporting previously published results and ideas in Sasaki et al. (2015).
2 Materials and Methods
2.1 Source of Animals Used
All specimens were from a population of B. philenor that thrives near the confluence of Mesquite Wash and Sycamore Creek in the Mazatzal Mountains, Arizona (33° 43′ 50″ N, 111° 30′ 50″ W). Animals used in the mating studies were reared from eggs and early instar larvae collected in the field from early June to mid-July in 2011. All larvae were reared in a walk-in environmental chamber, programmed for 14 h of light at 30 °C and 10 h of dark at 24 °C with relative humidity held constant at 55%, and were fed ad libitum on cuttings of the local larval food plant, Aristolochia watsonii. On the day of eclosion, males were weighed, their forewing length measured, and given an individual number. Sexes were kept separately in small flight cages (~1 m3) at room temperature (~24 °C) and individually fed 20% sucrose solution for about 20 min each day.
2.2 Examination of Reproductive Tracts of Virgin and Mated Males
To examine the effect of mating on the appearance of the male’s reproductive tract, we hand-paired males with 0–3-day-old virgin females using the method of Watanabe and Hirota (1999). Then, each male was dissected and his reproductive tract examined to assess changes in the appearance of simplex with age and with mating experience. We divided males into three experimental groups: (1) males that never mated and dissected on the day of eclosion or 3 or 6 days after eclosion; (2) males that mated 1, 3, or 5 days after eclosion and dissected immediately after the mating; and (3) males mated 1 day after eclosion and dissected right after the mating or 1, 2, 3, or 5 days after mating.
Before dissection, each male was immobilized by gently pinching their thorax. Each male’s abdomen was then removed from the body and placed in a petri dish filled with fresh insect Ringer’s solution. The reproductive organs including the simplex and duplex were carefully removed from his abdomen. To describe the simplex of each male, we measured its length, appearance, and mass. We first imaged each simplex after removing any fat bodies attached to it and then recorded its appearance with a digital camera attached to a microscope. After capturing images, each simplex was separated from the attached duplex and aedeagus. Wet mass of each simplex was then determined to the nearest 0.01 mg.
2.3 Estimation of Recent Mating Success of Field-Caught Male
Sixty-eight wild males were collected from 16 July to 1 August 2011 in the morning near Sunflower, Arizona. Each captured male was scored as to his wing wear as an indicator of his age. Age-class was scored on the scale (I (least worn) to V (most worn)) described by Watanabe et al. (1986). The forewing length of each male was measured from the wing base to the wing tip. All males were dissected on the day of capture. To assess recent mating success of males, the mass, length, and transparency differences of each male’s simplex were measured.
2.4 Spectral Analyses of Iridescent Wing Areas
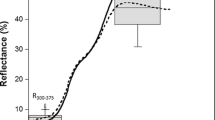
In preparation for spectral measurements, the left hindwing of each butterfly was removed from the thorax and mounted dorsal side up on black card stock with spray adhesive. Reflectance spectra were collected from these wings using techniques described in Rutowski et al. (2010). Reflectance relative to a magnesium oxide white standard was measured between 300 and 700 nm from the wings. Because the reflectance spectra of these iridescent wing surfaces are unimodal, we extracted three color parameters, intensity, hue, and chroma, to describe and analyze the properties of the wing reflectance (Montgomerie 2008).
3 Results
3.1 Virgin Male Reproductive Tract
For virgin males, the simplex mass adjusted for forewing length ((simplex mass)1/3/forewing length), simplex length adjusted for forewing length (simplex length/forewing length), and the transparency difference of simplex was approximately 0.5, 1.3, and 1.3, respectively, and these did not change with male age [mass (ANOVA, F 2,21=1.554, p = 0.237); length (ANOVA, F 2,21=2.276, p = 0.130); transparency difference (ANOVA, F 2,21=0.475, p = 0.629)]. Consequently, simplex of virgin males did not change in appearance with time since eclosion.
3.2 Reproductive Tract of Males Immediately After Mating
For males, immediately after the termination of copulation, the simplex mass adjusted for forewing length, the simplex length adjusted for forewing length, and the transparency difference were approximately 0.32, 0.5, and 0.3, respectively, and these did not vary with the age of the male at mating [mass (ANOVA, F 2,16=0.027, p = 0.974); length (ANOVA, F 2,16 = 1.331, p = 0.296); transparency difference (ANOVA, F 2,16=0.170, p = 0.845)]. Although the simplex of males just after the termination of copulation was different in appearance from that of virgin males, these were not affected by age at mating.
3.3 Changes in the Male’s Reproductive Tract with Time Since Mating
Although the simplex of males just after the termination of copulation was short, it lengthened and refilled again as time passed since mating. During this period, the color of the simplex turned from yellow to colorless, and the amount granular substances increased in the basal end of the tube. Statistically, the mass, length, and transparency difference of simplex all changed with time between mating and dissection (Fig. 14.2; mass: ANOVA: F 5,61 = 59.202, p < 0.001; length: ANOVA: F 5,55 = 42.770, p < 0.001; transparency difference: ANOVA: F 5,55 = 17.139, p < 0.001). After copulation, simplex mass (A) and length (B) dropped to half their precopulatory values, but returned to precopulatory values in about 2 days. The transparency difference decreased with mating but also returned to premating values within about 2 days (C).
Simplex mass (a), length (b), and transparency difference (c) of simplex for virgin males (V, 0, 3, 6 days old) and for mated males (1, 3, 5 days old) dissected at various number of days after mating (mean±S.D.) (After Sasaki et al. 2015). *,** and *** represent p < 0.05, p < 0.01, and p < 0.001 in Tukey’s HSD test, respectively
Using the results of these analyses, we developed criteria for assessing whether a male’s reproductive tract showed evidence of recent mating. The distribution of simplex mass adjusted for forewing length of males within 1 day after mating was 0.251–0.453, whereas that of virgin males was 0.465–0.565, with no overlap in these ranges. The observed ranges of simplex length adjusted for forewing length and the transparency difference of males within 1 day after mating and virgin males also did not overlap (length, 0.401–0.940 vs 1.084–1.628; transparency difference, 0.013–0.964 vs 1.105–2.148). So, we set the lower end of the ranges of values for simplex characteristics of virgin males as the value below which would indicate that the male had recently mated. That is, a field-caught male that had a simplex of less than 0.46 in mass, less than 1.0 in length, or less than 1.0 in transparency difference was taken as indicating that the male had recently mated.
3.4 Mating Success of Field-Caught Males
All field-caught males were evaluated and placed in groups based on which of the three criteria for recent mating they met and which they did not (Table 14.1). For the eight possible groups and to maximize contrasts any group that met two or more of the criteria (Groups E to H) we labeled as showing strong evidence of recent mating. However, because there were no individuals in Group G, we regarded males in Group E, F, and H as recently mated males. Of the 68 males in the list, 12 showed this strong evidence of having mated recently. We also confidently labeled as not recently mated, males that met none of the criteria (Group A).
Using GLM with binomial errors and a logit link function, we compared the phenotypic characteristics of those that had recently mated (Groups E, F, and H) with those that had not (Group A). The characters included in the analysis were the intensity and hue of the iridescent area of male dorsal hindwing and age-class. Chroma was not included as an independent variable in the analysis because there were significant correlations between chroma and all other characteristics (Table 14.2). As shown in Table 14.3, while intensity was not related to their recent mating success, hue and age-class significantly affect their recent mating success. Recently mated males were older and had a higher hue value (were greener) than males that had not recently mated (Figs. 14.3 and 14.4).
The hue (wavelength of maximum reflectance) of the dorsal hindwing for field-caught males that met the criteria for evidence of having recently mated and those males that did not meet the criteria (±S.E.) (After Sasaki et al. 2015)
Change with age-class in the number of clearly recently mated males (light-gray bar), clearly not recently mated males (black bar), and males of uncertain recent mating history (dark-gray bar) for field-caught males (After Sasaki et al. 2015)
4 Discussion
4.1 Assessing the Mating History of Male Butterflies in the Field
The most convincing demonstrations of the evolutionary significance of mating preferences are those in which the results of manipulative experiments are matched by observations in the wild or in wild-caught populations (Kemp 2007). In butterflies, many laboratory experiments have demonstrated the occurrence of female preference for particular male traits, including wing coloration (Krebs and West 1988; Robertson and Monteiro 2005; Andersson et al. 2007). However, the conclusions of these studies have rarely been validated against data obtained in more natural field-based settings (Kemp and Rutowski 2011). In other insects, the comparison of traits from copulating males and unattached males in the field has been extensively used to make inferences about population mating biology (Flecker et al. 1988; Harari et al. 1999; Alcock and Kemp 2006). However, this strategy has not been used in butterflies, except in cases of extremely high density, owing to the difficulty of observing butterfly mating in the field (Kemp and Rutowski 2011). Therefore, to determine the effect of female preference on the evolution of traits in male butterflies, it was necessary to establish an alternative method for evaluating male mating success.
Tsubaki and Matsumoto (1998) estimated the mating frequency of male Luehdorfia japonica by assessing the degree of scale loss from males’ claspers. In this species, males consume scales and use them to form a mating plug on the female abdomen during copulation. Kazuma (1987) reported that the degree of scale loss in laboratory-reared males increased with repeated hand pairing. The degree of scale loss was scored on a scale of 0 (slight scale loss) to 3 (almost all scales were lost), and each stage corresponded to 0, 1, 2, and 3 or more matings. Since mating frequency must have a strong relationship to lifetime reproductive success, this method may provide an accurate estimation of reproductive success in wild males. However, because the mating-related loss of scales is not the rule among lepidopterans, this method is not applicable to all species.
In the present study, we reported a new technique for assessing male mating success that relies on changes that occur in the appearance of internal reproductive organs during mating. Since the internal reproductive organs of butterflies are not much different between species and it is common for males to ejaculate spermatophores and accessory substances during mating (e.g., Drummond 1984), this method can likely be applied to any butterfly species. In fact, it is known that the status of the male simplex just after mating is also different from that before mating in Papilio xuthus (Sasaki, personal observation), P. machaon (Sasaki, personal observation), Byasa alcinous (Sasaki, personal observation), and Eurema hecabe (Konagaya, personal communication). In addition, this new strategy can be used at both middle- or low-density mating sites, since it can detect the occurrence of male mating within a few days, and even though the technique only distinguishes between males that have recently mated and those that have not, it still holds promise for examining variation in male mating success and, thereby, investigating traits that correlate with mating success and the intensity of sexual selection.
We note that, when using this method, there might be some traits that are not suitable for investigating the relationship with mating history. For example, it is impossible, in principle, to investigate the relationship between recent mating success and spermatophore production capacity, which is closely related to the reproductive success of both male and female butterflies.
4.2 Phenotypic Correlates of Mating Success in Male B. philenor in the Field
Our results indicate that males that exhibit signs of having recently mated differ from those that do not, in that they are older and have a greener coloration. This could mean that age and coloration are important determinants of male mating success and under selection, either in the context of (1) female choice, in which females prefer older and greener males, or (2) male competition, in which older and greener males are, for some reason, more effective competitors. In general, the results of the present study support the prediction that male coloration and mating success are related. However, Rutowski and Rajyaguru (2013) reported that the dorsal hindwings of successfully mated B. philenor males possessed more chromatic iridescence than those that failed to mate, rather than a different hue, as reported here.
Such differences between field and laboratory study have also been reported by previous studies. For example, in-copula males of Eurema hecabe were reportedly older than their free-flying counterparts and possessed significantly less bright markings, while brighter males were preferred by females in the laboratory (Kemp 2008). In this case, the difference was caused by the existence of newly emerged females that could not reject mating, and since the density of individuals at the study site was high and activity is centered at localized breeding sites, males could profitably locate such females.
The reasons for difference the color parameters correlated with the mating success of male B. philenor have not yet been clarified. It is possible that the data reported here about the specifics of male age and color associated with recent mating success were affected by the several significant correlations among color parameters and between coloration and age. We made efforts to control for these correlations by excluding variables, such as chroma, from our analysis, but in the end, it is difficult to make conclusions with confidence about the reasons for the connections between male color, age, and male mating success suggested by our data set. In addition, we have not controlled or taken into consideration several other variables that might affect male mating history, such as body size, population density, time during the breeding season, and weather. To convincingly identify factors that determine male mating success in B. philenor, the experimental manipulation of candidate variables is needed.
References
Alcock J, Kemp DJ (2006) The behavioral significance of male body size in the tarantula hawk wasp Hemipepsis ustulata (Hymenoptera: Pompilidae). Ethology 112:691–698
Allen CE, Zwaan BJ, Brakefield PM (2011) Evolution of sexual dimorphism in the Lepidoptera. Ann Rev Entomol 56:445–464
Andersson J, Karlson AKB, Vongvanich N, Wiklund C (2007) Male sex pheromone release and female mate choice in a butterfly. J Exp Biol 210:964–970
Beldade P, Koops K, Brakefield PM (2002) Developmental constraints versus flexibility in morphological evolution. Nature 416:844–847
Bissoondath CJ, Wiklund C (1996) Male butterfly investment in successive ejaculates in relation to mating system. Behav Ecol Sociobiol 39:285–292
Blount JD, Metcalfe NB, Birkhead TR, Surai PF (2003) Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science 300:125–127
Carroll SB, Gates J, Keys DN, Paddock SW, Panganiban GE, Selegue JE, Williams JA (1994) Pattern formation and eyespot determination in butterfly wings. Science 265:109–114
Darwin C (1874) The descent of man and selection in relation to sex. John Murray and Sons, London
Drummond BA (1984) Multiple mating and sperm competition in the Lepidoptera. In: Smith RL (ed) Sperm competition and the evolution of animal mating systems, pp 291–370
Flecker AS, Allan JD, McClintock NL (1988) Male body size and mating success in swarms of the mayfly Epeorus longimanus. Ecography 11:280–285
Grether GF, Cummings ME, Hudon J (2005) Countergradient variation in the sexual coloration of guppies (Poecilia reticulata): drosopterin synthesis balances carotenoid availability. Evolution 59:175–188
Harari AR, Handler AM, Landolt PJ (1999) Size-assortative mating, male choice and female choice in the curculionid beetle Diaprepes abbreviatus. Anim Behav 58:1191–1200
Hill GE, Montgomerie R (1994) Plumage color signals nutritional condition in the house finch. Proc R Soc Lond B 258:47–52
Kazuma M (1987) Mating patterns of a sphragis-bearing butterfly, Luehdorfia japonica Leech (Lepidoptera: Papilionidae), with descriptions of mating behavior. Res Popul Ecol 29:97-110
Kemp DJ (2007) Female butterflies prefer males bearing bright iridescent ornamentation. Proc R Soc London B 274:1043–1047
Kemp DJ (2008) Female mating biases for bright ultraviolet iridescence in the butterfly Eurema hecabe (Pieridae). Behav Ecol 19:1–8
Kemp DJ, Rutowski RL (2011) The role of coloration in mate choice and sexual interactions in butterflies. Adv Study Behav 43:55–92
Keyser AJ, Hill GE (1999) Condition-dependent variation in the blue-ultraviolet coloration of a structurally based plumage ornament. Proc R Soc Lond B 266:771–777
Koch PB, Keys DN, Rocheleau T, Aronstein K, Blackburn M, Carroll SB (1998) Regulation of dopa decarboxylase expression during colour pattern formation in wild-type and melanic tiger swallowtail butterflies. Development 125:2303–2313
Krebs RA, West AD (1988) Female mate preference and the evolution of female-limited Batesian mimicry. Evolution 42:1101–1104
LaChance LEO, Richard RD, Ruud RL (1977) Movement of eupyrene sperm bundles from the testis and storage in the ductus ejaculatoris duplex of the male pink bollworm: effects of age, strain, irradiation, and light. Ann Entomol Soc Am 70:647–651
Montgomerie R (2008) CLR, version 1.05. Queen’s University, Kingston, Canada. Available as of 14 December 2011 at http://post.queensu.ca/~mont/color/analyze.html
Nijhout HF (1990) A comprehensive model for colour pattern formation in butterflies. Proc R Soc London B 239:81–113
Riemann JG, Thorson BJ, Ruud RL (1974) Daily cycle of release of sperm from the testes of the Mediterranean flour moth. J Insect Physiol 20:195–207
Robertson KA, Monteiro A (2005) Female Bicyclus anynana butterflies choose males on the basis of their dorsal UV-reflective eyespot pupils. Proc R Soc London B 272:1541–1546
Rutowski RL (1997) Sexual dimorphism, mating systems and ecology in butterflies. In: Choe JC, Crespi BJ (eds) The evolution of mating systems in insects and arachnids. Cambridge University Press, Cambridge, pp 257–272
Rutowski RL, Nahm A, Macedonia JM (2010) Iridescent hindwing patches in the pipevine swallowtail: differences in dorsal and ventral surfaces relate to signal function and context. Funct Ecol 24:767–775
Rutowski RL, Newton M, Schaefer J (1983) Interspecific variation in the size of the nutrient investment made by male butterflies during copulation. Evolution 34:708–713
Rutowski RL, Rajyaguru PK (2013) Male-specific iridescent coloration in the pipevine swallowtail (Battus philenor) is used in mate choice by females but not sexual discrimination by males. J Insect Behav 26:200–211
Sasaki N, Konagaya T, Watanabe M, Rutowski RL (2015) Indicators of recent mating success in the pipevine swallowtail butterfly (Battus philenor) and their relationship to male phenotype. J Insect Physiol 83:30–36
Svärd L, Wiklund C (1989) Mass and production rate of ejaculates in relation to monandry/polyandry in butterflies. Behav Ecol Sociobiol 24:395–402
Takeuchi T (2016) Agonistic display or courtship behavior? A review of contests over mating opportunity in butterflies. J Ethol:1–10
Tsubaki Y, Matsumoto K (1998) Fluctuating asymmetry and male mating success in a sphragis-bearing butterfly Luehdorfia japonica (Lepidoptera: Papilionidae). J Insect Behav 11:571–582
Wallace AR (1889) Darwinism: an exposition of the theory of natural selection, with some of its applications. Macmillan & Co, London
Watanabe M, Hirota M (1999) Effects of sucrose intake on spermatophore mass produced by male swallowtail butterfly Papilio xuthus L. Zool Sci 16:55–61
Watanabe M, Sato K (1993) A spermatophore structured in the bursa copulatrix of the small white Pieris rapae (Lepidoptera, Pieridae) during copulation, and its sugar content. J Res Lepid 32:26–36
Watanabe M, Nozato K, Kiritani K (1986) Studies on ecology and behavior of Japanese black swallowtail butterflies (Lepidoptera: Papilionidae): V. Fecundity in summer generations. Appl Entomol Zool 21:448–453
Acknowledgments
We thank Masaru Hasegawa and the members of Rutowski Laboratory, especially Sean Hannam, for assistance in the field and laboratory work as well as for valuable discussions. This study was supported by the Japan Society for the Promotion of Science for the Institutional Program for Young Researcher Overseas Visits to NS and TK and in part by JSPS KAKENHI Grant Number 24570019 (MW) and by NSF Grant IOS 1145654 (to R. L. Rutowski). We would like to thank Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
This chapter is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
Copyright information
© 2017 The Author(s)
About this chapter
Cite this chapter
Sasaki, N., Konagaya, T., Watanabe, M., Rutowski, R.L. (2017). Estimating the Mating Success of Male Butterflies in the Field. In: Sekimura, T., Nijhout, H. (eds) Diversity and Evolution of Butterfly Wing Patterns. Springer, Singapore. https://doi.org/10.1007/978-981-10-4956-9_14
Download citation
DOI: https://doi.org/10.1007/978-981-10-4956-9_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-4955-2
Online ISBN: 978-981-10-4956-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)