Abstract
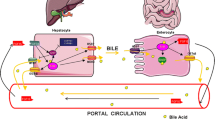
In animals, cholesterol is an essential molecule for membrane formation and synthesis of hormones and bile acids (BAs). Excess of cholesterol, either due to food consumption or endogenous synthesis, leads to gallstone formation and atherosclerosis. BAs produced from cholesterol and free cholesterol are secreted into bile and subsequently eliminated via feces, the only route to eliminate excess of cholesterol. As a major secretory pathway, the different steps of bile formation are precisely controlled and coordinated mostly via a complex network of nuclear receptors. Nuclear receptors (NRs) are transcription factors that, upon ligand binding and cofactors recruitment, modulate polymerase II activity and therefore gene expression, after binding to highly specific DNA response elements located in gene promoters. The Farnesoid X Receptor (FXR, NR1H4) [1] is a bile acid activated nuclear receptor [2–4], regulating several key steps of hepatic physiology such as bile formation, phase I/II metabolism, and glucose, lipid, and lipoprotein metabolisms [5]. Moreover, other NRs like Pregnane X Receptor (PXR; NRI2) [6, 7], Vitamin D Receptor (VDR; NR1I1) [8], and Constitutive Androstane Receptor (CAR; NR1I3) [9, 10] were identified as additional bile acid responsive NRs. In addition to NRs, BAs can also activate a membrane receptor for BAs (TGR5/BG37) [11, 12], a step which does not require bile acid uptake into target cells. Both FXR and TGR5 may play an important role in the pathogenesis and treatment of a variety of hepatic and extrahepatic metabolic disorders including cholestasis, fatty liver, diabetes, dylipidemia, and atherosclerosis.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Forman BM, Goode E, Chen J et al (1995) Identification of a nuclear receptor that is activated by farnesol metabolites. Cell 81(5):687–693
Makishima M, Okamoto AY, Repa JJ et al (1999) Identification of a nuclear receptor for bile acids. Science 284 (5418):1362–1365
Parks DJ, Blanchard SG, Bledsoe RK et al (1999) Bile acids: natural ligands for an orphan nuclear receptor. Science 284(5418):1365–1368
Wang H, Chen J, Hollister K, Sowers LC, Forman BM (1999) Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell 3(5):543–553
Claudel T, Staels B, Kuipers F (2005) The Farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler Thromb Vasc Biol 25(10):2020–2030
Staudinger JL, Goodwin B, Jones SA et al (2001) The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. PNAS 98(6):3369–3374
Xie W, Radominska-Pandya A, Shi Y et al (2001) An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci U S A 98(6): 3375–3380
Makishima M, Lu TT, Xie W et al (2002) Vitamin D receptor as an intestinal bile acid sensor. Science 296(5571): 1313–1316
Uppal H, Toma D, Saini SP, Ren S, Jones TJ, Xie W (2005) Combined loss of orphan receptors PXR and CAR heightens sensitivity to toxic bile acids in mice. Hepatology 41(1): 168–176
Zhang J, Huang W, Qatanani M, Evans RM, Moore DD (2004) The constitutive androstane receptor and pregnane X receptor function coordinately to prevent bile acid-induced hepatotoxicity. J Biol Chem 279(47):49517–49522
Maruyama T, Miyamoto Y, Nakamura T et al (2002) Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun 298(5):714–719
Kawamata Y, Fujii R, Hosoya M et al (2003) A G Protein-coupled receptor responsive to bile acids. J Biol Chem 278(11):9435–9440
Trauner M, Boyer JL (2003) Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev 83(2):633–671
Hofmann AF (1999) The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med 159(22): 2647–2658
Nathanson MH, Boyer JL (1991) Mechanisms and regulation of bile secretion. Hepatology 14(3):551–566
Baiocchi L, LeSage G, Glaser S, Alpini G (1999) Regulation of cholangiocyte bile secretion. J Hepatol 31(1): 179–191
Marzioni M, Glaser SS, Francis H, Phinizy JL, LeSage G, Alpini G (2002) Functional heterogeneity of cholangiocytes. Semin Liver Dis 22(3):227–240
Lazaridis KN, Strazzabosco M, Larusso NF (2004) The cholangiopathies: disorders of biliary epithelia. Gastroenterology 127(5):1565–1577
Kullak-Ublick GA, Stieger B, Meier PJ (2004) Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology 126(1):322–342
Trauner M, Wagner M, Fickert P, Zollner G (2005) Molecular regulation of hepatobiliary transport systems: clinical implications for understanding and treating cholestasis. J Clin Gastroenterol 39(4 Suppl 2):S111–124
Hofmann AF (1999) Bile acids: The good, the bad, and the ugly. News Physiol Sci 14:24–29
Fiorucci S, Rizzo G, Donini A, Distrutti E, Santucci L (2007) Targeting farnesoid X receptor for liver and metabolic disorders. Trends Mol Med 13(7):298–309
Russell DW (2003) The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 72:137–174
Chiang JY (2004) Regulation of bile acid synthesis: pathways, nuclear receptors, and mechanisms. J Hepatol 40(3): 539–551
Lehmann JM, Kliewer SA, Moore LB et al (1997) Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem 272(6):3137–3140
Peet DJ, Turley SD, Ma W et al (1998) Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell 93(5):693–704
Kern F Jr (1991) Normal plasma cholesterol in an 88-year-old man who eats 25 eggs a day Mechanisms of adaptation. N Engl J Med 324(13):896–899
Goodwin B, Jones SA, Price RR et al (2000) A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell 6(3):517–526
Lu TT, Makishima M, Repa JJ et al (2000) Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell 6(3):507–515
Hayhurst GP, Lee YH, Lambert G, Ward JM, Gonzalez FJ (2001) Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol Cell Biol 21(4):1393–1403
Davis RA, Miyake JH, Hui TY, Spann NJ (2002) Regulation of cholesterol-7alpha-hydroxylase: BAREly missing a SHP. J Lipid Res 43(4):533–543
Mataki C, Magnier BC, Houten SM et al (2007) Compromised intestinal lipid absorption in mice with a liver-specific deficiency of liver receptor homolog 1. Mol Cell Biol 27(23): 8330–8339
Lee YK, Schmidt DR, Cummins CL et al (2008) Liver receptor homolog-1 regulates bile acid homeostasis but is not essential for feedback regulation of bile acid synthesis. Mol Endocrinol 22(6):1345–1356
Li-Hawkins J, Lund EG, Turley SD, Russell DW (2000) Disruption of the oxysterol 7alpha-hydroxylase gene in mice. J Biol Chem 275(22):16536–16542
Kerr TA, Saeki S, Schneider M et al (2002) Loss of Nuclear Receptor SHP Impairs but Does Not Eliminate Negative Feedback Regulation of Bile Acid Synthesis. Dev Cell 2(6): 713–720
Wang L, Lee YK, Bundman D et al (2002) Redundant pathways for negative feedback regulation of bile Acid production. Dev Cell 2(6):721–731
Kim I, Ahn SH, Inagaki T et al (2007) Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res 48(12):2664–2672
Jung D, Kullak-Ublick GA (2003) Hepatocyte nuclear factor 1 alpha: a key mediator of the effect of bile acids on gene expression. Hepatology 37(3):622–631
De Fabiani E, Mitro N, Gilardi F, Caruso D, Galli G, Crestani M (2003) Coordinated control of cholesterol catabolism to bile acids and of gluconeogenesis via a novel mechanism of transcription regulation linked to the fasted-to-fed cycle. J Biol Chem 278(40):39124–39132
Li T, Chiang JY (2005) Mechanism of rifampicin and pregnane X receptor inhibition of human cholesterol 7 alpha-hydroxylase gene transcription. Am J Physiol Gastrointest Liver Physiol 288(1):G74–84
Kesaniemi YA, Grundy SM (1984) Influence of gemfibrozil and clofibrate on metabolism of cholesterol and plasma triglycerides in man. Jama 251(17):2241–2246
Marrapodi M, Chiang JY (2000) Peroxisome proliferator-activated receptor alpha (PPARalpha) and agonist inhibit cholesterol 7alpha-hydroxylase gene (CYP7A1) transcription. J Lipid Res 41(4):514–520
Hunt MC, Yang YZ, Eggertsen G et al (2000) The peroxisome proliferator-activated receptor alpha (PPARalpha) regulates bile acid biosynthesis. J Biol Chem 275(37): 28947–28953
Gupta S, Stravitz RT, Dent P, Hylemon PB (2001) Down-regulation of cholesterol 7alpha-hydroxylase (CYP7A1) gene expression by bile acids in primary rat hepatocytes is mediated by the c-Jun N-terminal kinase pathway. J Biol Chem 276(19):15816–15822
De Fabiani E, Mitro N, Anzulovich AC, Pinelli A, Galli G, Crestani M (2001) The negative effects of bile acids and tumor necrosis factor-alpha on the transcription of cholesterol 7alpha-hydroxylase gene (CYP7A1) converge to hepatic nuclear factor-4: a novel mechanism of feedback regulation of bile acid synthesis mediated by nuclear receptors. J Biol Chem 276(33):30708–30716
Pandak WM, Li YC, Chiang JY et al (1991) Regulation of cholesterol 7 alpha-hydroxylase mRNA and transcriptional activity by taurocholate and cholesterol in the chronic biliary diverted rat. J Biol Chem 266(6):3416–3421
Inagaki T, Choi M, Moschetta A et al (2005) Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2(4):217–225
Holt JA, Luo G, Billin AN et al (2003) Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev 17(13):1581–1591
Yu C, Wang F, Kan M et al (2000) Elevated cholesterol metabolism and bile acid synthesis in mice lacking membrane tyrosine kinase receptor FGFR4. J Biol Chem 275(20): 15482–15489
Ito S, Fujimori T, Furuya A, Satoh J, Nabeshima Y, Nabeshima Y (2005) Impaired negative feedback suppression of bile acid synthesis in mice lacking betaKlotho. J Clin Invest 115(8):2202–2208
Lin BC, Wang M, Blackmore C, Desnoyers LR (2007) Liver-specific activities of FGF19 require Klotho beta. J Biol Chem 282(37):27277–27284
Kurosu H, Choi M, Ogawa Y et al (2007) Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem 282(37):26687–26695
Wu X, Ge H, Gupte J et al (2007) Co-receptor requirements for fibroblast growth factor-19 signaling. J Biol Chem 282(40):29069–29072
Jung D, Inagaki T, Dawson PA, Kliewer SA, Mangelsdorf DJ, Moschetta A (2007) FXR agonists and FGF15 reduce fecal bile acid excretion in a mouse model of bile acid malabsorption. J Lipid Res. 2007 Dec;48(12):2693–2700
Zhang M, Chiang JY (2001) Transcriptional Regulation of the Human Sterol 12alpha Hydroxylase Gene (CYP8B1) Roles of Hepatocyte Nuclear Receptor 4alpha in mediating bile acid repression. J Biol Chem 276(45):41690–41699
Shoda J, Kano M, Oda K et al (2001) The expression levels of plasma membrane transporters in the cholestatic liver of patients undergoing biliary drainage and their association with the impairment of biliary secretory function. Am J Gastroenterol 96(12):3368–3378
Bremmelgaard A, Sjovall J (1979) Bile acid profiles in urine of patients with liver diseases. Eur J Clin Invest 9(5):341–348
Bodin K, Lindbom U, Diczfalusy U (2005) Novel pathways of bile acid metabolism involving CYP3A4. Biochim Biophys Acta 1687(1–3):84–93
Handschin C, Meyer UA (2003) Induction of drug metabolism: the role of nuclear receptors. Pharmacol Rev 55(4): 649–673
Gnerre C, Blattler S, Kaufmann MR, Looser R, Meyer UA (2004) Regulation of CYP3A4 by the bile acid receptor FXR: evidence for functional binding sites in the CYP3A4 gene. Pharmacogenetics 14(10):635–645
Drocourt L, Ourlin JC, Pascussi JM, Maurel P, Vilarem MJ (2002) Expression of CYP3A4, CYP2B6, and CYP2C9 is regulated by the vitamin D receptor pathway in primary human hepatocytes. J Biol Chem 277(28):25125–25132
Goodwin B, Hodgson E, D’Costa DJ, Robertson GR, Liddle C (2002) Transcriptional regulation of the human CYP3A4 gene by the constitutive androstane receptor. Mol Pharmacol 62(2):359–365
Weinshilboum RM, Otterness DM, Aksoy IA, Wood TC, Her C, Raftogianis RB (1997) Sulfation and sulfotransferases 1: Sulfotransferase molecular biology: cDNAs and genes. Faseb J 11(1):3–14
Falany CN (1997) Enzymology of human cytosolic sulfotransferases. Faseb J 11(4):206–216
Thomassen PA (1979) Urinary bile acids in late pregnancy and in recurrent cholestasis of pregnancy. Eur J Clin Invest 9(6):425–432
Makino I, Hashimoto H, Shinozaki K, Yoshino K, Nakagawa S (1975) Sulfated and nonsulfated bile acids in urine, serum, and bile of patients with hepatobiliary diseases. Gastroenterology 68(3):545–553
van Berge Henegouwen GP, Brandt KH, Eyssen H, Parmentier G (1976) Sulphated and unsulphated bile acids in serum, bile, and urine of patients with cholestasis. Gut 17(11):861–869
Song CS, Echchgadda I, Baek BS et al (2001) Dehydroepiandrosterone sulfotransferase gene induction by bile acid activated farnesoid x receptor. J Biol Chem 276(45):42549–42556
Sonoda J, Xie W, Rosenfeld JM, Barwick JL, Guzelian PS, Evans RM (2002) Regulation of a xenobiotic sulfonation cascade by nuclear pregnane X receptor (PXR). Proc Natl Acad Sci U S A 99(21):13801–13806
Echchgadda I, Song CS, Roy AK, Chatterjee B (2004) Dehydroepiandrosterone sulfotransferase is a target for transcriptional induction by the vitamin D receptor. Mol Pharmacol 65(3):720–729
Assem M, Schuetz EG, Leggas M et al (2004) Interactions between hepatic Mrp4 and Sult2a as revealed by the constitutive androstane receptor and Mrp4 knockout mice. J Biol Chem 279(21):22250–22257
Saini SP, Sonoda J, Xu L et al (2004) A novel constitutive androstane receptor-mediated and CYP3A-independent pathway of bile acid detoxification. Mol Pharmacol 65(2): 292–300
Makishima M (2005) Nuclear receptors as targets for drug development: regulation of cholesterol and bile acid metabolism by nuclear receptors. J Pharmacol Sci 97(2): 177–183
Takikawa H, Beppu T, Seyama Y (1984) Urinary concentrations of bile acid glucuronides and sulfates in hepatobiliary diseases. Gastroenterol Jpn 19(2):104–109
Frohling W, Stiehl A (1976) Bile salt glucuronides: identification and quantitative analysis in the urine of patients with cholestasis. Eur J Clin Invest 6(1):67–74
Barbier O, Torra IP, Sirvent A et al (2003) FXR induces the UGT2B4 enzyme in hepatocytes: a potential mechanism of negative feedback control of FXR activity. Gastroenterology 124(7):1926–1940
Barbier O, Duran-Sandoval D, Pineda-Torra I, Kosykh V, Fruchart JC, Staels B (2003) Peroxisome proliferator-activated receptor alpha induces hepatic expression of the human bile acid glucuronidating UDP-glucuronosyltransferase 2B4 enzyme. J Biol Chem 278(35):32852–32860
Pineda Torra I, Claudel T, Duval C, Kosykh V, Fruchart JC, Staels B (2003) Bile Acids Induce the Expression of the Human Peroxisome Proliferator- Activated Receptor alpha Gene via Activation of the Farnesoid X Receptor. Mol Endocrinol 17(2):259–272
Lu Y, Heydel JM, Li X, Bratton S, Lindblom T, Radominska-Pandya A (2005) Lithocholic Acid decreases expression of ugt2b7 in caco-2 cells: a potential role for a negative farnesoid x receptor response element. Drug Metab Dispos 33(7): 937–946
Jung D, Hagenbuch B, Fried M, Meier PJ, Kullak-Ublick GA (2004) Role of liver-enriched transcription factors and nuclear receptors in regulating the human, mouse, and rat NTCP gene. Am J Physiol Gastrointest Liver Physiol 286(5): G752–761
Geier A, Wagner M, Dietrich CG, Trauner M (2007) Principles of hepatic organic anion transporter regulation during cholestasis, inflammation and liver regeneration. Biochim Biophys Acta 1773(3):283–308
Zollner G, Fickert P, Silbert D et al (2002) Induction of short heterodimer partner 1 precedes downregulation of Ntcp in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol 282(1):G184–191
Geier A, Zollner G, Dietrich CG et al (2005) Cytokine-independent repression of rodent Ntcp in obstructive cholestasis. Hepatology 41(3):470–477
Denson LA, Sturm E, Echevarria W et al (2001) The orphan nuclear receptor, shp, mediates bile acid-induced inhibition of the rat bile acid transporter, ntcp. Gastroenterology 121(1):140–147
Lee YK, Dell H, Dowhan DH, Hadzopoulou-Cladaras M, Moore DD (2000) The orphan nuclear receptor SHP inhibits hepatocyte nuclear factor 4 and retinoid X receptor transactivation: two mechanisms for repression. Mol Cell Biol 20(1):187–195
Li D, Zimmerman TL, Thevananther S, Lee HY, Kurie JM, Karpen SJ (2002) Interleukin-1 beta-mediated suppression of RXR:RAR transactivation of the Ntcp promoter is JNK-dependent. J Biol Chem 277(35):31416–31422
Zollner G, Wagner M, Fickert P et al (2005) Role of nuclear receptors and hepatocyte-enriched transcription factors for Ntcp repression in biliary obstruction in mouse liver. Am J Physiol Gastrointest Liver Physiol 289(5):G798–805
Eloranta JJ, Jung D, Kullak-Ublick GA (2006) The human Na+-taurocholate cotransporting polypeptide gene is activated by glucocorticoid receptor and peroxisome proliferator-activated receptor-gamma coactivator-1alpha, and suppressed by bile acids via a small heterodimer partner-depende. Mol Endocrinol 20(1):65–79
Shneider BL, Fox VL, Schwarz KB et al (1997) Hepatic basolateral sodium-dependent-bile acid transporter expression in two unusual cases of hypercholanemia and in extrahepatic biliary atresia. Hepatology 25(5):1176–1183
Zollner G, Fickert P, Zenz R et al (2001) Hepatobiliary transporter expression in percutaneous liver biopsies of patients with cholestatic liver diseases. Hepatology 33(3): 633–646
Zollner G, Fickert P, Silbert D et al (2003) Adaptive changes in hepatobiliary transporter expression in primary biliary cirrhosis. J Hepatol 38(6):717–727
Zollner G, Marschall HU, Wagner M, Trauner M (2006) Role of nuclear receptors in the adaptive response to bile acids and cholestasis: pathogenetic and therapeutic considerations. Mol Pharm 3(3):231–251
Jung D, Podvinec M, Meyer UA et al (2002) Human organic anion transporting polypeptide 8 promoter is transactivated by the farnesoid X receptor/bile acid receptor. Gastroenterology 122(7):1954–1966
Shi X, Bai S, Ford AC et al (1995) Stable inducible expression of a functional rat liver organic anion transport protein in HeLa cells. J Biol Chem 270(43):25591–25595
Kullak-Ublick GA, Stieger B, Hagenbuch B, Meier PJ (2000) Hepatic transport of bile salts. Semin Liver Dis 20(3): 273–292
Meier PJ, Stieger B (2002) Bile salt transporters. Annu Rev Physiol 64:635–661
Keppler D, Konig J (2000) Hepatic secretion of conjugated drugs and endogenous substances. Semin Liver Dis 20(3): 265–272
Cui Y, Konig J, Buchholz JK, Spring H, Leier I, Keppler D (1999) Drug resistance and ATP-dependent conjugate transport mediated by the apical multidrug resistance protein, MRP2, permanently expressed in human and canine cells. Mol Pharmacol 55(5):929–937
Dubuisson C, Cresteil D, Desrochers M, Decimo D, Hadchouel M, Jacquemin E (1996) Ontogenic expression of the Na(+)-independent organic anion transporting polypeptide (oatp) in rat liver and kidney. J Hepatol 25(6):932–940
Elferink RP, Ottenhoff R, van Marle J, Frijters CM, Smith AJ, Groen AK (1998) Class III P-glycoproteins mediate the formation of lipoprotein X in the mouse. J Clin Invest 102(9):1749–1757
Eloranta JJ, Kullak-Ublick GA (2005) Coordinate transcriptional regulation of bile acid homeostasis and drug metabolism. Arch Biochem Biophys 433(2):397–412
Trauner M, Fickert P, Wagner M (2007) MDR3 (ABCB4) defects: a paradigm for the genetics of adult cholestatic syndromes. Semin Liver Dis 27(1):77–98
Hazard SE, Patel SB (2007) Sterolins ABCG5 and ABCG8: regulators of whole body dietary sterols. Pflugers Arch 453(5):745–752
Banales JM, Prieto J, Medina JF (2006) Cholangiocyte anion exchange and biliary bicarbonate excretion. WorldJ Gastroenterol 12(22):3496–3511
Fickert P, Zollner G, Fuchsbichler A et al (2002) Ursodeoxycholic acid aggravates bile infarcts in bile duct-ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology 123(4):1238–1251
Zollner G, Fickert P, Fuchsbichler A et al (2003) Role of nuclear bile acid receptor, FXR, in adaptive ABC transporter regulation by cholic and ursodeoxycholic acid in mouse liver, kidney and intestine. J Hepatol 39(4):480–488
Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ (2001) Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J Biol Chem 276(31):28857–28865
Gerloff T, Stieger B, Hagenbuch B et al (1998) The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J Biol Chem 273(16):10046–10050
Plass JR, Mol O, Heegsma J et al (2002) Farnesoid X receptor and bile salts are involved in transcriptional regulation of the gene encoding the human bile salt export pump. Hepatology 35(3):589–596
Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ (2000) Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102(6):731–744
Wagner M, Fickert P, Zollner G et al (2003) Role of farnesoid X receptor in determining hepatic ABC transporter expression and liver injury in bile duct-ligated mice. Gastroenterology 125(3):825–838
Honjo Y, Sasaki S, Kobayashi Y, Misawa H, Nakamura H (2006) 1, 25-dihydroxyvitamin D3 and its receptor inhibit the chenodeoxycholic acid-dependent transactivation by farnesoid X receptor. J Endocrinol 188(3):635–643
Gascon-Barre M, Demers C, Mirshahi A, Neron S, Zalzal S, Nanci A (2003) The normal liver harbors the vitamin D nuclear receptor in nonparenchymal and biliary epithelial cells. Hepatology 37(5):1034–1042
Kast HR, Goodwin B, Tarr PT et al (2001) Regulation of multidrug resistance-associated protein 2 (MRP2;ABCC2) by the nuclear receptors PXR, FXR, and CAR. J Biol Chem 12:12
Huang L, Zhao A, Lew JL, et al (2003) Farnesoid X-receptor activates transcription of the phospholipid pump MDR3. J Biol Chem. 2003 Dec 19;278(51):51085–51090
Kok T, Bloks VW, Wolters H et al (2003) Peroxisome proliferator-activated receptor alpha (PPARalpha)-mediated regulation of multidrug resistance 2 (Mdr2) expression and function in mice. Biochem J 369(3):539–547
Liu Y, Binz J, Numerick MJ, et al (2003) Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J Clin Invest. 2003 Dec;112(11):1678–1687
Schuetz EG, Strom S, Yasuda K et al (2001) Disrupted bile acid homeostasis reveals an unexpected interaction among nuclear hormone receptors, transporters, and cytochrome P450. J Biol Chem 276(42):39411–39418
Teng S, Jekerle V, Piquette-Miller M (2003) Induction of ABCC3 (MRP3) by pregnane X receptor activators. Drug Metab Dispos 31(11):1296–1299
McCarthy TC, Li X, Sinal CJ (2005) Vitamin D receptor-dependent regulation of colon multidrug resistance-associated protein 3 gene expression by bile acids. J Biol Chem 280(24):23232–23242
Wagner M, Halilbasic E, Marschall HU et al (2005) CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology 42(2):420–430
Cherrington NJ, Hartley DP, Li N, Johnson DR, Klaassen CD (2002) Organ distribution of multidrug resistance proteins 1, 2, and 3 (Mrp1, 2, and 3) mRNA and hepatic induction of Mrp3 by constitutive androstane receptor activators in rats. J Pharmacol Exp Ther 300(1):97–104
Maher JM, Cheng X, Slitt AL, Dieter MZ, Klaassen CD (2005) Induction of the multidrug resistance-associated protein family of transporters by chemical activators of receptor-mediated pathways in mouse liver. Drug Metab Dispos 33(7):956–962
Zollner G, Wagner M, Moustafa T et al (2006) Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-alpha/beta in the adaptive response to bile acids. Am J Physiol Gastrointest Liver Physiol 290(5): G923–932
Boyer JL, Trauner M, Mennone A et al (2006) Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTalpha-OSTbeta in cholestasis in humans and rodents. Am J Physiol Gastrointest Liver Physiol 290(6): G1124–1130
Lee H, Zhang Y, Lee FY, Nelson SF, Gonzalez FJ, Edwards PA (2006) FXR regulates organic solute transporters alpha and beta in the adrenal gland, kidney, and intestine. J Lipid Res 47(1):201–214
Baumgartner G, Pusl T (2008) Medical treatment of cholestatic liver disease. Clin Liver Dis 12(1):53–80
Tanaka H, Makino I (1992) Ursodeoxycholic acid-dependent activation of the glucocorticoid receptor. Biochem Biophys Res Commun 188(2):942–948
Miura T, Ouchida R, Yoshikawa N et al (2001) Functional modulation of the glucocorticoid receptor and suppression of NF-kappaB-dependent transcription by ursodeoxycholic acid. J Biol Chem 276(50):47371–47378
Pascussi JM, Busson-Le Coniat M, Maurel P, Vilarem MJ (2003) Transcriptional analysis of the orphan nuclear receptor constitutive androstane receptor (NR1I3) gene promoter: identification of a distal glucocorticoid response element. Mol Endocrinol 17(1):42–55
Bodin K, Bretillon L, Aden Y et al (2001) Antiepileptic drugs increase plasma levels of 4beta-hydroxycholesterol in humans: evidence for involvement of cytochrome p450 3A4. J Biol Chem 276(42):38685–38689
Ellis E, Axelson M, Abrahamsson A et al (2003) Feedback regulation of bile acid synthesis in primary human hepatocytes: evidence that CDCA is the strongest inhibitor. Hepatology 38(4):930–938
Lew JL, Zhao A, Yu J et al (2004) The farnesoid X receptor controls gene expression in a ligand- and promoter-selective fashion. J Biol Chem 279(10):8856–8861
Gerk PM, Li W, Megaraj V, Vore M (2007) Human multidrug resistance protein 2 transports the therapeutic bile salt tauroursodeoxycholate. J Pharmacol Exp Ther 320(2): 893–899
Paumgartner G, Beuers U (2002) Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology 36(3):525–531
Trauner M, Graziadei IW (1999) Review article: mechanisms of action and therapeutic applications of ursodeoxycholic acid in chronic liver diseases. Aliment Pharmacol Ther 13(8):979–996
Kast HR, Goodwin B, Tarr PT et al (2002) Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem 277(4):2908–2915
Yu J, Lo JL, Huang L et al (2002) Lithocholic acid decreases expression of bile salt export pump through farnesoid X receptor antagonist activity. J Biol Chem 277(35):31441–31447
Claudel T, Sturm E, Kuipers F, Staels B (2004) The farnesoid X receptor: a novel drug target? Expert Opin Investig Drugs 13(9):1135–1148
Moschetta A, Bookout AL, Mangelsdorf DJ (2004) Prevention of cholesterol gallstone disease by FXR agonists in a mouse model. Nat Med 10(12):1352–1358
Grundy SM, Ahrens EH Jr, Salen G (1971) Interruption of the enterohepatic circulation of bile acids in man: comparative effects of cholestyramine and ileal exclusion on cholesterol metabolism. J Lab Clin Med 78(1):94–121
Heaton KW (1977) Disturbances of bile acid metabolism in intestinal disease. Clin Gastroenterol 6(1):69–89
Buchwald H, Varco RL, Matts JP et al (1990) Effect of partial ileal bypass surgery on mortality and morbidity from coronary heart disease in patients with hypercholesterolemia. Report of the Program on the Surgical Control of the Hyperlipidemias (POSCH). N Engl J Med 323(14): 946–955
Shepherd J, Packard CJ, Morgan HG, Third JL, Stewart JM, Lawrie TD (1979) The effects of cholestyramine on high density lipoprotein metabolism. Atherosclerosis 33(4):433–444
Brensike JF, Levy RI, Kelsey SF et al (1984) Effects of therapy with cholestyramine on progression of coronary arteriosclerosis: results of the NHLBI Type II Coronary Intervention Study. Circulation 69(2):313–324
Levy RI, Brensike JF, Epstein SE et al (1984) The influence of changes in lipid values induced by cholestyramine and diet on progression of coronary artery disease: results of NHLBI Type II Coronary Intervention Study. Circulation 69(2):325–337
Kuriyama M, Tokimura Y, Fujiyama J, Utatsu Y, Osame M (1994) Treatment of cerebrotendinous xanthomatosis: effects of chenodeoxycholic acid, pravastatin, and combined use. J Neurol Sci 125(1):22–28
Leiss O, von Bergmann K (1982) Different effects of chenodeoxycholic acid and ursodeoxycholic acid on serum lipoprotein concentrations in patients with radiolucent gallstones. Scand J Gastroenterol 17(5):587–592
Iglesias A, Arranz M, Alvarez JJ et al (1996) Cholesteryl ester transfer activity in liver disease and cholestasis, and its relation with fatty acid composition of lipoprotein lipids. Clin Chim Acta 248(2):157–174
Claudel T, Sturm E, Duez H et al (2002) Bile acid-activated nuclear receptor FXR suppresses apolipoprotein A-I transcription via a negative FXR response element. J Clin Invest 109(7):961–971
Melter M, Rodeck B, Kardorff R et al (2000) Progressive familial intrahepatic cholestasis: partial biliary diversion normalizes serum lipids and improves growth in noncirrhotic patients. Am J Gastroenterol 95(12):3522–3528
Albrink MJ, Man EB (1959) Serum triglycerides in coronary artery disease. Archives of Internal Medicine. 103: 4–8
Goldstein JL, Schrott HG, Hazzard WR, Bierman EL, Motulsky AG (1973) Hyperlipidemia in coronary heart disease II Genetic analysis of lipid levels in 176 families and delineation of a new inherited disorder, combined hyperlipidemia. J Clin Invest 52(7):1544–1568
Brunzell JD, Schrott HG, Motulsky AG, Bierman EL (1976) Myocardial infarction in the familial forms of hypertriglyceridemia. Metabolism 25(3):313–320
Genest JJ Jr, Martin-Munley SS, McNamara JR et al (1992) Familial lipoprotein disorders in patients with premature coronary artery disease. Circulation 85(6):2025–2033
Bateson MC, Maclean D, Evans JR, Bouchier IA (1978) Chenodeoxycholic acid therapy for hypertriglyceridaemia in men. Br J Clin Pharmacol 5(3):249–254
Begemann F (1978) Influence of chenodeoxycholic acid on the kinetics of endogenous triglyceride transport in man. Eur J Clin Invest 8(5):283–288
Camarri E, Fici F, Marcolongo R (1978) Influence of chenodeoxycholic acid on serum triglycerides in patients with primary hypertriglyceridemia. Int J Clin Pharmacol Biopharm 16(11):523–526
Camarri E, Marcolongo R, Zaccherotti L, Marini G (1978) The hypotriglyceridemic effect of chenodeoxycholic acid in type IV hyperlipemia. Biomedicine 29(6):193–198
Duane WC (1995) Abnormal bile acid absorption in familial hypertriglyceridemia. J Lipid Res 36(1):96–107
Duane WC, Hartich LA, Bartman AE, Ho SB (2000) Diminished gene expression of ileal apical sodium bile acid transporter explains impaired absorption of bile acid in patients with hypertriglyceridemia. J Lipid Res 41(9): 1384–1389
Angelin B (1995) 1994 Mack-Forster Award Lecture Review Studies on the regulation of hepatic cholesterol metabolism in humans. Eur J Clin Invest 25(4):215–224
Angelin B, Einarsson K, Hellstrom K, Leijd B (1978) Effects of cholestyramine and chenodeoxycholic acid on the metabolism of endogenous triglyceride in hyperlipoproteinemia. J Lipid Res 19(8):1017–1024
Molgaard J, von Schenck H, Olsson AG (1989) Comparative effects of simvastatin and cholestyramine in treatment of patients with hypercholesterolaemia. Eur J Clin Pharmacol 36(5):455–460
Pullinger CR, Eng C, Salen G et al (2002) Human cholesterol 7alpha-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J Clin Invest 110(1): 109–117
Claudel T, Inoue Y, Barbier O et al (2003) Farnesoid X receptor agonists suppress hepatic apolipoprotein CIII expression. Gastroenterology 125(2):544–555
Duran-Sandoval D, Mautino G, Martin G et al (2004) Glucose regulates the expression of the farnesoid X receptor in liver. Diabetes 53(4):890–898
DenBesten L, Reyna RH, Connor WE, Stegink LD (1973) The different effects on the serum lipids and fecal steroids of high carbohydrate diets given orally or intravenously.J Clin Invest 52(6):1384–1393
Stacpoole PW, Grundy SM, Swift LL, Greene HL, Sloni AE, Burr IM (1981) Elevated cholesterol and bile acid synthesis in an adult patient with homozygous familial hypercholesterolemia Reduction by a high glucose diet. J Clin Invest 68(5):1166–1171
Dawes LG, Laut HC, Woodruff M (2007) Decreased bile acid synthesis with total parenteral nutrition. Am J Surg 194(5):623–627
Ma K, Saha PK, Chan L, Moore DD (2006) Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest 116(4):1102–1109
Zhang Y, Lee FY, Barrera G et al (2006) Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A 103(4):1006–1011
Ludwig J, Viggiano TR, McGill DB, Oh BJ (1980) Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc 55(7): 434–438
Shibata M, Kihara Y, Taguchi M, Tashiro M, Otsuki M (2007) Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle-aged Japanese men. Diabetes Care 30(11):2940–2944
Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S (2005) Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology 42(1):44–52
Bedogni G, Miglioli L, Masutti F et al (2007) Incidence and natural course of fatty liver in the general population: the Dionysos study. Hepatology 46(5):1387–1391
Neuschwander-Tetri BA, Caldwell SH (2003) Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology 37(5):1202–1219
Adams LA, Lymp JF, St Sauver J et al (2005) The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 129(1):113–121
Ekstedt M, Franzen LE, Mathiesen UL et al (2006) Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 44(4):865–873
Targher G, Bertolini L, Poli F et al (2005) Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes 54(12): 3541–3546
Huang J, Iqbal J, Saha PK et al (2007) Molecular characterization of the role of orphan receptor small heterodimer partner in development of fatty liver. Hepatology 46(1): 147–157
Nishigori H, Tomura H, Tonooka N et al (2001) Mutations in the small heterodimer partner gene are associated with mild obesity in Japanese subjects. Proc Natl Acad Sci U S A 98(2):575–580
Echwald SM, Andersen KL, Sorensen TI et al (2004) Mutation analysis of NR0B2 among 1545 Danish men identifies a novel c.278G>A (p.G93D) variant with reduced functional activity. Hum Mutat 24(5):381–387
Hung CC, Farooqi IS, Ong K et al (2003) Contribution of variants in the small heterodimer partner gene to birthweight, adiposity, and insulin levels: mutational analysis and association studies in multiple populations. Diabetes 52(5):1288–1291
Mitchell SM, Weedon MN, Owen KR et al (2003) Genetic variation in the small heterodimer partner gene and young-onset type 2 diabetes, obesity, and birth weight in U.K. subjects. Diabetes 52(5):1276–1279
Maruyama T, Tanaka K, Suzuki J et al (2006) Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. J Endocrinol 191(1):197–205
Vassileva G, Golovko A, Markowitz L et al (2006) Targeted deletion of Gpbar1 protects mice from cholesterol gallstone formation. Biochem J 398(3):423–430
Watanabe M, Houten SM, Mataki C et al (2006) Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439(7075): 484–489
Lean ME, James WP, Jennings G, Trayhurn P (1986) Brown adipose tissue uncoupling protein content in human infants, children and adults. Clin Sci (Lond) 71(3): 291–297
Oberkofler H, Dallinger G, Liu YM, Hell E, Krempler F, Patsch W (1997) Uncoupling protein gene: quantification of expression levels in adipose tissues of obese and non-obese humans. J Lipid Res 38(10):2125–2133
Huttunen P, Hirvonen J, Kinnula V (1981) The occurrence of brown adipose tissue in outdoor workers. Eur J Appl Physiol Occup Physiol 46(4):339–345
Zancanaro C, Pelosi G, Accordini C, Balercia G, Sbabo L, Cinti S (1994) Immunohistochemical identification of the uncoupling protein in human hibernoma. Biol Cell 80(1): 75–78
Lean ME, James WP, Jennings G, Trayhurn P (1986) Brown adipose tissue in patients with phaeochromocytoma. Int J Obes 10(3):219–227
Ricquier D, Nechad M, Mory G (1982) Ultrastructural and biochemical characterization of human brown adipose tissue in pheochromocytoma. J Clin Endocrinol Metab 54(4): 803–807
Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84(1): 277–359
Yoshimura T, Kurita C, Nagao T et al (1997) Inhibition of tumor necrosis factor-alpha and interleukin-1-beta production by beta-adrenoceptor agonists from lipopolysaccharide-stimulated human peripheral blood mononuclear cells. Pharmacology 54(3):144–152
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Claudel, T., Trauner, M. (2010). Bile Acids and Their Receptors. In: Dufour, JF., Clavien, PA. (eds) Signaling Pathways in Liver Diseases. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-00150-5_21
Download citation
DOI: https://doi.org/10.1007/978-3-642-00150-5_21
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-00149-9
Online ISBN: 978-3-642-00150-5
eBook Packages: MedicineMedicine (R0)