Abstract
In recent decades, probiotics have shown beneficial effects on animal and human health. Probiotics can protect the host against several health threats, including infectious diseases. Before 1995, researchers believed that the effect of probiotics was only on gut microbiota which can restore the gut flora and thus prevent pathogenic bacteria from triggering gastroenteritis. Recent studies have shown that the immunomodulatory activity is the most important mechanism of action of probiotics. From this information, researchers started to evaluate the effect of some immunobiotics, not only on pathogenic bacteria but also on viruses, including enteric and respiratory viruses. Several studies have confirmed the potential antiviral activity of some probiotics due to the immunomodulatory effect. These studies were conducted on humans (clinical trials) and in animal models. In this chapter, probiotics with antiviral effect against respiratory and enteric viruses will be presented and discussed, as well as their mechanisms of action.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Antiviral probiotics
- Respiratory viruses
- Immunomodulation
- Gut microbiota
- Immunobiotics
- Enteric viruses
- Antiviral probiotics
- Viral trapping
- Norovirus
- Rotavirus
- Immunomodulation
- Gut microbiota
1.1 Overview
Respiratory infections and gastroenteritis constitute the major causes of mortality and morbidity worldwide, both in developing and developed countries [1]. Despite the widespread adoption of vaccines strategies, some pathogens remain a threat to public health worldwide. The US National Institutes of Health (NIH; Bethesda, MD, USA) declared the emergence of 16 new infectious diseases, six of which have been considered reemerging infections [2]. In the United States of America (USA), mortality caused by infectious diseases (IDs) was amounted to 170,000 deaths in 2000 [3]. An increase in immunocompromised patients plays a crucial role in the emergence and/or reemergence of IDs; therefore, post-infection complications can lead to death. Public health is faced with two major obstacles to eradicating IDs: (1) Antibiotic therapies, which have been saving infected patient for several years. Unfortunately, the rapid emergence of resistant bacteria is occurring worldwide, endangering the efficacy of antibiotics, which have transformed medicine and saved millions of lives [4]. (2) The lack of antiviral agents against infectious viruses, which leads to a high treatment level between populations even in the presence of some vaccines covering a few virus types [5]. Several strategies have been developed to overcome this crisis, e.g., (i) the use of bacteriophages as antibacterial agents [4], (ii) the extraction and purification of antimicrobial peptides [6], and (iii) the prevention of IDs by using vaccines and/or recombinant vaccine strategies [7]. Preventing infectious diseases occurring seems to be the perfect method of avoiding ID complications, since all of the abovementioned strategies have inconveniences such as side effects and stability in the host.
Immune system boosting is the essential key factor in ID prevention. Dietary balance in meals, administration of supplements such as fiber, and probiotics are three methods to enhance and stimulate the immune system, thus protecting the mucosa against the entry of pathogens. Probiotics have demonstrated their capacity to stimulate and modulate the immune system [8]. In addition to the antibacterial activity of probiotics, some strains showed an effective antiviral activity which can be a solution to the lack of antiviral agents [9].
In this chapter, we focus on probiotics which have been shown to be effective as antiviral agents against respiratory and enteric viruses. In addition, we give details of some clinical trials and both in vitro and in vivo experiments which have confirmed this efficacy.
1.2 Part I-A: Probiotics and Respiratory Infections
1.2.1 Introduction
Lactic acid bacteria (LAB) can be found in many ecosystems, including human and animal flora. LAB, as well as their metabolites such as bacteriocins, are generally recognized as safe (GRAS) [10]. The antibacterial activity of probiotics has been confirmed in a large number of research studies. This activity may occur through several mechanisms: (1) Pathogens exclusion: probiotic strains have a high affinity for adhesion to epithelial cells. Thus, probiotics will saturate the receptors and exert a barrier effect against the pathogens involved in infections. (2) Nutrient competition: probiotic strains can ingest many essential molecules, and consequently pathogens cannot grow in this ecosystem. (3) Production of antimicrobial compounds, such as lactic acid, hydrogen peroxide, bacteriocin-like inhibitory substances (BLISs), NRPS, and bacteriocins [11–13] (Fig. 1.1).
Potential functions attributed to LAB probiotics [5]
In addition to food applications, the use of LAB is growing, in particular as probiotics for controlling, for example, gastroenteritis, inflammatory pathologies of the digestive tract [14, 15] and to stimulate the local and systemic immune response [16, 17].
In recent decades, some probiotics have shown an antiviral activity and several mechanisms have been demonstrated. In respiratory tract infections (RTIs), the majority of probiotics can inhibit the most important respiratory viruses (RVs) by immunomodulatory mechanisms [18] (Fig. 1.2). This antiviral mechanism might be explained due to the entry routes of RVs. RVs infect the mucosal cells of the RT, and for this reason, probiotic strains and their antimicrobial compounds cannot directly interact with viruses by physical contact. Probiotic strains can finally reduce or eradicate virus infectivity by immunomodulatory activity, which has led scientists to call them “immunobiotics” [19]. In this chapter, the majority of anti-RV probiotics will be mentioned with other information, such as the source of probiotic strain, target virus, experimental model, and mode of antiviral activity (Table 1.1).
Suggested mechanisms of antiviral probiotics against respiratory viruses. This figure shows the antiviral mechanisms of some probiotic strains used against viral respiratory infections. Although there is a difference between the probiotic colonization ecosystem and the target RV ecosystem, several studies have showed that there is a relationship between gut microbiota and other tissues. Probiotics can inhibit viruses and/or help the immune system defend itself against RVs. First, the RVs interact with the respiratory epithelium, which generates an innate immune response by activating the IFN signaling and other proinflammatory cytokines. Once cytokines have been secreted, macrophages and NK cells will be recruited to phagocytize and kill both viruses and viral-infected cells. To trigger a specific immune response, the immune system needs proinflammatory cytokines, energy, and some cofactor elements. Hence, probiotics can provide some elements to boost the immune response: A. Probiotics interact with the gut epithelium and are recognized by intestinal DCs (IDCs); this interaction results in the production of IL-12 and IFNγ by IDCs, which can modulate both the respiratory and gut immune response. B . Secretion of IFNγ and IL-12 by intestinal DCs; these two proinflammatory cytokines have dual functions: IFNγ and IL-12 can circulate in the bloodstream to reach the respiratory epithelium and therefore help alveolar macrophages and NK cells eliminate RVs. C. The proinflammatory cytokines (IFNγ and IL-12) secreted in the gut ecosystem after colonization of some probiotic strains help the immune system to generate a specific Th1/Th17 immune response; the number of CD4+ and CD8+ increases and becomes more efficient. In addition, CD4+ will secrete IL-17, which enhances the innate immune response. D. Some probiotic strains, via induction of IFNγ and IL-17 production, can stimulate the overexpression of innate immunity-related genes such as the overexpression of TLR7, even in the lung. This overexpression of TLR7 amplifies the innate immune responses. E. Probiotics can help B lymphocytes differentiate and become plasma cells, which can secrete specific sIgA. In our case, some studies showed the impact of some probiotics in increasing sIgA in lung tissues. However, until now there is no explanation of the real mechanisms of how intestinal probiotics can help secretion of sIgA which are specific to elimination of RVs. This effect can be explained by the capacity of some probiotics to enhance cytokine production, which can improve the rapid differentiation of B lymphocytes to plasma cells in lung tissues.
1.2.1.1 Lactobacillus Probiotic Strains in Viral Respiratory Infections
Lactobacillus is the most studied genus of anti-RVs probiotics, followed by the Bifidobacterium genus. It was reported that Lactobacillus plantarum L-137 (L. plantarum L-137) isolated from fermented foods showed proinflammatory activity which can decrease the titer of influenza virus type A (IVA-H1N1) in mouse lungs [20]. Another strain, L. plantarum YU, isolated from Japanese fermented foods, showed anti-H1N1 activity by activating the Th1 immune response [22].
Recently, several studies have reported that L. plantarum species reduce the signs of influenza-like symptoms and even increase body weight and survival rate in a mouse model [21, 23, 24]. In addition, mice infected by a lethal pneumovirus survived when they were protected using a combination of two lactobacilli strains, L. plantarum NCIMB 8826 isolated from human saliva and L. reuteri F275 isolated from the human gastrointestinal tract (GIT) [25]. It has not been reported that L. plantarum probiotic strains were assessed in a human experimental model, which may be due to the undesirable acid or metabolites secreted by this species.
L. rhamnosus strains are the most important probiotics for human applications. Furthermore, the majority of L. rhamnosus strains are immunobiotics, due to their ability to stimulate and enhance the host immune system. In animal experiments, L. rhamnosus GG (LGG), a famous probiotic strain, was evaluated and showed an anti-influenza virus activity on intranasal and oral administration [26, 27].
In mice infected with respiratory syncytial virus (RSV), heat-killed L. rhamnosus CRL1505 and CRL1506 showed a good inhibitory effect and increased the body weight of mice [34]. These two strains were considered good immunobiotics in RSV infection due to their IFN-α stimulation, which decreases the viral load in mouse lungs [34]. The majority of clinical trials in children were performed using LGG [28, 29]. LGG reduces the number of upper and lower viral RTIs in children, reduces the days of absence from daycare and decreases antibiotic use [28]. The administration route of LGG in clinical trials in children was often by drinking probiotic-inoculated milk [28, 30–32].
The antiviral activity of LGG was also assessed in adults and the elderly. Several clinical trials were conducted in order to improve the beneficial effect of this strain in the treatment and prevention of viral infections. Kekkonen et al. studied 141 marathon runners (22–69 years old) who drank two bottles of milk daily. The probiotic group drank a milk inoculated with LGG (4.1010 CFUs). The results showed that the ingestion of LGG did not decrease RTI episodes or the severity of symptoms [33]. However, the combination of LGG with Bifidobacterium animalis ssp. Lactis Bb12 (B. animalis ssp. Lactis Bb12) decreased the duration and symptom severity of URTIs significantly [62].
Lactobacillus casei (L. casei) is a beneficial bacterium found naturally in both the mouth and intestines of humans. L. casei may be found in “raw or fermented dairy and fresh or fermented plant products” [63].
L. casei Shirota (LcS) is the major probiotic strain among this species. This strain has been isolated from mouth flora [64]. The intranasal administration of LcS in H1N1-infected mice showed a decrease in the viral titer in a nasal wash. Moreover, LcS increases the secretion of antiviral cytokines such as interferon alpha (IFN-α). Furthermore, LcS stimulates the innate immune response [35]. LcS has shown an immunomodulatory activity against RVs. However, in clinical trials, in particular in the elderly group, LcS has shown insignificant results in comparison with the placebo group [65, 66].
Another probiotic strain, L. casei DN-114,001, showed good antiviral activity in clinical trials. L. casei DN-114,001 was evaluated in children, adults, and the elderly in separate studies. In children clinical trials, L. casei DN-114,001 decreased the symptoms and duration of RTIs significantly [36, 37]. In the adult and elderly groups, the administration of L. casei DN-114,001 decreased the duration of RTIs and common infectious diseases (CIDs) [38–40, 67].
L. paracasei, in particular L. paracasei ssp. Paracasei (Lpp), was evaluated for its antimicrobial activity in animal models [24, 41]. After oral administration in mice, the Lpp 06Tca19 and Lpp 06Tca22 strains, isolated from fermented camel milk, showed a significant decrease in TNF-α in bronchoalveolar lavage fluid (BALF). This effect led to an increase in the mice’s survival and a decrease in the macrophage and neutrophil concentrations in BALF [41].
L. fermentum is a species which can be found in human and animal flora [68]. This species is usually used as a probiotic in humans. In RTIs, L. fermentum was evaluated in both human clinical trials, in particular in children and adults [45, 46], and in animal models in order to investigate the mechanism of viral inhibition [42, 43]. L. fermentum-1 and L. fermentum CJL-112 were assessed in H1N1-infected mice. The results have shown an important reduction in the viral load, with high stimulation of IgA and Il-12 secretion which allows an increase in the mice’s survival [42, 43].
L. fermentum CECT5716 was evaluated only in human clinical trials [44, 45]. Two hundred and fifteen healthy infants (6 months old) took 2.108 CFUs/daily with galactooligosaccharides as prebiotics. This trial showed a significant decrease in the incidence of URTIs and LRTIs in infants [44]. L. fermentum VRI003 and L. fermentum PCC are two probiotic strains which showed a significant decrease in the duration of RTI symptoms in healthy, physically active adults. However, these two strains did not reduce the incidence of RTIs [46, 47].
L. acidophilus is a famous probiotic strain used in pharmaceutical supplements [69]. A few studies evaluated the antiviral activity of L. acidophilus, because this species is usually used for gastrointestinal problems [47, 60]. All antiviral clinical trials used in humans were conducted using a combination formula with other probiotic strains, while one animal experiment was conducted using L. acidophilus L-92, isolated from a healthy Japanese volunteer, which showed an anti-IFV A (H1N1) activity by increasing active NK cells in lungs. Moreover, L. acidophilus L-92 showed an increase in IFN-α secretion [48].
L. salivarius resides in the mouth and small intestine. It mainly plays a role in protection against several kinds of pathogens [70]. Sixty-six endurance athletes participated in a clinical trial; 33 of them have taken 2.1010 CFUs of L. salivarius probiotic strains daily for 16 weeks, while the control group (n =33) took a placebo. The results showed a nonsignificant change in comparison with the placebo group [49]. These results can lead to the conclusion that the influence of probiotics in RTIs could be directly related to the species or to a new characteristic presented in a specific strain.
L. brevis KB-290 (isolated from a traditional Japanese pickle called “suguki”), L. gasseri TMC0356 (isolated from the intestine of healthy adults), L. pentosus S-PT84 (isolated from Kyoto pickles), and L. pentosus b240 (isolated from fermented tea leaves) are probiotic strains evaluated in mouse model experiments [27, 50–52]. These strains showed a strong anti-IFV activity, in particular against the H1N1 strain. The anti-H1N1 activity of L. brevis KB-290 was reported to increase IFN-α and IgA secretion in mouse lungs after oral administration of this strain [50]. The oral administration of L. gasseri TMC0356 in intranasally H1N1-infected mice showed a positive effect on influenza symptoms. L. gasseri TMC0356 can decrease the viral titers by interacting with the intestinal immunity system, in particular in Peyer’s patch, resulting in high production of IL-12, IL-6, IFN-c, and IgA [27]. Kiso et al. reported that the oral administration of L. pentosus b240 increased protection in mice against a lethal dose of H1N1. The primary mechanism of this effect was by upregulation of antiviral genes such as Egr1 (a critical regulator of host inflammatory chemokines) and Rsad2 (an interferon-stimulated gene (ISG)) [52]. Izumo et al. reported a new antiviral activity mechanism in a mouse experimental model infected by the H1N1 strain. They showed that the antiviral activity was created by activation of lung NK cells after intranasal administration of L. pentosus S-PT84 in BALB/c mice. Moreover, this strain can increase the production of IFN-α and IgA and decrease the allergic reaction by modulating the Th1/Th2 balance [51].
1.2.1.2 Bifidobacteria Probiotic Strains in Viral Respiratory Infections
Bifidobacterium is a very important bacterium in animal and human flora. This genus has beneficial effects for the host, and it was used for the first time as a commercial probiotic [71]. Bifidobacteria promote good digestion, boost the immune system, and inhibit almost all intestinal pathogens [72].
In viral RTIs, the bifidobacterial strains were used in combination in several human clinical trials to assess antiviral activity and investigate the mechanism of such activity [55, 60]. The combination was conducted using lactobacilli probiotic strains [28, 58, 60]. In a few studies, the antiviral mechanisms of bifidobacteria were investigated in animal models, in particular mouse experiments.
Bifidobacterium longum (B. longum) BB536, isolated from Japanese healthy infants, showed an anti-IFV A/PR/8/34 (H1N1) activity after oral administration for 2 weeks before infection. This antiviral activity was created by decreasing proinflammatory cytokines, such as IFNγ and IL-6, in BALB/c mice. Furthermore, this strain showed the capability to significantly decrease the symptom score and body weight loss [53]. In another BALB/c mouse experiment, Wu et al. reported that the administration of a Bifico® strains mixture, in particular B. longum, led to upregulation of the expression of several genes involved in antiviral responses, such as TLR7, MyD88, IRAK4, TRAF6, and NF-KB [54].
In a double-blind, placebo-controlled study, 109 healthy newborns aged 1 month participated; 55 candidates were subjected to 109 CFUs/day of B. animalis ssp. Lactis BB12 and the control group (n =54) received control tablets as a placebo. Taipale et al. reported that the B. animalis ssp. Lactis BB12 strain reduced the number of viral RTI episodes, while there was no effect on the occurrence of acute otitis [55].
Several studies investigated the combination of B. animalis ssp. Lactis BB12 with other probiotic strains to determine the possibility of the strongest antiviral activity.
The combination of L. reuteri ATCC DSM 1793 (isolated from Peruvian mother’s milk) with B. animalis ssp. Lactis BB12 was evaluated in a double-blind, placebo-controlled, randomized trial of 201 healthy infants aged between 4 and 10 months. This combination reduced the viral RTI symptoms, fever, and antibiotic consumption [59].
Rautava et al. showed that the combination of B. animalis ssp. Lactis BB12 and the LGG strain reduced antibiotic consumptions. Moreover, this combination reduced the incidence of acute otitis media in the first 7 months of life [58].
In another double-blind randomized controlled trial, a combination of B. bifidum with L. acidophilus probiotic strains (Infloran, Bern, Switzerland) was administered to 80 healthy children aged between 8 and 13 years old. Reduced viral RTI symptoms and a decrease in the school absence rate were the main outcomes of this combination [60].
B. animalis ssp. Lactis B1–04 reduced viral URTI episodes in a clinical trial of 460 physically active adults (18–60 years old) [56]. Lehtoranta et al. reported the efficacy of a combination of LGG, L. rhamnosus Lc705, B. breve 99, and Propionibacterium freudenreichii JS in nasopharynx bocavirus infection, in particular in otitis-prone patients [61]. This study was a randomized, double-blind, placebo-controlled trial on 269 otitis-prone children (aged 9 months to 5.6 years) with 6 months of probiotic intervention. The authors showed the specific antiviral activity of this combination against bocavirus but not picornavirus. Moreover, this combination seems to be effective in children, but not in the elderly [61].
Each of the abovementioned probiotic strains seems to have one or many specific antiviral mechanisms. Furthermore, the viral specificity is directly related to the strain used or the combination of several probiotic strains in the same or different genus types. Moreover, the antiviral effect of probiotics by immunomodulatory mechanisms depends on the immune system status, which can be explained in the study conducted by Lehtoranta et al., who showed that the combination of four probiotic strains worked very well in children but not in the elderly [61].
1.2.2 Conclusion and Perspectives
Lactobacillus and Bifidobacterium genera have the strongest antiviral activity against respiratory viruses, in particular against influenza virus type A. This antiviral activity depends on the strain’s specificity and the situation of the host immune system. Clinical trials (CTs) are the most used evaluation method in these studies. The alleviation of symptoms was measured in these CTs in order to investigate the impact of suggested probiotic strains against RVs. The main mechanism of such probiotics is immunomodulation. The use of probiotics in respiratory infections leads to the activation of many signals for innate immunity and the production of IgA antibodies in respiratory tissue. Anti-inflammatory probiotics in respiratory viral infections are not welcome, since they block the immune responses against the virus. However, in respiratory inflammation, probiotics that stimulate the production of anti-inflammatory (IL-10, TGFβ) cytokines play a crucial role in suppressing inflammation.
We recommend avoiding antibiotic treatment for respiratory infections except in the case of a confirmed bacterial infection. Although the antibiotic treatment may prevent respiratory bacterial superinfection, this antibiotic therapy eradicates the microbiota, in particular Gram-positive probiotic strains. Anti-RoV probiotics should be evaluated in depth in germ-free mice and/or antibiotic-treated mice in order to determine the complete mechanism of such probiotics. Furthermore, the molecules responsible for this immunomodulatory activity should be investigated and purified for in vivo experiments.
1.3 Part I-B Probiotics and Viral Gastroenteritis
1.3.1 Introduction
In 1907, Elie Metchnikoff observed the healthy effect of fermented dairy products in his population. Recently, researchers have confirmed the beneficial effects of fermented foods, in particular the role of lactic acid bacteria (LAB), such as Lactobacillus, Bifidobacterium and other LAB, which have probiotic properties [73]. Gram-positive Lactobacillus and Bifidobacterium bacteria are not occasional contaminants, but they are two genera that colonize the primary microbiota of humans.
The colonization, development, and maturation of the newborn’s gastrointestinal tract that begins immediately at birth and continues for 2 years is modulated by numerous factors, including mode of delivery, feeding regime, maternal diet/weight, probiotic and prebiotic use, and antibiotic exposure pre-, peri-, and postnatally [74]. This microbiota plays a major role in the host’s defense against pathogens [75]. This microbiota can maintain the health of the gut ecosystem and preserve defensive readiness against enteric pathogens and sometimes prevent enteric chronic diseases [76]. Microbial concentrations are distributed along the digestive tract, with 103 bacterial cells/mm3 in duodenum and stomach, 102 to 103 bacterial cells/mm3 in the fasting ileus and distal ileum, and 1010 to 1012 bacterial cells/mm3 in the colon [77].
Unfortunately, due to an incorrect feeding regime, humans are losing the primary microbiota which is related to an increase in diseases, including infectious diseases. To restore this primary microbiota, several health organizations suggested using probiotics as a dietary supplement.
1.3.1.1 The Importance of Microbiota
Recent studies have demonstrated the symbiotic relationship between intestinal microorganisms that benefits their human host. Intestinal microorganisms, referred to as intestinal microbiota, have several mechanisms of maintaining gut health. The intestinal microbiota degrades indigestible dietary substances, such as fiber, and converts it into an energy source for gut cells and the immune system [78, 79].
The other role of intestinal microbiota is to develop the gut immune system. Hooper et al. showed that germ-free infected mice have severely compromised immune responses and a reduction in the level of secretory immunoglobulin A (IgA) and the number of intestinal T cells compared with wild-type mice [80, 81]. In addition, intestinal microbiota can protect the host against pathogens by inducing intestinal epithelial cells to secrete antimicrobial proteins, such as angionin and C-type lectin RegIIIγ [82, 83].
1.3.1.2 Histo-Blood Group Antigens (HBGAs) and Gram-Negative Bacteria in Gut Microbiota
The ABO groups or “blood group antigens” in human red cells were discovered by Karl Landsteiner in 1900 [84]. Subsequently, this kind of antigens, called histo-blood group antigens (HBGAs), has been found in other tissues and biological fluids, such as gut and saliva [85]. Some hosts lack the function of the fut1 gene and are called “nonsecretory hosts” [86]. The biological role of HBGAs has not yet been completely defined. In infectious diseases, the presence of A and B HBGAs can inhibit the in vitro motility of carcinoma cells, and their absence is associated with an unfavorable prognosis [86]. Returning to infectious diseases, HBGAs play a crucial role in bacterial and viral pathogenesis. Specific strains of pathogens bind to carbohydrates of the HBG family. Several studies have shown that a large number of pathogens bind HBG as the first step of pathogenesis, such as uropathogenic strain of E. coli R45, S. pneumoniae, S. aureus, Salmonella typhimirium, and Campylobacter jejuni [87–90].
The HBGAs may not only provide an attachment receptor to pathogens, since they may be present on the pathogens themselves. Gram-negative bacteria can present this type of antigen (in some strains may can be on the LPS molecules) [91]; humoral immune responses can thus be generated. For example, E. coli 086 presents B HBGA; thus, an anti-B response will be evoked in A and O HBGA individuals. Thus, B group individuals were more susceptible to infection caused by E. coli 086 [92]. In this chapter, the role of HBGA in viral infection will be discussed.
1.3.1.3 Viral Gastroenteritis and the Role of Gram-Negative Bacteria of the Gut Microbiota
The gut microbiota contains a large number of microbes, forming the biggest ecosystem in humans and animals [73]. Gastrointestinal infections have a great impact on public health both in developing and developed countries [93]. Viruses are the most frequent causative agent involved in gastroenteritis, in particular in infants and children [94]. The sources of enteric viruses (EnVs) are usually contaminated food and water through ingestion by the orofecal route [95].
After the occurrence of infection, treatment of the symptoms is the only way to prevent infection complications. In addition to antipyretic drugs, rehydration therapy is the most common treatment for viral gastroenteritis. The absence of specific antiviral agents against EnVs requires scientists to find an alternative which can prevent or help in the treatment of such infections [5].
The definition of EnVs is viruses that are able to replicate in the intestinal epithelium, even if only several types are the causing of gastroenteritis [96]. Noroviruses (NoVs), rotaviruses (RoVs), arboviruses (AVs), enteric adenoviruses (AdVs), and enteroviruses (EVs) are the viruses most frequently responsible for gastrointestinal infections worldwide [97]. Reoviruses are one of the many EnVs that replicate in the intestinal tract, but they are generally asymptomatic. Another type of enteric virus, such as poliovirus, can cause severe disease after dissemination to peripheral tissues [97]. Certain retroviruses, including mouse mammary tumor virus (MMTV), can be transmitted orally from an infected mother through her milk, after which they infect the gastrointestinal tract [98].
1.3.1.3.1 Does “Gut Microbiota” Enhance or Inhibit Viral Gastroenteritis?
Upon EnVs ingestion, viruses will “communicate” with gut microbiota resident in the intestinal lumen, which vary from one host to another. The result of this interaction seems to be dependent on the composition of the microbiota. In some hosts, good microbiota (a high number of commensal bacteria) is an inconvenience; while in other hosts, a complex microbiota (containing a large variety of microbial genera and species) is beneficial in defending against EnVs and preventing viral gastroenteritis. This hypothesis is supported by several studies that have demonstrated that the intestinal microbiota is important and plays various roles in reducing infection by enteric viruses [97].
The role of commensal bacteria in the persistence of enteric viral infections has previously been shown in a series of recent studies published in 2011, using poliovirus, reovirus and mouse mammary tumor virus (MMTV) as EnV models [99–101].
The replication of poliovirus in the intestine was reduced in antibiotic-treated mice, while the reconstitution of the intestinal microbiota restored poliovirus infection [100]. Moreover, Kuss et al. showed that intraperitoneal infection with poliovirus was independent of the presence or absence of intestinal microbiota, which highlights the important role of the microbiota in reducing poliovirus infection [100]. In a recent study [101], showed that the use of broad-spectrum antibiotics in a mouse model infected with rotavirus decreased the infectivity of the latter. They reported that viral antigens were reduced in feces, and there was delayed shedding of viruses compared with the control mice group [101].
In another study, murine Norovirus (MuNoV) was used to investigate the importance of intestinal microbiota in viral infectivity [102–104]. Jones et al. showed that depletion of intestinal microbiota reduced the replication of MuNoV in the distal ileum, mesenteric lymph nodes, and colon compared with the control group [103]. The MuNoV was reduced in feces shedding in antibiotic-treated mice compared with colonized mice as shown by Kernbauer et al. [104]. A recent study conducted by Baldridge et al. showed that the use of a broad-spectrum antibiotic in mice did not help MuNoV to establish persistent infection, while the transplantation of intestinal microbiota from another healthy mouse rescued the infectivity of this virus. Moreover, they reported that systemic infection with the MuNoV was independent of the presence or absence of gut microbiota as shown by poliovirus infection [102].
1.3.1.3.2 Direct and Indirect Mechanisms of Intestinal Microbiota, Which Enhance EnV Infection
1.3.1.3.2.1 What Is the Direct Mechanism of the Gut Microbiota in Viral Infectivity?
Virion stabilization and promotion of virus attachment are the two direct mechanisms by which the intestinal microbiota enhance EnV infections [100, 105]. These findings were investigated using an in vivo poliovirus model (in mice) and an in vitro model (cell culture testing). Kuss et al. studied the stability of the poliovirus virion in the presence or absence of intestinal microbiota. They found that the isolation of poliovirus (before progeny virion production) from colonized mice was more viable and resistant to high temperatures and became bleach resistant. These results were also seen when the poliovirus was incubated with dead Gram-negative bacteria [100].
Robinson et al. conducted an in-depth study of the mechanisms of intestinal microbiota. They found that surface compounds of Gram-negative bacteria played a crucial role in poliovirus stability. The authors showed that the bacterial LPS bound the viral protein 1 (VP1) at threonine 99. The LPS-bound virus increases the thermostability and chlorine resistance as well as decreasing the viral genome release [105]. Moreover, the pretreatment of poliovirus particles with Gram-negative bacteria/or LPS molecules promotes poliovirus attachment to the host cells [105]. The poliovirus receptor (PVR) seems to be an important element in the abovementioned host cell attachment. The authors showed that poliovirus cannot attach to the permissive cells which were pretreated with anti-PVR antibodies. This result was independent of the presence of LPS binding to poliovirus. Moreover, non-PVR-expressing cells also showed the same results [105]. Using another viral model and after a long history of in vitro culture difficulties, B cells seem to be permissive cells for MuNoV [103].
Tan and Jiang showed that human and MuNoV required commensal bacteria to infect human B cells. These findings were supported by the reduction of norovirus (NoV) infectivity when infected stool was filtered with a 0.22 μm filter. The infectivity was rescued when live/dead commensal bacteria were added to the stool [106]. The authors showed that LPS molecules were not the bacterial compound that played the cofactor role in norovirus infection. Norovirus and B cells were incubated with LPS, and the results showed that the LPS did not initiate the norovirus infection. Moreover, the authors showed that the histo-blood group antigen (HBGA) glycan was a cofactor of the norovirus infection [106]. A recent study showed that a variety of commensal bacteria express these glycans and can bind norovirus in a virus-strain-specific manner [107]. The majority of Gram-negative bacteria express this kind of glycan, as shown in several recent studies [103, 108].
1.3.1.3.3 Indirect Mechanisms
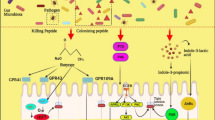
As shown in Fig. 1.3, the gut microbiota can inhibit and/or reduce the antiviral immune response via an indirect mechanism. The gut microbiota can sometimes create a tolerogenic microenvironment that helps the virus infect cells and suppress antiviral antibody production, and sometimes it can block virus-induced IFN signaling [97].
Suggested mechanisms of antiviral probiotics against enteric viruses. Since probiotics and EnVs have the same route of entry, probiotics can interact with viral particles in several ways. The advantage here is the capacity of probiotics to colonize the gut ecosystem, which is the target of EnVs: A. Some probiotic strains can colonize the gut ecosystem and then form a carbohydrate biofilm which probably saturates host IEC receptors as well as viral receptors. B. Probiotics protect the host IECs against damage and lesions. Several studies have confirmed that some probiotic strains play a crucial role in tissue restoration, especially by inducing mucin secretion by IECs and strengthening cell tight junctions. C. The immunomodulatory effect is the principal mechanism of antiviral probiotics (AvPr). These probiotics can stimulate the secretion of proinflammatory cytokines, especially from DCs such as IL-6, Il-12, and IFNγ. In addition, AvPr can boost innate immune cells, such as macrophages and NK cells. The latter also produce IFN-α, which is an antiviral cytokine. D. AvPr help the immune system to react with more rapid specific responses. The Th2 response is essential for B lymphocytes to be able to differentiate into plasma cells with specific sIgA secretion. E. AvPr can inhibit or decrease viral infectivity and spreading by superproduction of mucin and by changing the morphology of villi, which can skew viral attachment. F. TLR3 is the PPR of viral MAMPs, especially for RNA viruses. Hence, the overexpression of TLR3 induced by some AvPr can amplify the innate immune response by catching a large number of viral particles. G. Some AvPr interact physically with viral particles. Indeed, several studies have showed that some probiotic strains can bind or trap viral particles on their cell wall. Moreover, these trapped viruses lose some pathogenic characteristics and consequently lose cell infectivity. H. Finally, AvPr can play an indirect role in preventing and/or decreasing viral infection, especially against enteric viruses, by excluding the colonization of Gram-negative bacteria (See Fig. 1.3).
-
(i)
Tolerogenic microenvironment
The gut microbiota, in particular Gram-negative bacteria, induces a tolerogenic microenvironment that allows persistent enteric virus infection [109, 110]. Briefly, the capacity of enteric viruses to bind Gram-negative bacteria by LPS-VP (viral protein) binding can skew the immune response. The story begins when the enteric virus binds the microbiota LPS. The LPS will be recognized by the TLR4, which induces the production of IL-6. The B cells have IL-6R; when the IL-6 binds to the IL-6R of the B cells, the B cells produce IL-10, which is an anti-inflammatory cytokine. This action blocks the antiviral immunity response and leads to viral persistence. This information is supported by several studies using an MMTV and norovirus model in solenocyte and B-cell culturing, respectively [99, 111, 112]. In another study, the norovirus infection occurred in germ-free mice which were also deficient in production of IL-10. This study supports and confirms that the production of IL-10 by the presence of gut microbiota, in particular Gram-negative bacteria, was the essential key to viral persistence through the creation of a tolerogenic microenvironment [113, 114].
-
(ii)
Viral antibody production
[101] in the case of rotavirus infection, showed that the fecal and serum IgA titer was higher in germ-free mice compared with the control mice group. This data suggests that gut microbiota suppress the antiviral humoral response. In contrast to the rotavirus case, the MuNoV infection of antibiotic-treated mice reduced the serum IgG titer after 35 days of infection compared with the colonized mice group [101]. These findings will be investigated in depth to identify exactly which bacterial compound is responsible for this mechanism and to determine if this interaction occurred in a virus-strain-specific manner.
-
(iii)
Blocking of the IFN signaling
Several studies have reported that the IFNλ, which is considered to be in type III of IFNs, activates the same intracellular signaling pathway and many of the same biological activities as other IFN types, including antiviral activity, in a wide variety of target cells [115]. Baldridge et al. reported the importance of IFN type I, II, and III responses in reducing MuNoV infection as well as viral persistence. TLR4 is required for bacterial regulation of viral persistence. Therefore, the presence of LPS/Gram-negative bacteria were dispensable in this regulation [102].
Moreover, a recent study showed that the type III IFN response was essential in reducing MuNoV infectivity in the colon [116]. Briefly, EnVs are recognized by a variety of TLRs, such as TLR3, TLR9, etc. These TLRs stimulate the IFN production by the B cells or other secretory cells. The IFNs, in particular type III IFNγ, bind to the IFN receptor present on the enterocytes, which can reduce the viral persistence. Several studies have reported that commensal bacteria, in particular Gram-negative bacteria recognized by TLR4, bind to the EnVs and then the immune system will be skewed. Thus, the TLR4s inhibit the production of IFNγ, allowing viral persistence. This data was confirmed using MuNoVs as an EnV model [102, 116–118]. Pott et al. reported that IFNγ also controls rotavirus infection in mice; thus, it will be interesting to determine whether this response is similarly regulated by the interactions between the enteric virus and commensal bacteria [119].
1.3.1.4 Role of Probiotics in Gut Microbiota
In addition to the immunomodulatory effect of probiotics, these beneficial bacteria have several mechanisms to defend gut pathogens and infections.
In the previous part, the studies have shown that the composition of the intestinal microbiota can help EnVs to persist and sometimes amplifies their infectivity. Remarkably, the presence of Gram-negative bacteria in the microbiota is essential to save the infectivity of EnVs. Therefore, changing the intestinal microbiota composition seems to be effective in preventing or inhibiting enteric viral infection. Otherwise, a high percentage of Gram-positive bacteria may be a solution in viral gastroenteritis treatment and/or prevention. From this hypothesis, the importance of Gram-positive bacteria, in particular lactic acid bacteria (LABs) – which is considered to be GRAS – in preventing and even treating this type of infection will be discussed in this part.
The implantation of probiotics in the digestive tube is clearly beneficial, since they have the ability to form a biofilm on the enterocytes and prevent the adhesion and proliferation of other bacteria such as Gram-negatives [120]. Moreover, as shown in Fig. 1.3, probiotics can exclude commensal and pathogenic bacteria by several mechanisms such as the immunomodulatory effect (immunobiotic action), reduction of pH, production of antimicrobial compounds (hydrogen peroxide, lactic acid, NRPS, bacteriocins, etc.), trophic competition, and biofilm formation (receptor competing) [121].
1.3.1.4.1 Anti-enteric Viruses Probiotics
Anti-EnV probiotics (AEnPs) are divided into two categories according to the direct or indirect antiviral mechanisms. In this section, direct mechanisms will be discussed in detail.
The probiotics with antiviral effects, called further antiviral/anti-EnV probiotics, can inhibit viral infections with several direct mechanisms. The antiviral compound secreted by these probiotics will be discussed in Chap. 4. In this section, the immunomodulation and physical interaction will be presented and discussed (Table 1.2).
1.3.1.4.2 Indirect Mechanism of Anti-EnV Probiotics
The microbiota diversity and composition are directly related to the incidence of gastroenteritis, including viral infection [163]. For example, the presence of bifidobacteria genera in the first months in the gut microbiota of infants prevents the majority of intestinal infections [164]. Therefore, almost all intestinal probiotics can play a crucial role in preventing or treating viral gastroenteritis by indirect mechanisms. These probiotics reduce the “viral infection cofactor,” which is the LPS and HBGA molecules present in Gram-negative bacteria and some commensal bacteria, respectively [108]. Otherwise, orally administered probiotics can change the composition of the gut microbiota by increasing the number of probiotic cells and decreasing commensal and Gram-negative bacteria.
1.3.1.4.3 Direct Mechanism of Anti-EnV Probiotics
The meaning of direct mechanism is when the EnVs interact directly with probiotic cells and/or their metabolic compounds. As shown in Fig. 1.4, probiotics can interact and inhibit EnVs by several mechanisms. Indeed, it is depending to the specificity probiotic strain and viral type. Before talking about the direct mechanism or direct interaction of these probiotics, the viral infection steps should be presented.
Exclusion of commensal bacteria by probiotics in the gut ecosystem. AvPr can play an indirect role in preventing and/or decreasing viral infection, especially against enteric viruses, by excluding the colonization of Gram-negative bacteria (see Fig. 1.3) in the gut ecosystem, which were considered a cofactor in some enteric virus infections. Thus, proinflammatory probiotics (which induce a proinflammatory response) are welcome in viral gastroenteritis because they can trigger proinflammatory immunity to eliminate EnVs. Probiotic strains capable of binding host cells very well and then creating a microenvironment which prevents many kinds of commensal and pathogenic bacteria from proliferating, including Gram-negative bacteria. Probiotics have a stronger capacity to adhere to host cells than Gram-negative bacteria (probably because of the high hydrophobicity of their cell walls), which can decrease the number of Gram-negative bacteria. Probiotics can act in different ways: A. Biofilm formation: This biofilm can protect host cells against other commensal bacteria, because this biofilm covers the majority of host cell receptors. B. By the immunomodulatory effect, probiotics can stimulate the innate immune response, especially of phagocytes. C. At the same time, probiotics induce the secretion of antimicrobial peptides (AMPs) such as β-defensins and cathelicidins which target commensal bacteria. However, there is no explanation of the resistance of some probiotic strains against these AMPs. D. Overproduction of mucin can also prevent commensal bacteria adhesion. E. The co-aggregation capacity of probiotic strains leads to the trapping of other microbes, as well as commensal or Gram-negative bacteria. F. Probiotics can secrete several enzymes to compete with other commensal bacteria for nutrients present in the gut ecosystem. In addition, the majority of probiotic strains possess arginine dehydrogenase, which is important in this mechanism. G. Probiotic strains can secrete a variety of antimicrobial substances, such as hydrogen peroxide, lactic acid, non-ribosomal peptide synthetase (NRPS), bacteriocins, and bacteriocin-like inhibitory substances (BLIS).
In general, EnVs can infect target cells by five steps called the viral replication cycle. The viral replication cycle starts by viral attachment to host cells (1), followed by penetration and uncoating (2), viroplasm formation (3), and finishing with virus particle maturation (4) and release (5) [165]. Each EnV has its own specificity in infection mechanisms and/or the replication cycle. For this reason, the following information will discuss the direct mechanism of probiotics regarding the type of EnV.
1.3.1.5 Probiotic Strains Against Rotavirus (RoV) Infections
RoVs are the major cause of diarrhea and acute gastroenteritis in infants and young children [165]. RoVs are naked viruses containing dsRNA. The RoV virion or particle consists of three protein layers called a triple-layered particle (TLP) [166]. The viral protein (VP) and nonstructural protein (NSP) are the two main viral proteins found in RoVs. For TLP, the main protein forming the external layer is VP7, with VP4 which forms the viral spike. VP6 forms the second layer of the RoV particle. Thus, the VP6 layer constitutes the double-layered particle (DLP) of the RoV. Kam et al. (2014) showed that, in actively transcribing DLP, the middle VP6 layer order decreased, while the number of cores increased. Thus, the transcribed mRNAs released from these cores translated later to the viral protein (VP and NSP) in host cells [167].
The RoV replication cycle starts with the attachment to the host cells mediated by VP4 and VP7 molecules which play a role in the penetration and uncoating of RoV. The third step consists of the synthesis of ssRNA (mRNA), which is mediated by VP1, VP3, and VP2 molecules. Viroplasm formation (viral protein (NSP2, NSP5) and viral RNA interact with each other to form cytoplasmic inclusion bodies), RNA packaging, minus ssRNA synthesis (RNA replication), and DLP formation constitutes the fourth step. Finally, RoV will be released from host cells after maturation of virus particles (from DLPs to TLPs) [165, 168].
Several studies have shown the effectiveness of probiotics in the treatment and prevention of acute diarrhea including RoV infections. Human, murine, and porcine rotaviruses were used in these studies. The majority of investigations were based on the symptoms, such as duration of diarrhea, duration of hospitalization, virus shedding in feces, and sometimes immunomodulation. A few studies conducted an in-depth investigation of the mechanism of action of some probiotics, in particular the interaction between virus-probiotic-host cells.
1.3.1.5.1 Clinical Trials (CTs)
Lactobacillus and Bifidobacterium strains were the most studied genera in rotavirus infections. Lactobacillus rhamnosus GG (LGG) is the best studied probiotic which showed a significant reduction of diarrhea duration and rotavirus infectivity [122, 123]. Effects of various probiotic strains on rotaviruses have been conducted using double-blind placebo-controlled randomized trials since 1991 [124, 125, 134]. Guandalini et al. showed that LGG administration reduced the diarrhea duration in neonatal patients with rotavirus infection [124].
In 49 children, the administration of 1010–1011 CFUs/ml of LGG twice daily for 5 days reduced the duration of acute diarrhea from 2.7 to 1.8 days, accompanied by an increase of IgA-specific responses [126]. In other RCTs, LGG reduced the duration of diarrhea caused by rotavirus gastroenteritis and improved the health recovery of infected children [127–132].
The L. reuteri SD 2222 strain was administered in patients aged 6–36 months with watery diarrhea caused by rotavirus. This strain showed a strongly reduction of diarrhea duration up to 5 days [134]. Saavedra et al., Shornikova et al., and Sugita and Togawa showed the anti-rotaviral activity in clinical trials of the following probiotic strains: Streptococcus thermophilus (S. thermophiles), L. reuteri DSM 12246, and L. acidophilus La5 [135–137]. Another study showed that LGG strains and L. casei Shirota (LcS) have an antiviral activity against rotaviruses and transmissible gastroenteritis virus (TGEV). The LGG strain showed the strongest activity, because of their strongest attachment capability to different cell lines. In addition to the attachment effect, the induction of reactive oxygen species (ROS) release seems to play a role in such activity [138]. Teran et al. conducted a randomized single-blind controlled trial (RSBCT) in 75 Bolivian children aged from 28 days to 24 months. A 1-gram mix of probiotic strains was administered to the probiotic group (n =25) for 5 days. The mix contained the following strains: L. acidophilus, L. rhamnosus, B. longum, and Saccharomyces boulardii (S. boulardii). The second group (n =25) was given nitazoxanide (an antiparasitic agent) at the dose of 15 mg/kg. The third group (n =25) was subjected to the normal protocol of rehydration. The results showed that the duration of diarrhea was reduced to 48 h compared with 54 h and 79 h for the nitazoxanide and rehydration groups, respectively [162]. Moreover, a study conducted by Grandy et al. in RDBPC trial showed the effectiveness of probiotic strains against rotavirus infections using S. boulardii alone and S. boulardii with a mixture of probiotic strains. The results showed that the two probiotic preparations reduced the infection symptoms (p = 0.0042) [122]. L. reuteri, called Probio-16, showed antiviral activity against porcine rotavirus; this activity was poorly demonstrated [139].
L. reuteri DSM 17938 was evaluated in RCTs on 74 children with rotavirus infection, the results showed a decrease in the number of patients with acute diarrhea [140]. Simakachorn et al. conducted an RCT on 73 children with rotavirus infection. The children were administered six sachets containing 109 of heat-treated L. acidophilus LB cells and 160 mg of twofold concentrated neutralized CFCS. The duration of the diarrhea decreased from 74 to 42.9 h [141].
Recent studies have started in-depth investigations of the interaction between rotaviral particles and probiotic strains. The cell culture was used to demonstrate what is happening between the rotaviral particles and probiotic strains. Various cell lines were evaluated; pig and human epithelial cells were used in some studies. Maragkoudakis et al. showed that LGG and LcS presence decreased the ROS release which can reduce cell damage [138]. Liu et al. studied the mechanism of antiviral activity of LGG in a new cell line called the porcine small intestinal epithelial cell line (IPEC-J2) as a model to study the impact of LGG on innate immunity during a rotavirus infection. They demonstrated that LGG presence reduced the rotavirus-induced IL-6 response [133].
1.3.1.5.2 Animal Models (AMs)
The animal model was established for several reasons. First, the animal model allows us to conduct an in-depth investigation of the mechanism of action of probiotic strains before and after viral infection. Moreover, the animal model (in vivo model) facilitates monitoring of the probiotic’s effect during the animal’s life cycle. The probiotics with antiviral activity were evaluated in vivo using a mouse model in most studies. Hagbom et al. confirmed that the neonatal mice and rats provide a reliable animal model for studying the rotavirus infection and also immune responses during this infection [142]. In a murine infected model, LGG has decreased both the barrier permeability in murine intestine and epithelium vacuolation in the jejunum. Furthermore, LGG was able to reduce the duration of acute diarrhea, and finally LGG was able to stimulate the secretion of IgA [143, 144]. L. casei DN-114,001 was administered in germ-free suckling rats infected further by rotavirus. The results showed that L. casei DN-114,001 changed the morphology of the intestinal villi and decreased intestinal cell lesions [145]. L. reuteri DSM 17938 was also evaluated in normal mice infected by rotavirus. The results showed that L. reuteri DSM 17938 has decreased the intestinal cell lesions and consequently reduced the duration of acute diarrhea [146].
Recently, Mao et al. studied the effect of LGG on the intestinal physiology, morphology and primary immune-specific responses of weaned piglets infected by the porcine rotavirus. This study showed that LGG administration in the weaned piglets group enhances specific immune responses by increasing rotavirus-specific IgA secretion. In addition, LGG decreased the NSP4 (rotavirus enterotoxin) – considered an intracellular receptor essential for DLP particles to interact with viroplasms and modulate intracellular Ca2+ and RNA replication [165] – in the jejunal mucosa induced by rotavirus infection [147]. The production of mucin 1 and mucin 2 and morphological improvement of the jejunal mucosa were evaluated in the presence of LGG. The results showed that LGG enhanced the production of mucin and recuperated the integrity of both the villus and the tight junction by stimulation of occlusion and other gene expression assisting the morphological jejunal defense against rotavirus [147].
E. coli Nissle (EcN) – Gram-negative probiotic strain – was evaluated alone or in combination with LGG in neonatal gnotobiotic piglets. The viral shedding titer was lower using EcN in comparison with LGG, LGG+EcN, and without probiotic strains. This result was correlated with the reduction of the specific IgA responses in the small intestine in EcN colonized piglets. The in vitro investigation using mononuclear cell culture, EcN, showed stimulation effects on the production of anti-inflammatory cytokines such as IL-6 and IL-10 [148]. These findings support the hypothesis conducted by Stephanie Karst in 2016 which showed that Gram-negative bacteria improve viral infection by various direct and indirect mechanisms [97].
Yang et al. evaluated the impact of dietary rice bran (RB) on the human rotavirus vaccine (HRoV) in vaccinated gnotobiotic pigs. They found that the RB-supplemented diet enhanced the vaccination responses in gnotobiotic pigs. In addition, the levels of IFNγ production from CD4+ and CD8+ were increased in intestinal and systemic lymphoid tissues [169]. In 2015, the authors showed that RB plays a role as a prebiotic for some probiotic strains. The LGG+EcN colonized gnotobiotic pigs were supplemented with RB daily, followed by human rotavirus (HuRoV) orally challenged. The RB showed a prebiotic effect promoting the growth of LGG and EcN in the gut. Moreover, RB-fed pigs had a lower mitotic index and villus width. The RB and/or probiotic strains increased immunomodulation by enhancing the secretion of IFNγ and HuRoV-Ab [150].
L. ruminis species have shown antiviral activity for the first time against the human rotavirus Wa strain. L. ruminis SPM0211 showed an anti-HuRoV activity which was explained by an immunomodulatory effect enhancing the Type I IFNs immune response [149].
To finish the last investigation of the antiviral mechanism of LGG, a new experimental model was developed in order to understand the beneficial interaction between pathogens and probiotics. An ex vivo experiment called intestinal organoid (derived from Lgr5+ stem cells) was conducted by Aoki-Yoshida et al. [151]. The LGG strains showed an increase in TLR3 gene expression – TLR3 is the essential key in innate immune responses following the recognition of rotavirus – in murine intestine both in in vivo and ex vivo experiments, without alteration of other TLR gene expressions. Moreover, LGG increased the mRNA levels of interferon-α (IFN-α) and a neutrophil chemokine (CXCL1). Furthermore, other probiotic strains, B. bifidum and L. paracasei, failed to increase the TLR3 mRNA levels ex vivo [151]. These findings confirm the hypothesis about the specificity of probiotic strains against viruses. Thus the antiviral activity occurred in a “virus-strain-specific manner.”
Bifidobacterial probiotic strains were also evaluated against RoVs using in vitro and in vivo experiments. B. longum SPM1205 and SPM1206 showed antiviral activity against the HuRoV Wa strain in an infected neonatal mouse model and Caco-2 cells. The two bifidobacterial strains showed an immunomodulatory effect on the type I IFNs immune responses [152]. A complete genome sequence of B. longum subsp. Infantis CECT 7210 was conducted in 2015 by [170]. This strain had previously showed, in a study conducted by Muñoz et al. [153], a direct effect on rotavirus strains in both in vitro (MA-104 and HT-29 cell culture) and in vivo (McN mouse model) experiments. The immunomodulatory mechanism was the main effect of this strain [153]. After complete sequencing of the B. longum subsp. Infantis CECT 7210 strain, they reported that there were 360 more elements (genes) in this strain compared with the complete genome sequence of B. longum 157F [170]. Thus, more in-depth research must be conducted on this strain to identify the detailed mechanism of antiviral activity, and more specifically the anti-HRoV activity.
1.3.1.6 Probiotic Strains Against Norovirus Infections
Noroviruses (NoVs) are naked RNA viruses belonging to the calicivirus family. NoVs are transmitted via the fecal–oral route and cause gastrointestinal disease with vomiting and acute diarrhea lasting 24–48 h [57]. NoVs cause 267 million infections each year and over 200,000 deaths, mostly in infants and the elderly [171, 172]. NoVs need host receptors to start the infection cycle. Debbink et al. reported that HBGA (See sec. I-B2) is a diverse family of carbohydrates expressed in mucosal surfaces, which are the main receptors of NoVs, in particular for the GII.4 genotype considered to cause the majority of human NoV infection because they can bind to A, B, and O secretors which are the majority (80 %) of the population [57]. The expression of HBGAs depend on the fut2 gene which codes for an enzyme called fucosyltransferase. The GI.1 genotype (Norwalk virus) cannot infect patients with a nonfunctional fut2 gene (called a “nonsecretory host”). However, some NoV strains are capable of binding other receptors such as Lewis carbohydrates [173, 174].
The immune responses are very important to blockade NoVs infection and viral spreading. The IgA genogroup-specific secretion is the main humoral immune response against NoVs [175]. The CD4+Th1 response is essential in the cellular immune response against NoVs which increases IFNγ and IL-2 production [176].
The development of antiviral treatments and vaccines to fight NoV infection has been hindered because of their extreme genetic diversity. Recently, the uncultivable nature of NoVs has been resolved by using a B-cell model. Thereby, the pathogenesis and replication cycle have been understood deeply in cell cultures and animal models [177]. The prevention strategies seem to be most effective mainly in infants and the elderly. To prevent and treat HuNoVs, several researchers have worked on the role of probiotics in such infection. The probiotic effectiveness in NoV infections was evaluated using both in vitro and in vivo experiments and clinical trials.
LcS introduced in fermented milk alleviated fever in NoV-infected elderly patients. The probiotic group (n =39) showed fast recuperation compared with the control group. Moreover, the acetic acid concentration in feces has increased, and thereby Bifidobacterium and Lactobacillus genera became dominant [154]. Takeda et al. reported that the administration of LcS improves the natural killer (NK) cell activity by producing the IL-12 by macrophages in response to LcS [155].
Lactococcus lactis ssp. Lactis LM0230 (L. lactis ssp. Lactis LM0230) – probiotic strains – were evaluated for antiviral activity against feline calicivirus (FCV), a HuNoV surrogate. This strain, “bacterial cell suspension (BCS)” and its metabolites “bacterial growth medium cell-free filtrate (BGMF)” were added to Crandell-Reese feline kidney (CRFK) cells line. The results showed that CRFK pretreated by BCS and BGMF caused nonsignificant decreases in the FCV titer. The pretreatment of FCV by BCS resulted in a decreased FCV titer after 24 h. The co-incubation of FCV and BCS in CRFK cells showed 100 % virus titer reduction (7.5 log TCID50/0.1 ml) [156]. The effect of BGMF will be discussed in Chap. 4.
In order to investigate the physical interaction between probiotic cells and NoV particles, Rubio-del-Campo et al. used a p-particles model designed from the C-terminal protruding P-domain of the NoV VP1 capsid protein. The p-particles exhibit the same surface conformation of viruslike particles (VLPs), and therefore these p-particles can bind to the HBGAs. In this study, 11 probiotic strains were tested: E. coli Nissle 1917 L. lactis MG1363, L. acidophilus LA-5, L. bulgaricus ATCC11842T, L. plantarum 299v, L. plantarum 299v Adh- (an isogenic derivative of 299v strain with decreased adhesion capacities), L. casei 431 ATCC55544, L. casei BL23 CECT5275, L. casei VSL#3,
LGG ATCC53103, and L. rhamnosus HN001. The Norwalk virus (GI.1) and GII.4 (HuNoV) were used in these experiments. The results showed that the probiotic strains possessed the capacity to bind to both GI.1 and GII.4 p-particles. Furthermore, L. rhamnosus, L. casei BL23 CECT5275, L. casei VSL#3 showed the highest binding effect of both p-particles. As unexpected results, the E. coli Nissle 1917 – Gram-negative probiotic – showed the poorest binding capacity to GI.1 and GII.4, although other studies showed that Gram-negative bacteria can bind enteroviruses via LPS molecules or HBGAs [97, 107]. In contrast, in HT-29 culture cells, E. coli Nissle 1917 was more efficient in NoV p-particles blocking, resulting in low host cell binding, while the other probiotic strains showed a low inhibition effect. The low adhesion capacity of probiotic strains to host cells did not affect p-particles binding; this suggestion was confirmed by the L. plantarum 299v adh- (probiotic strain with low attachment capacity) which showed high GI.1 p-particles binding compared with L. plantarum 99v (normal attachment capacity). In order to investigate the interaction between probiotic strains and NoV p-particles in more depth, an exclusion assay (HT-29 cells incubated with bacteria followed by P-particles challenge) and displacement test (HT-29 cells incubated with p-particles followed by bacterial challenge) were performed. The results showed that the probiotic strains enhanced the NoV p-particle attachment of monolayer surfaces. These results are not clear, since they disagree with other studies. The probable hypothesis is that the attached probiotic strains can bind to the NoV p-particles on their peptidoglycans (teichoic acid), which can lead to higher p-particle retention on the HT-29 surfaces [157].
A recent study has evaluated an engineered probiotic strain of L. paracasei which can produce the 3D8 scFv protein (an antiviral protein that can penetrate into host cells and hydrolyze nucleic acid molecules) against MuNoV. The results showed that L. paracasei 3D8 scFv retained its cell-penetrating effect, and therefore the intracellular nucleic acids have been hydrolyzed. The pretreatment of RAW264.7 cells with this engineered probiotic strain prevented the cell apoptosis caused by MuNoV infection. Moreover, L. paracasei 3D8 scFv has decreased mRNA expression of the viral capsid protein (VP1) [158].
Recently, B. adolescentis showed an antiviral activity against MuNoV as a HuNoV surrogate. The results showed that the inhibition did not occur in the viral binding step. Using VLPs as model, B. adolescentis decreased the attachment of HuNoV GI.1 VLPs to both Caco-2 and HT-29 cells, while no effect was shown in the presence of GII.4 VLPs [159].
1.3.1.7 Probiotics and Other Enteric Viruses
Astroviruses are nonenveloped viruses with positive-sense ssRNA. The Astroviridae family consists of two genera, Mamastrovirus (MAstV) and Avastrovirus (AAstV), based on mammalian and avian species, respectively [178]. Astroviruses can infect a wide variety of mammalian species, such as cats [179], dogs [180], mice [181], sheep [182], and cattle [183]. These mammals are always in direct contact with humans. HAstVs are one of the most important causes of acute gastroenteritis in newborn and infant patients [184]. Cross-species transmission is frequent, in particular in poultry as avian species [185] and between pigs, cats, and humans as mammalian species [186]. Thus, the zoonotic potential of these viruses is high, and future nonhuman-to-human transmissions are likely to occur [178]. Some authors have speculated that probiotics, which may interfere with the biological cycle of enteric viruses at many different stages, may be useful as a measure to prevent and/or treat intestinal viral infections [187, 188].
E. faecium NCIMB 10415 is the first probiotic strain authorized by the European Union (EU) as a probiotic feed additive for animals, including piglets. E. faecium NCIMB 10415 has shown an immunomodulatory effect in several studies [189]. Transmissible gastroenteritis virus (TGEV), an enteropathogenic coronavirus, causes 100 % mortality in newborn piglets after severe gastroenteritis. TGEV can also infect respiratory tissues in some cases [190]. Chai et al. showed the antiviral activity of E. faecium NCIMB 10415 against TGEV using in vitro swine testicle (ST) cell lines. They showed that this strain has a double antiviral mechanism. First, the strain can trap virus particles on its cell wall and consequently prevent infection. The second mechanism is the stimulation of eukaryotic cells that produce NO, IL-6, and IL-8 [160].
In addition to gastrointestinal infections, enteroviruses can cause extraintestinal infections. Via the orofecal route, Coxsackievirus type A strain 16 (CA16) and enterovirus 71 (EV71) cause hand, foot, and mouth disease (HFMD) [191]. This viral infection results in morbidity and mortality in several regions, including Asia Pacific and Europe [192]. HFMD can lead to neurological complications and cardiopulmonary dysfunction resulting from acute EV71 infection [193].
Liu et al. evaluated a bivalent vaccine against EV71, which has completed the phase III clinical trials [161, 194]. Since CA16 and EV71 act by the orofecal route, Ang Yin et al. evaluated the impact of colonization of the probiotic strain on HFMD using in vitro human skeletal muscle and colon cell lines. The authors showed that the use of L. reuteri Protectis (ATCC 55730) [195], decreased the viral load. Moreover, this antiviral activity is dose-dependent. The authors suggested that L. reuteri Protectis interacted physically with CA6, CA16, and EV71 and impaired viral entry to eukaryotic cells. This antiviral activity seems to be virus probiotic strain specific, since no antiviral effect was shown using Coxsackievirus B strain 2 (target virus) in the presence of another probiotic strain LcS [161].
1.3.2 Conclusion and Perspectives
Probiotics exhibit direct and indirect mechanisms in eradicating enteric viruses. The effectiveness of probiotics in the gut ecosystem is more relevant, since they interact with viral infections by several mechanisms, including immunomodulation, which is almost the only mechanism available for probiotics in respiratory infections.
The impact of enteric viruses can be decreased by changing the microbiota composition. Otherwise, HBGA and LPS are molecules that can be presented by Gram-negative bacteria and are considered a secondary receptor for enteric viruses such as NoVs and RoVs. For this reason, using probiotics can change the microbiota to Gram-positive dominant flora, which blocks the Gram-negative cofactor of viral infection.
Furthermore, the physical interaction of probiotics has been confirmed in several studies which confirm the capacity of some probiotic strains to trap viruses.
The use of antibiotics in viral gastroenteritis is a double-edged sword. Broad-spectrum antibiotic therapy kills probiotic strains or inhibits their multiplication. In contrast, using anti-Gram-negative antibiotics such as polymyxin B or other non-broad-spectrum antibiotics can be a crucial factor in blocking the viral cycle. Moreover, using probiotic strains with antibiotic resistance should be taken into consideration when treating viral gastroenteritis to keep probiotics live and eradicate Gram-negative resident flora. The antibiotic resistance of commercial probiotic strains can be found in a review conducted by Sharma et al. [196].
Abbreviations
- AAstV:
-
Avastrovirus
- AdVs:
-
Enteric adenoviruses
- AEnP:
-
Anti-EnV probiotics
- AMPs:
-
Antimicrobial peptides
- AMs:
-
Animal models
- AVs:
-
Arboviruses
- BALF:
-
Bronchoalveolar lavage fluid
- BCS:
-
Bacterial cell suspension
- BLISs:
-
Bacteriocin-like inhibitory substances
- CA16:
-
Coxsackievirus type A strain 16
- CFU:
-
Colony-forming unit
- CRFK:
-
Crandell-Reese feline kidney
- CTs:
-
Clinical trials
- CXCL1:
-
Neutrophil chemokine
- DLP:
-
Double-layered particle
- EnVs:
-
Enteric viruses
- EU:
-
European Union
- EV71:
-
Enterovirus 71
- EVs:
-
Enteroviruses
- GIT:
-
Human gastrointestinal tract
- GRAS:
-
Generally recognized as safe
- HBGAs:
-
Histo-blood group antigens
- HFMD:
-
Hand, foot, and mouth disease
- HRoV:
-
Human rotavirus vaccine
- ID:
-
Infectious diseases
- IFN-α:
-
Interferon-α
- IgA:
-
Immunoglobulin A
- IL-10:
-
Interleukin 10
- IL-12:
-
Interleukin 12
- IL-17:
-
Interleukin 17
- IL-2:
-
Interleukin 2
- IL-6:
-
Interleukin 6
- IL-8:
-
Interleukin 8
- IVA-H1N1:
-
Influenza virus type A
- LAB:
-
Lactic acid bacteria
- LPS:
-
Lipopolysaccharide
- MAstV:
-
Mamastrovirus
- MMTV:
-
Mouse mammary tumor virus
- MuNoVs:
-
Murine noroviruses
- NK cells:
-
Natural killer cells
- NRPS:
-
Non-ribosomal peptide synthetase
- NSP:
-
Nonstructural protein
- NVs:
-
Noroviruses
- PVR:
-
Poliovirus receptor
- RB:
-
Rice bran
- ROS:
-
Reactive oxygen species
- RoVs:
-
Rotaviruses
- RSBCT:
-
Randomized single-blind controlled trial
- RSV:
-
Respiratory syncytial virus
- RTIs:
-
Respiratory tract infections
- RVs:
-
Respiratory viruses
- TGEV:
-
Transmissible gastroenteritis virus
- TGFβ:
-
Transforming growth factor beta
- TLR:
-
Toll like receptors
- TNF-α:
-
Tumor necrosis factor alpha
- VLPs:
-
Viruslike particles
- VP:
-
Viral protein
- VP1:
-
Viral protein 1
References
Fonkwo PN. Pricing infectious disease. EMBO Rep. 2008;9:S13–7. doi:10.1038/embor.2008.110.
Fauci AS, Touchette NA, Folkers GK. Emerging infectious diseases: a 10-year perspective from the National Institute of Allergy and Infectious Diseases. Emerg Infect Dis. 2005;11:519–25. doi:10.3201/eid1104.041167.
United States. National Intelligence Council. National intelligence estimate: the global infectious disease threat and its implications for the United States. Environ Change Secur Proj Rep. 2000;6:33–65.
Golkar Z, Bagasra O, Pace DG. Bacteriophage therapy: a potential solution for the antibiotic resistance crisis. J Infect Dev Ctries. 2014;8:129–36.
Al Kassaa I, Hober D, Hamze M, Chihib NE, Drider D. Antiviral potential of lactic acid bacteria and their bacteriocins. Probiotics Antimicrob Proteins. 2014;6:177–85. doi:10.1007/s12602-014-9162-6.
Gordon YJ, Romanowski EG, McDermott AM. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr Eye Res. 2005;30:505–15. doi:10.1080/02713680590968637.
Rappuoli R, Pizza M, Del Giudice G, De Gregorio E. Vaccines, new opportunities for a new society. Proc Natl Acad Sci U S A. 2014;111:12288–93. doi:10.1073/pnas.1402981111.
Hardy H, Harris J, Lyon E, Beal J, Foey AD. Probiotics, prebiotics and immunomodulation of gut mucosal defences: homeostasis and immunopathology. Nutrients. 2013;5:1869–912. doi:10.3390/nu5061869.
Kassaa IA, Hober D, Hamze M, Caloone D, Dewilde A, Chihib N, et al. Vaginal Lactobacillus gasseri CMUL57 can inhibit herpes simplex type 2 but not Coxsackievirus B4E2. Arch Microbiol. 2015;197:657–64. doi:10.1007/s00203-015-1101-8.
WHO Guidelines for the evaluation of probiotics in food, report of a joint FAO/WHO Working Group on drafting guidelines for the evaluation of probiotics in food 2002: http://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf?ua=1.
Boris S, Barbés C. Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes Infect Inst Pasteur. 2000;2:543–6.
Green G, Dicks LM, Bruggeman G, Vandamme EJ, Chikindas ML. Pediocin PD-1, a bactericidal antimicrobial peptide from Pediococcus damnosus NCFB 1832. J Appl Microbiol. 1997;83:127–32.
Strieker M, Tanović A, Marahiel MA. Nonribosomal peptide synthetases: structures and dynamics. Curr Opin Struct Biol. 2010;20:234–40. doi:10.1016/j.sbi.2010.01.009.
Ahmad K, Fatemeh F, Mehri N, Maryam S. Probiotics for the treatment of pediatric helicobacter pylori infection: a randomized double blind clinical trial. Iran J Pediatr. 2013;23:79–84.
Liu Y, Tran DQ, Fatheree NY, Marc Rhoads J. Lactobacillus reuteri DSM 17938 differentially modulates effector memory T cells and Foxp3+ regulatory T cells in a mouse model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2014;307:G177–86. doi:10.1152/ajpgi.00038.2014.
Valeur N, Engel P, Carbajal N, Connolly E, Ladefoged K. Colonization and immunomodulation by Lactobacillus reuteri ATCC 55730 in the human gastrointestinal tract. Appl Environ Microbiol. 2004;70:1176–81.
Vitaliti G, Pavone P, Guglielmo F, Spataro G, Falsaperla R. The immunomodulatory effect of probiotics beyond atopy: an update. J Asthma Off J Assoc Care Asthma. 2014;51:320–32. doi:10.3109/02770903.2013.862259.
Lehtoranta L, Pitkäranta A, Korpela R. Probiotics in respiratory virus infections. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2014;33:1289–302. doi:10.1007/s10096-014-2086-y.
Kitazawa H, Villena J, Alvarez S. Probiotics: immunobiotics and immunogenics. NewYork, USA: CRC Press; 2013.
Murosaki S, Yamamoto Y, Ito K, Inokuchi T, Kusaka H, Ikeda H, et al. Heat-killed Lactobacillus plantarum L-137 suppresses naturally fed antigen-specific IgE production by stimulation of IL-12 production in mice. J Allergy Clin Immunol. 1998;102:57–64.
Maeda N, Nakamura R, Hirose Y, Murosaki S, Yamamoto Y, Kase T, et al. Oral administration of heat-killed Lactobacillus plantarum L-137 enhances protection against influenza virus infection by stimulation of type I interferon production in mice. Int Immunopharmacol. 2009;9:1122–5. doi:10.1016/j.intimp.2009.04.015.
Kawashima T, Hayashi K, Kosaka A, Kawashima M, Igarashi T, Tsutsui H, et al. Lactobacillus plantarum strain YU from fermented foods activates Th1 and protective immune responses. Int Immunopharmacol. 2011;11:2017–24. doi:10.1016/j.intimp.2011.08.013.
Kechaou N, Chain F, Gratadoux J-J, Blugeon S, Bertho N, Chevalier C, et al. Identification of one novel candidate probiotic Lactobacillus plantarum strain active against influenza virus infection in mice by a large-scale screening. Appl Environ Microbiol. 2013;79:1491–9. doi:10.1128/AEM.03075-12.
Park M-K, Ngo V, Kwon Y-M, Lee Y-T, Yoo S, Cho Y-H, et al. Lactobacillus plantarum DK119 as a probiotic confers protection against influenza virus by modulating innate immunity. PLoS One. 2013;8:e75368. doi:10.1371/journal.pone.0075368.
Gabryszewski SJ, Bachar O, Dyer KD, Percopo CM, Killoran KE, Domachowske JB, et al. Lactobacillus-mediated priming of the respiratory mucosa protects against lethal pneumovirus infection. J Immunol Baltim Md 1950 2011;186:1151–61. doi:10.4049/jimmunol.1001751.
Harata G, He F, Hiruta N, Kawase M, Kubota A, Hiramatsu M, et al. Intranasal administration of Lactobacillus rhamnosus GG protects mice from H1N1 influenza virus infection by regulating respiratory immune responses. Lett Appl Microbiol. 2010;50:597–602. doi:10.1111/j.1472-765X.2010.02844.x.
Kawase M, He F, Kubota A, Harata G, Hiramatsu M. Oral administration of lactobacilli from human intestinal tract protects mice against influenza virus infection. Lett Appl Microbiol. 2010;51:6–10. doi:10.1111/j.1472-765X.2010.02849.x.
Hatakka K, Savilahti E, Pönkä A, Meurman JH, Poussa T, Näse L, et al. Effect of long term consumption of probiotic milk on infections in children attending day care centres: double blind, randomised trial. BMJ. 2001;322:1327.
Luoto R, Ruuskanen O, Waris M, Kalliomäki M, Salminen S, Isolauri E. Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2014;133:405–13. doi:10.1016/j.jaci.2013.08.020.
Hojsak I, Abdović S, Szajewska H, Milosević M, Krznarić Z, Kolacek S. Lactobacillus GG in the prevention of nosocomial gastrointestinal and respiratory tract infections. Pediatrics. 2010;125:e1171–7. doi:10.1542/peds.2009-2568.
Hojsak I, Snovak N, Abdović S, Szajewska H, Misak Z, Kolacek S. Lactobacillus GG in the prevention of gastrointestinal and respiratory tract infections in children who attend day care centers: a randomized, double-blind, placebo-controlled trial. Clin Nutr Edinb Scotl. 2010;29:312–6. doi:10.1016/j.clnu.2009.09.008.
Kumpu M, Kekkonen RA, Kautiainen H, Järvenpää S, Kristo A, Huovinen P, et al. Milk containing probiotic Lactobacillus rhamnosus GG and respiratory illness in children: a randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr. 2012;66:1020–3. doi:10.1038/ejcn.2012.62.
Kekkonen RA, Vasankari TJ, Vuorimaa T, Haahtela T, Julkunen I, Korpela R. The effect of probiotics on respiratory infections and gastrointestinal symptoms during training in marathon runners. Int J Sport Nutr Exerc Metab. 2007;17:352–63.
Tomosada Y, Chiba E, Zelaya H, Takahashi T, Tsukida K, Kitazawa H, et al. Nasally administered Lactobacillus rhamnosus strains differentially modulate respiratory antiviral immune responses and induce protection against respiratory syncytial virus infection. BMC Immunol. 2013;14:40. doi:10.1186/1471-2172-14-40.
Hori T, Kiyoshima J, Shida K, Yasui H. Effect of intranasal administration of Lactobacillus casei Shirota on influenza virus infection of upper respiratory tract in mice. Clin Diagn Lab Immunol. 2001;8:593–7. doi:10.1128/CDLI.8.3.593-597.2001.
Cobo Sanz JM, Mateos JA, Muñoz Conejo A. Effect of Lactobacillus casei on the incidence of infectious conditions in children. Nutr Hosp. 2006;21:547–51.
Merenstein D, Murphy M, Fokar A, Hernandez RK, Park H, Nsouli H, et al. Use of a fermented dairy probiotic drink containing Lactobacillus casei (DN-114001) to decrease the rate of illness in kids: the DRINK study. A patient-oriented, double-blind, cluster-randomized, placebo-controlled, clinical trial. Eur J Clin Nutr. 2010;64:669–77. doi:10.1038/ejcn.2010.65.
Guillemard E, Tondu F, Lacoin F, Schrezenmeir J. Consumption of a fermented dairy product containing the probiotic Lactobacillus casei DN-114001 reduces the duration of respiratory infections in the elderly in a randomised controlled trial. Br J Nutr. 2010;103:58–68. doi:10.1017/S0007114509991395.
Tiollier E, Chennaoui M, Gomez-Merino D, Drogou C, Filaire E, Guezennec CY. Effect of a probiotics supplementation on respiratory infections and immune and hormonal parameters during intense military training. Mil Med. 2007;172:1006–11.
Turchet P, Laurenzano M, Auboiron S, Antoine JM. Effect of fermented milk containing the probiotic Lactobacillus casei DN-114001 on winter infections in free-living elderly subjects: a randomised, controlled pilot study. J Nutr Health Aging. 2003;7:75–7.
Takeda S, Takeshita M, Kikuchi Y, Dashnyam B, Kawahara S, Yoshida H, et al. Efficacy of oral administration of heat-killed probiotics from Mongolian dairy products against influenza infection in mice: alleviation of influenza infection by its immunomodulatory activity through intestinal immunity. Int Immunopharmacol. 2011;11:1976–83. doi:10.1016/j.intimp.2011.08.007.
Yeo J-M, Lee H-J, Kim J-W, Lee J-B, Park S-Y, Choi I-S, et al. Lactobacillus fermentum CJL-112 protects mice against influenza virus infection by activating T-helper 1 and eliciting a protective immune response. Int Immunopharmacol. 2014;18:50–4. doi:10.1016/j.intimp.2013.10.020.
Youn H-N, Lee D-H, Lee Y-N, Park J-K, Yuk S-S, Yang S-Y, et al. Intranasal administration of live Lactobacillus species facilitates protection against influenza virus infection in mice. Antiviral Res. 2012;93:138–43. doi:10.1016/j.antiviral.2011.11.004.
Maldonado J, Cañabate F, Sempere L, Vela F, Sánchez AR, Narbona E, et al. Human milk probiotic Lactobacillus fermentum CECT5716 reduces the incidence of gastrointestinal and upper respiratory tract infections in infants. J Pediatr Gastroenterol Nutr. 2012;54:55–61. doi:10.1097/MPG.0b013e3182333f18.
Olivares M, Díaz-Ropero MP, Sierra S, Lara-Villoslada F, Fonollá J, Navas M, et al. Oral intake of Lactobacillus fermentum CECT5716 enhances the effects of influenza vaccination. Nutr Burbank Los Angel Cty Calif. 2007;23:254–60. doi:10.1016/j.nut.2007.01.004.
Cox AJ, Pyne DB, Saunders PU, Fricker PA. Oral administration of the probiotic Lactobacillus fermentum VRI-003 and mucosal immunity in endurance athletes. Br J Sports Med. 2010;44:222–6. doi:10.1136/bjsm.2007.044628.
West NP, Pyne DB, Cripps AW, Hopkins WG, Eskesen DC, Jairath A, et al. Lactobacillus fermentum (PCC®) supplementation and gastrointestinal and respiratory-tract illness symptoms: a randomised control trial in athletes. Nutr J. 2011;10:30. doi:10.1186/1475-2891-10-30.
Goto H, Sagitani A, Ashida N, Kato S, Hirota T, Shinoda T, et al. Anti-influenza virus effects of both live and non-live Lactobacillus acidophilus L-92 accompanied by the activation of innate immunity. Br J Nutr. 2013;110:1810–8. doi:10.1017/S0007114513001104.
Gleeson M, Bishop NC, Oliveira M, McCauley T, Tauler P, Lawrence C. Effects of a Lactobacillus salivarius probiotic intervention on infection, cold symptom duration and severity, and mucosal immunity in endurance athletes. Int J Sport Nutr Exerc Metab. 2012;22:235–42.
Waki N, Yajima N, Suganuma H, Buddle BM, Luo D, Heiser A, et al. Oral administration of Lactobacillus brevis KB290 to mice alleviates clinical symptoms following influenza virus infection. Lett Appl Microbiol. 2014;58:87–93. doi:10.1111/lam.12160.
Izumo T, Maekawa T, Ida M, Noguchi A, Kitagawa Y, Shibata H, et al. Effect of intranasal administration of Lactobacillus pentosus S-PT84 on influenza virus infection in mice. Int Immunopharmacol. 2010;10:1101–6. doi:10.1016/j.intimp.2010.06.012.
Kiso M, Takano R, Sakabe S, Katsura H, Shinya K, Uraki R, et al. Protective efficacy of orally administered, heat-killed Lactobacillus pentosus b240 against influenza A virus. Sci Rep. 2013;3:1563. doi:10.1038/srep01563.
Iwabuchi N, Xiao J-Z, Yaeshima T, Iwatsuki K. Oral administration of Bifidobacterium longum ameliorates influenza virus infection in mice. Biol Pharm Bull. 2011;34:1352–5.
Wu S, Jiang Z-Y, Sun Y-F, Yu B, Chen J, Dai C-Q, et al. Microbiota regulates the TLR7 signaling pathway against respiratory tract influenza A virus infection. Curr Microbiol. 2013;67:414–22. doi:10.1007/s00284-013-0380-z.
Taipale T, Pienihäkkinen K, Isolauri E, Larsen C, Brockmann E, Alanen P, et al. Bifidobacterium animalis subsp. lactis BB-12 in reducing the risk of infections in infancy. Br J Nutr. 2011;105:409–16. doi:10.1017/S0007114510003685.
West NP, Horn PL, Pyne DB, Gebski VJ, Lahtinen SJ, Fricker PA, et al. Probiotic supplementation for respiratory and gastrointestinal illness symptoms in healthy physically active individuals. Clin Nutr Edinb Scotl. 2014;33:581–7. doi:10.1016/j.clnu.2013.10.002.
Debbink K, Lindesmith LC, Donaldson EF, Baric RS. Norovirus immunity and the great escape. PLoS Pathog. 2012;8:e10029e21. doi:10.1371/journal.ppat.1002921.
Rautava S, Salminen S, Isolauri E. Specific probiotics in reducing the risk of acute infections in infancy--a randomised, double-blind, placebo-controlled study. Br J Nutr. 2009;101:1722–6. doi:10.1017/S0007114508116282.
Weizman Z, Asli G, Alsheikh A. Effect of a probiotic infant formula on infections in child care centers: comparison of two probiotic agents. Pediatrics. 2005;115:5–9. doi:10.1542/peds.2004-1815.
Rerksuppaphol S, Rerksuppaphol L. Randomized controlled trial of probiotics to reduce common cold in schoolchildren. Pediatr Int Off J Jpn Pediatr Soc. 2012;54:682–7. doi:10.1111/j.1442-200X.2012.03647.x.
Lehtoranta L, Söderlund-Venermo M, Nokso-Koivisto J, Toivola H, Blomgren K, Hatakka K, et al. Human bocavirus in the nasopharynx of otitis-prone children. Int J Pediatr Otorhinolaryngol. 2012;76:206–11. doi:10.1016/j.ijporl.2011.10.025.
Smith TJ, Rigassio-Radler D, Denmark R, Haley T, Touger-Decker R. Effect of Lactobacillus rhamnosus LGG® and Bifidobacterium animalis ssp. lactis BB-12® on health-related quality of life in college students affected by upper respiratory infections. Br J Nutr. 2013;109:1999–2007. doi:10.1017/S0007114512004138.
Randazzo CL, Restuccia C, Romano AD, Caggia C. Lactobacillus casei, dominant species in naturally fermented Sicilian green olives. Int J Food Microbiol. 2004;90:9–14.
Sutula J, Coulthwaite L, Verran J. Culture media for differential isolation of Lactobacillus casei Shirota from oral samples. J Microbiol Methods. 2012;90:65–71. doi:10.1016/j.mimet.2012.03.015.
Fujita R, Iimuro S, Shinozaki T, Sakamaki K, Uemura Y, Takeuchi A, et al. Decreased duration of acute upper respiratory tract infections with daily intake of fermented milk: a multicenter, double-blinded, randomized comparative study in users of day care facilities for the elderly population. Am J Infect Control. 2013;41:1231–5. doi:10.1016/j.ajic.2013.04.005.
Van Puyenbroeck K, Hens N, Coenen S, Michiels B, Beunckens C, Molenberghs G, et al. Efficacy of daily intake of Lactobacillus casei Shirota on respiratory symptoms and influenza vaccination immune response: a randomized, double-blind, placebo-controlled trial in healthy elderly nursing home residents. Am J Clin Nutr. 2012;95:1165–71. doi:10.3945/ajcn.111.026831.
Guillemard E, Tanguy J, Flavigny A, de la Motte S, Schrezenmeir J. Effects of consumption of a fermented dairy product containing the probiotic Lactobacillus casei DN-114001 on common respiratory and gastrointestinal infections in shift workers in a randomized controlled trial. J Am Coll Nutr. 2010;29:455–68.
Dickson EM, Riggio MP, Macpherson L. A novel species-specific PCR assay for identifying Lactobacillus fermentum. J Med Microbiol. 2005;54:299–303. doi:10.1099/jmm.0.45770-0.
Ljungh A, Wadström T. Lactic acid bacteria as probiotics. Curr Issues Intest Microbiol. 2006;7:73–89.
Mayanagi G, Kimura M, Nakaya S, Hirata H, Sakamoto M, Benno Y, et al. Probiotic effects of orally administered Lactobacillus salivarius WB21-containing tablets on periodontopathic bacteria: a double-blinded, placebo-controlled, randomized clinical trial. J Clin Periodontol. 2009;36:506–13. doi:10.1111/j.1600-051X.2009.01392.x.
Pochart P, Marteau P, Bouhnik Y, Goderel I, Bourlioux P, Rambaud JC. Survival of bifidobacteria ingested via fermented milk during their passage through the human small intestine: an in vivo study using intestinal perfusion. Am J Clin Nutr. 1992;55:78–80.
Mayo, B., & Sinderen, D. van (Eds.). (2010). Bifidobacteria: genomics and molecular aspects. Norfolk, UK: Caister Academic.
Gordon S. Elie Metchnikoff: father of natural immunity. Eur J Immunol. 2008;38:3257–64. doi:10.1002/eji.200838855.
Fouhy F, Ross RP, Fitzgerald GF, Stanton C, Cotter PD. Composition of the early intestinal microbiota. Gut Microbes. 2012;3:203–20. doi:10.4161/gmic.20169.
Aureli P, Capurso L, Castellazzi AM, Clerici M, Giovannini M, Morelli L, et al. Probiotics and health: an evidence-based review. Pharmacol Res. 2011;63:366–76. doi:10.1016/j.phrs.2011.02.006.
Collado MC, Isolauri E, Salminen S, Sanz Y. The impact of probiotic on gut health. Curr Drug Metab. 2009;10:68–78.
Rodríguez JM, Murphy K, Stanton C, Ross RP, Kober OI, Juge N, et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Health Dis. 2015;26:26050. doi:10.3402/mehd.v26.26050.
Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, et al. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–7. doi:10.1126/science.1223813.
Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–9. doi:10.1038/nature11552.
Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–73. doi:10.1126/science.1223490.
Kamada N, Núñez G. Regulation of the immune system by the resident intestinal bacteria. Gastroenterology. 2014;146:1477–88. doi:10.1053/j.gastro.2014.01.060.
Hooper LV, Stappenbeck TS, Hong CV, Gordon JI. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol. 2003;4:269–73. doi:10.1038/ni888.
Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–8. doi:10.1126/science.1209791.
Kantha SS. The blood revolution initiated by the famous footnote of Karl Landsteiner’s 1900 paper. Ceylon Med J. 1995;40:123–5.
Marionneau S, Cailleau-Thomas A, Rocher J, Le Moullac-Vaidye B, Ruvoën N, Clément M, et al. ABH and Lewis histo-blood group antigens, a model for the meaning of oligosaccharide diversity in the face of a changing world. Biochimie. 2001;83:565–73.
Hakomori S. Antigen structure and genetic basis of histo-blood groups A, B and O: their changes associated with human cancer. Biochim Biophys Acta. 1999;1473:247–66.
Barthelson R, Mobasseri A, Zopf D, Simon P. Adherence of Streptococcus pneumoniae to respiratory epithelial cells is inhibited by sialylated oligosaccharides. Infect Immun. 1998;66:1439–44.
Giannasca KT, Giannasca PJ, Neutra MR. Adherence of Salmonella typhimurium to Caco-2 cells: identification of a glycoconjugate receptor. Infect Immun. 1996;64:135–45.
Newburg DS. Oligosaccharides in human milk and bacterial colonization. J Pediatr Gastroenterol Nutr. 2000;30(Suppl 2):S8–17.
Saadi AT, Weir DM, Poxton IR, Stewart J, Essery SD, Blackwell CC, et al. Isolation of an adhesin from Staphylococcus aureus that binds Lewis a blood group antigen and its relevance to sudden infant death syndrome. FEMS Immunol Med Microbiol. 1994;8:315–20.
Aspinall GO, Monteiro MA. Lipopolysaccharides of Helicobacter pylori strains P466 and MO19: structures of the O antigen and core oligosaccharide regions. Biochemistry (Mosc). 1996;35:2498–504. doi:10.1021/bi951853k.
Andersson M, Carlin N, Leontein K, Lindquist U, Slettengren K. Structural studies of the O-antigenic polysaccharide of Escherichia coli O86, which possesses blood-group B activity. Carbohydr Res. 1989;185:211–23.
Wardlaw T, Salama P, Brocklehurst C, Chopra M, Mason E. Diarrhoea: why children are still dying and what can be done. Lancet Lond Engl. 2010;375:870–2. doi:10.1016/S0140-6736(09)61798-0.
Menon VK, George S, Sarkar R, Giri S, Samuel P, Vivek R, et al. Norovirus gastroenteritis in a birth cohort in Southern India. PLoS One. 2016;11:e0157007. doi:10.1371/journal.pone.0157007.
Joshi SS, Howell AB, D’Souza DH. Reduction of enteric viruses by blueberry juice and blueberry proanthocyanidins. Food Environ Virol. 2016;8:235–243. doi:10.1007/s12560-016-9247-3.
Buesa J, Rodrı’guez-Dı’az J. Molecular virology of enteric viruses (with emphasis on caliciviruses). Viruses foods. New York: Springer; 2006. p. 43–100.
Karst SM. The influence of commensal bacteria on infection with enteric viruses. Nat Rev Microbiol. 2016;14:197–204. doi:10.1038/nrmicro.2015.25.
Ross SR. Mouse mammary tumor virus molecular biology and oncogenesis. Viruses. 2010;2:2000–12. doi:10.3390/v2092000.
Kane M, Case LK, Kopaskie K, Kozlova A, MacDearmid C, Chervonsky AV, et al. Successful transmission of a retrovirus depends on the commensal microbiota. Science. 2011;334:245–9. doi:10.1126/science.1210718.
Kuss SK, Best GT, Etheredge CA, Pruijssers AJ, Frierson JM, Hooper LV, et al. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science. 2011;334:249–52. doi:10.1126/science.1211057.
Uchiyama R, Chassaing B, Zhang B, Gewirtz AT. Antibiotic treatment suppresses rotavirus infection and enhances specific humoral immunity. J Infect Dis. 2014;210:171–82. doi:10.1093/infdis/jiu037.
Baldridge MT, Nice TJ, McCune BT, Yokoyama CC, Kambal A, Wheadon M, et al. Commensal microbes and interferon-λ determine persistence of enteric murine norovirus infection. Science. 2015;347:266–9. doi:10.1126/science.1258025.
Jones MK, Watanabe M, Zhu S, Graves CL, Keyes LR, Grau KR, et al. Enteric bacteria promote human and mouse norovirus infection of B cells. Science. 2014;346:755–9. doi:10.1126/science.1257147.
Kernbauer E, Ding Y, Cadwell K. An enteric virus can replace the beneficial function of commensal bacteria. Nature. 2014;516:94–8. doi:10.1038/nature13960.
Robinson CM, Jesudhasan PR, Pfeiffer JK. Bacterial lipopolysaccharide binding enhances virion stability and promotes environmental fitness of an enteric virus. Cell Host Microbe. 2014;15:36–46. doi:10.1016/j.chom.2013.12.004.
Tan M, Jiang X. Norovirus and its histo-blood group antigen receptors: an answer to a historical puzzle. Trends Microbiol. 2005;13:285–93. doi:10.1016/j.tim.2005.04.004.
Miura T, Sano D, Suenaga A, Yoshimura T, Fuzawa M, Nakagomi T, et al. Histo-blood group antigen-like substances of human enteric bacteria as specific adsorbents for human noroviruses. J Virol. 2013;87:9441–51. doi:10.1128/JVI.01060-13.
Karst SM. Identification of a novel cellular target and a co-factor for norovirus infection - B cells & commensal bacteria. Gut Microbes. 2015;6:266–71. doi:10.1080/19490976.2015.1052211.
Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–44. doi:10.1038/nri2707.
Mukherji A, Kobiita A, Ye T, Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell. 2013;153:812–27. doi:10.1016/j.cell.2013.04.020.
Jude BA, Pobezinskaya Y, Bishop J, Parke S, Medzhitov RM, Chervonsky AV, et al. Subversion of the innate immune system by a retrovirus. Nat Immunol. 2003;4:573.
Wilks J, Lien E, Jacobson AN, Fischbach MA, Qureshi N, Chervonsky AV, et al. Mammalian lipopolysaccharide receptors incorporated into the retroviral envelope augment virus transmission. Cell Host Microbe. 2015;18:456–62. doi:10.1016/j.chom.2015.09.005.
Blacklow NR et al. Acute infectious nonbacterial gastroenteritis: etiology and pathogenesis. Ann Intern Med. 1972;76:993–1008.
Kahan SM, Liu G, Reinhard MK, Hsu CC, Livingston RS, Karst SM. Comparative murine norovirus studies reveal a lack of correlation between intestinal virus titers and enteric pathology. Virology. 2011;421:202–10. doi:10.1016/j.virol.2011.09.030.
Donnelly RP, Kotenko SV. Interferon-lambda: a new addition to an old family. J Interferon Cytokine Res Off J Int Soc Interferon Cytokine Res. 2010;30:555–64. doi:10.1089/jir.2010.0078.
Nice TJ, Baldridge MT, McCune BT, Norman JM, Lazear HM, Artyomov M, et al. Interferon-λ cures persistent murine norovirus infection in the absence of adaptive immunity. Science. 2015;347:269–73. doi:10.1126/science.1258100.
Bok K, Parra GI, Mitra T, Abente E, Shaver CK, Boon D, et al. Chimpanzees as an animal model for human norovirus infection and vaccine development. Proc Natl Acad Sci U S A. 2011;108:325–30. doi:10.1073/pnas.1014577107.
Duizer E, Schwab KJ, Neill FH, Atmar RL, Koopmans MPG, Estes MK. Laboratory efforts to cultivate noroviruses. J Gen Virol. 2004;85:79–87. doi:10.1099/vir.0.19478-0.
Pott J, Mahlakõiv T, Mordstein M, Duerr CU, Michiels T, Stockinger S, et al. IFN-lambda determines the intestinal epithelial antiviral host defense. Proc Natl Acad Sci U S A. 2011;108:7944–9. doi:10.1073/pnas.1100552108.
Howarth GS, Wang H. Role of endogenous microbiota, probiotics and their biological products in human ealth. Nutrients. 2013;5:58–81. doi:10.3390/nu5010058.
Al Kassaa I, Hamze M, Hober D, Chihib N-E, Drider D. Identification of vaginal lactobacilli with potential probiotic properties isolated from women in North Lebanon. Microb Ecol. 2014;67:722–34. doi:10.1007/s00248-014-0384-7.
Grandy G, Medina M, Soria R, Terán CG, Araya M. Probiotics in the treatment of acute rotavirus diarrhoea. A randomized, double-blind, controlled trial using two different probiotic preparations in Bolivian children. BMC Infect Dis. 2010;10:253. doi:10.1186/1471-2334-10-253.
Szajewska H, Wanke M, Patro B. Meta-analysis: the effects of Lactobacillus rhamnosus GG supplementation for the prevention of healthcare-associated diarrhoea in children. Aliment Pharmacol Ther. 2011;34:1079–87. doi:10.1111/j.1365-2036.2011.04837.x.
Guandalini S, Pensabene L, Zikri MA, Dias JA, Casali LG, Hoekstra H, et al. Lactobacillus GG administered in oral rehydration solution to children with acute diarrhea: a multicenter European trial. J Pediatr Gastroenterol Nutr. 2000;30:54–60.
Isolauri E, Juntunen M, Rautanen T, Sillanaukee P, Koivula T. A human Lactobacillus strain (Lactobacillus casei sp strain GG) promotes recovery from acute diarrhea in children. Pediatrics. 1991;88:90–7.
Majamaa H, Isolauri E, Saxelin M, Vesikari T. Lactic acid bacteria in the treatment of acute rotavirus gastroenteritis. J Pediatr Gastroenterol Nutr. 1995;20:333–8.
Oberhelman RA, Gilman RH, Sheen P, Taylor DN, Black RE, Cabrera L, et al. A placebo-controlled trial of Lactobacillus GG to prevent diarrhea in undernourished Peruvian children. J Pediatr. 1999;134:15–20.
Pant AR, Graham SM, Allen SJ, Harikul S, Sabchareon A, Cuevas L, et al. Lactobacillus GG and acute diarrhoea in young children in the tropics. J Trop Pediatr. 1996;42:162–5.
Rautanen T, Isolauri E, Salo E, Vesikari T. Management of acute diarrhoea with low osmolarity oral rehydration solutions and Lactobacillus strain GG. Arch Dis Child. 1998;79:157–60.
Raza S, Graham SM, Allen SJ, Sultana S, Cuevas L, Hart CA. Lactobacillus GG promotes recovery from acute nonbloody diarrhea in Pakistan. Pediatr Infect Dis J. 1995;14:107–11.
Ritchie BK, Brewster DR, Tran CD, Davidson GP, McNeil Y, Butler RN. Efficacy of Lactobacillus GG in aboriginal children with acute diarrhoeal disease: a randomised clinical trial. J Pediatr Gastroenterol Nutr. 2010;50:619–24. doi:10.1097/MPG.0b013e3181bbf53d.
Salazar-Lindo E, Miranda-Langschwager P, Campos-Sanchez M, Chea-Woo E, Sack RB. Lactobacillus casei strain GG in the treatment of infants with acute watery diarrhea: a randomized, double-blind, placebo controlled clinical trial [ISRCTN67363048]. BMC Pediatr. 2004;4:18. doi:10.1186/1471-2431-4-18.
Liu F, Li G, Wen K, Bui T, Cao D, Zhang Y, et al. Porcine small intestinal epithelial cell line (IPEC-J2) of rotavirus infection as a new model for the study of innate immune responses to rotaviruses and probiotics. Viral Immunol. 2010;23:135–49. doi:10.1089/vim.2009.0088.
Shornikova AV, Casas IA, Isolauri E, Mykkänen H, Vesikari T. Lactobacillus reuteri as a therapeutic agent in acute diarrhea in young children. J Pediatr Gastroenterol Nutr. 1997;24:399–404.
Saavedra JM, Bauman NA, Oung I, Perman JA, Yolken RH. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet Lond Engl. 1994;344:1046–9.
Shornikova AV, Casas IA, Mykkänen H, Salo E, Vesikari T. Bacteriotherapy with Lactobacillus reuteri in rotavirus gastroenteritis. Pediatr Infect Dis J. 1997;16:1103–7.
Sugita T, Togawa M. Efficacy of Lactobacillus preparation Biolactis powder in children with rotavirus enteritis. Japanese J Pediatr. 1994;47:2755–62.
Maragkoudakis PA, Chingwaru W, Gradisnik L, Tsakalidou E, Cencic A. Lactic acid bacteria efficiently protect human and animal intestinal epithelial and immune cells from enteric virus infection. Int J Food Microbiol. 2010;141(Suppl 1):S91–7. doi:10.1016/j.ijfoodmicro.2009.12.024.
Seo BJ, Mun MR, J RKV, Kim C-J, Lee I, Chang Y-H, et al. Bile tolerant Lactobacillus reuteri isolated from pig feces inhibits enteric bacterial pathogens and porcine rotavirus. Vet Res Commun. 2010;34:323–33. doi:10.1007/s11259-010-9357-6.
Francavilla R, Lionetti E, Castellaneta S, Ciruzzi F, Indrio F, Masciale A, et al. Randomised clinical trial: Lactobacillus reuteri DSM 17938 vs. placebo in children with acute diarrhoea--a double-blind study. Aliment Pharmacol Ther. 2012;36:363–9. doi:10.1111/j.1365-2036.2012.05180.x.
Simakachorn N, Pichaipat V, Rithipornpaisarn P, Kongkaew C, Tongpradit P, Varavithya W. Clinical evaluation of the addition of lyophilized, heat-killed Lactobacillus acidophilus LB to oral rehydration therapy in the treatment of acute diarrhea in children. J Pediatr Gastroenterol Nutr. 2000;30:68–72.
Hagbom M, Sharma S, Lundgren O, Svensson L. Towards a human rotavirus disease model. Curr Opin Virol. 2012;2:408–18. doi:10.1016/j.coviro.2012.05.006.
Pant N, Marcotte H, Brüssow H, Svensson L, Hammarström L. Effective prophylaxis against rotavirus diarrhea using a combination of Lactobacillus rhamnosus GG and antibodies. BMC Microbiol. 2007;7:86. doi:10.1186/1471-2180-7-86.
Zhang Z, Xiang Y, Li N, Wang B, Ai H, Wang X, et al. Protective effects of Lactobacillus rhamnosus GG against human rotavirus-induced diarrhoea in a neonatal mouse model. Pathog Dis. 2013;67:184–91. doi:10.1111/2049-632X.12030.
Guérin-Danan C, Meslin JC, Chambard A, Charpilienne A, Relano P, Bouley C, et al. Food supplementation with milk fermented by Lactobacillus casei DN-114001 protects suckling rats from rotavirus-associated diarrhea. J Nutr. 2001;131:111–7.
Preidis GA, Saulnier DM, Blutt SE, Mistretta T-A, Riehle KP, Major AM, et al. Host response to probiotics determined by nutritional status of rotavirus-infected neonatal mice. J Pediatr Gastroenterol Nutr. 2012;55:299–307. doi:10.1097/MPG.0b013e31824d2548.
Mao X, Gu C, Hu H, Tang J, Chen D, Yu B, et al. Dietary Lactobacillus rhamnosus GG Supplementation Improves the Mucosal Barrier Function in the Intestine of Weaned Piglets Challenged by Porcine Rotavirus. PLoS One. 2016;11:e0146312. doi:10.1371/journal.pone.0146312.
Kandasamy S, Vlasova AN, Fischer D, Kumar A, Chattha KS, Rauf A, et al. Differential effects of Escherichia coli Nissle and Lactobacillus rhamnosus Strain GG on human rotavirus binding, infection, and B cell immunity. J Immunol Baltim Md 1950 2016;196:1780–9. doi:10.4049/jimmunol.1501705.
Kang JY, Lee DK, Ha NJ, Shin HS. Antiviral effects of Lactobacillus ruminis. J Microbiol. 2015;53:796–803. doi:10.1007/s12275-015-5302-2.
Yang X, Twitchell E, Li G, Wen K, Weiss M, Kocher J, et al. High protective efficacy of rice bran against human rotavirus diarrhea via enhancing probiotic growth, gut barrier function, and innate immunity. Sci Rep. 2015;5:15004. doi:10.1038/srep15004.
Aoki-Yoshida A, Saito S, Fukiya S, Aoki R, Takayama Y, Suzuki C, et al. Lactobacillus rhamnosus GG increases Toll-like receptor 3 gene expression in murine small intestine ex vivo and in vivo. Benef Microbes. 2016;7:421–9. doi:10.3920/BM2015.0169.
Kang JY, Lee DK, Ha NJ, Shin HS. Antiviral effects of Lactobacillus ruminis SPM0211 and Bifidobacterium longum SPM1205 and SPM1206 on rotavirus-infected Caco-2 cells and a neonatal mouse model. J Microbiol Seoul Korea. 2015;53:796–803. doi:10.1007/s12275-015-5302-2.
Muñoz JAM, Chenoll E, Casinos B, Bataller E, Ramón D, Genovés S, et al. Novel probiotic Bifidobacterium longum subsp. infantis CECT 7210 strain active against rotavirus infections. Appl Environ Microbiol. 2011;77:8775–83. doi:10.1128/AEM.05548-11.
Nagata S, Asahara T, Ohta T, Yamada T, Kondo S, Bian L, et al. Effect of the continuous intake of probiotic-fermented milk containing Lactobacillus casei strain Shirota on fever in a mass outbreak of norovirus gastroenteritis and the faecal microflora in a health service facility for the aged. Br J Nutr. 2011;106:549–56. doi:10.1017/S000711451100064X.
Takeda K, Suzuki T, Shimada S-I, Shida K, Nanno M, Okumura K. Interleukin-12 is involved in the enhancement of human natural killer cell activity by Lactobacillus casei Shirota. Clin Exp Immunol. 2006;146:109–15. doi:10.1111/j.1365-2249.2006.03165.x.
Aboubakr HA, El-Banna AA, Youssef MM, Al-Sohaimy SAA, Goyal SM. Antiviral effects of Lactococcus lactis on feline calicivirus, a human norovirus surrogate. Food Environ Virol. 2014; doi:10.1007/s12560-014-9164-2.
Rubio-del-Campo A, Coll-Marqués JM, Yebra MJ, Buesa J, Pérez-Martínez G, Monedero V, et al. Noroviral p-particles as an in vitro model to assess the interactions of noroviruses with probiotics. PLoS One. 2014;9:e89586. doi:10.1371/journal.pone.0089586.
Hoang PM, Cho S, Kim KE, Byun SJ, Lee T-K, Lee S. Development of Lactobacillus paracasei harboring nucleic acid-hydrolyzing 3D8 scFv as a preventive probiotic against murine norovirus infection. Appl Microbiol Biotechnol. 2015;99:2793–803. doi:10.1007/s00253-014-6257-7.
Li D, Breiman A, le Pendu J, Uyttendaele M. Anti-viral effect of Bifidobacterium adolescentis against Noroviruses. Front Microbiol. 2016;7:864. doi:10.3389/fmicb.2016.00864.
Chai W, Burwinkel M, Wang Z, Palissa C, Esch B, Twardziok S, et al. Antiviral effects of a probiotic Enterococcus faecium strain against transmissible gastroenteritis coronavirus. Arch Virol. 2013;158:799–807. doi:10.1007/s00705-012-1543-0.
Ang LYE, Too HKI, Tan EL, Chow T-KV, Shek P-CL, Tham E, et al. Antiviral activity of Lactobacillus reuteri Protectis against Coxsackievirus A and Enterovirus 71 infection in human skeletal muscle and colon cell lines. Virol J. 2016;13:111. doi:10.1186/s12985-016-0567-6.
Teran CG, Teran-Escalera CN, Villarroel P. Nitazoxanide vs. probiotics for the treatment of acute rotavirus diarrhea in children: a randomized, single-blind, controlled trial in Bolivian children. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2009;13:518–23. doi:10.1016/j.ijid.2008.09.014.
Picard C, Fioramonti J, Francois A, Robinson T, Neant F, Matuchansky C. Review article: bifidobacteria as probiotic agents – physiological effects and clinical benefits. Aliment Pharmacol Ther. 2005;22:495–512. doi:10.1111/j.1365-2036.2005.02615.x.
Liepke C, Adermann K, Raida M, Mägert H-J, Forssmann W-G, Zucht H-D. Human milk provides peptides highly stimulating the growth of bifidobacteria. Eur J Biochem. 2002;269:712–8. doi:10.1046/j.0014-2956.2001.02712.x.
Desselberger U. Review: Rotaviruses. Virus Res. 2014;190:75–96. doi:10.1016/j.virusres.2014.06.016.
Jayaram H, Estes M, Prasad BVV. Emerging themes in rotavirus cell entry, genome organization, transcription and replication. Virus Res. 2004;101:67–81. doi:10.1016/j.virusres.2003.12.007.
Kam J, Demmert AC, Tanner JR, McDonald SM, Kelly DF. Structural dynamics of viral nanomachines. Technology. 2014;2:44.
Fabbretti E, Afrikanova I, Vascotto F, Burrone OR. Two non-structural rotavirus proteins, NSP2 and NSP5, form viroplasm-like structures in vivo. J Gen Virol. 1999;80(Pt 2):333–9. doi:10.1099/0022-1317-80-2-333.
Yang X, Wen K, Tin C, Li G, Wang H, Kocher J, et al. Dietary rice bran protects against rotavirus diarrhea and promotes Th1-type immune responses to human rotavirus vaccine in gnotobiotic pigs. Clin Vaccine Immunol CVI. 2014;21:1396–403. doi:10.1128/CVI.00210-14.
Chenoll E, Rivero M, Codoñer FM, Martinez-Blanch JF, Ramón D, Genovés S, et al. Complete Genome Sequence of Bifidobacterium longum subsp. infantis Strain CECT 7210, a Probiotic Strain Active against Rotavirus Infections. Genome Announc. 2015:3. doi:10.1128/genomeA.00105-15.
Donaldson EF, Lindesmith LC, Lobue AD, Baric RS. Viral shape-shifting: norovirus evasion of the human immune system. Nat Rev Microbiol. 2010;8:231–41. doi:10.1038/nrmicro2296.
Patel MM, Widdowson M-A, Glass RI, Akazawa K, Vinjé J, Parashar UD. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis. 2008;14:1224–31. doi:10.3201/eid1408.071114.
de Rougemont A, Ruvoen-Clouet N, Simon B, Estienney M, Elie-Caille C, Aho S, et al. Qualitative and quantitative analysis of the binding of GII.4 norovirus variants onto human blood group antigens. J Virol. 2011;85:4057–70. doi:10.1128/JVI.02077-10.
Lindesmith L, Moe C, Marionneau S, Ruvoen N, Jiang X, Lindblad L, et al. Human susceptibility and resistance to Norwalk virus infection. Nat Med. 2003;9:548–53. doi:10.1038/nm860.
Reeck A, Kavanagh O, Estes MK, Opekun AR, Gilger MA, Graham DY, et al. Serological correlate of protection against norovirus-induced gastroenteritis. J Infect Dis. 2010;202:1212–8. doi:10.1086/656364.
Lindesmith LC, Donaldson E, Leon J, Moe CL, Frelinger JA, Johnston RE, et al. Heterotypic humoral and cellular immune responses following Norwalk virus infection. J Virol. 2010;84:1800–15. doi:10.1128/JVI.02179-09.
Karst SM, Wobus CE, Goodfellow IG, Green KY, Virgin HW. Advances in norovirus biology. Cell Host Microbe. 2014;15:668–80. doi:10.1016/j.chom.2014.05.015.
Bosch A, Pintó RM, Guix S. Human Astroviruses. Clin Microbiol Rev. 2014;27:1048–74. doi:10.1128/CMR.00013-14.
Hoshino Y, Zimmer JF, Moise NS, Scott FW. Detection of astroviruses in feces of a cat with diarrhea. Brief report Arch Virol. 1981;70:373–6.
Castro TX, Cubel Garcia RCN, Costa EM, Leal RM, Xavier Mda PT, Leite JPG. Molecular characterisation of calicivirus and astrovirus in puppies with enteritis. Vet Rec. 2013;172:557. doi:10.1136/vr.101566.
Kjeldsberg E, Hem A. Detection of astroviruses in gut contents of nude and normal mice. Brief report. Arch Virol. 1985;84:135–40.
Jonassen CM, Jonassen TTØ, Sveen TM, Grinde B. Complete genomic sequences of astroviruses from sheep and turkey: comparison with related viruses. Virus Res. 2003;91:195–201.
Woode GN, Bridger JC. Isolation of small viruses resembling astroviruses and caliciviruses from acute enteritis of calves. J Med Microbiol. 1978;11:441–52. doi:10.1099/00222615-11-4-441.
Walter JE, Mitchell DK. Astrovirus infection in children. Curr Opin Infect Dis. 2003;16:247–53. doi:10.1097/01.qco.0000073775.11390.60.
Biđin M, Biđin Z, Majnarić D, Tišljar M, Lojkić I. Circulation and phylogenetic relationship of chicken and turkey-origin astroviruses detected in domestic ducks (Anas platyrhynchos domesticus). Avian Pathol J WVPA. 2012;41:555–62. doi:10.1080/03079457.2012.733340.
Ulloa JC, Gutiérrez MF. Genomic analysis of two ORF2 segments of new porcine astrovirus isolates and their close relationship with human astroviruses. Can J Microbiol. 2010;56:569–77. doi:10.1139/w10-042.
Britton RA, Versalovic J. Probiotics and gastrointestinal infections. Interdiscip Perspect Infect Dis. 2008;2008:290769. doi:10.1155/2008/290769.
Colbère-Garapin F, Martin-Latil S, Blondel B, Mousson L, Pelletier I, Autret A, et al. Prevention and treatment of enteric viral infections: possible benefits of probiotic bacteria. Microbes Infect Inst Pasteur. 2007;9:1623–31. doi:10.1016/j.micinf.2007.09.016.
Ganesh BP, Richter JF, Blaut M, Loh G. Enterococcus faecium NCIMB 10415 does not protect interleukin-10 knock-out mice from chronic gut inflammation. Benef Microbes. 2012;3:43–50. doi:10.3920/BM2011.0050.
Sola I, Castilla J, Pintado B, Sánchez-Morgado JM, Whitelaw CB, Clark AJ, et al. Transgenic mice secreting coronavirus neutralizing antibodies into the milk. J Virol. 1998;72:3762–72.
Bruu A-L. Enteroviruses: polioviruses, coxsackieviruses, echoviruses and newer enteroviruses. In: Microbiology LRH of M, Information essor SJRPD of R Analysis and, Whitley essor RJ, editors. A practice guide to Clinical Virology. Wiley; 2002. p. 44–5.
Nguyen NTB, Pham HV, Hoang CQ, Nguyen TM, Nguyen LT, Phan HC, et al. Epidemiological and clinical characteristics of children who died from hand, foot and mouth disease in Vietnam, 2011. BMC Infect Dis. 2014;14:341. doi:10.1186/1471-2334-14-341.
Wang S-M, Liu C-C. Update of enterovirus 71 infection: epidemiology, pathogenesis and vaccine. Expert Rev Anti Infect Ther. 2014;12:447–56. doi:10.1586/14787210.2014.895666.
Sun S, Jiang L, Liang Z, Mao Q, Su W, Zhang H, et al. Evaluation of monovalent and bivalent vaccines against lethal Enterovirus 71 and Coxsackievirus A16 infection in newborn mice. Hum Vaccin Immunother. 2014;10:2885–95. doi:10.4161/hv.29823.
Rosander A, Connolly E, Roos S. Removal of antibiotic resistance gene-carrying plasmids from Lactobacillus reuteri ATCC 55730 and characterization of the resulting daughter strain, L. reuteri DSM 17938. Appl Environ Microbiol. 2008;74:6032–40. doi:10.1128/AEM.00991-08.
Sharma P, Tomar SK, Goswami P, Sangwan V, Singh R. Antibiotic resistance among commercially available probiotics. Food Res Int. 2014;57:176–95. doi:10.1016/j.foodres.2014.01.025.
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
AL KASSAA, I. (2017). Antiviral Probiotics: A New Concept in Medical Sciences. In: New Insights on Antiviral Probiotics. Springer, Cham. https://doi.org/10.1007/978-3-319-49688-7_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-49688-7_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-49687-0
Online ISBN: 978-3-319-49688-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)