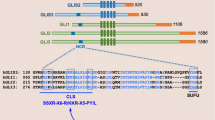
Abstract
The SLC26A4 gene’s 21 exons on chromosome 7q31.1 encode the pendrin (SLC26A4) Cl−/anion exchanger. SLC26A4 is highly expressed in the epithelial cells of kidney, thyroid, inner ear and airways, in which the pendrin polypeptide is essential for normal chloride reabsorption, bicarbonate secretion and iodide accumulation. These activities regulate systemic volume and acid-base status, airway surface liquid composition, thyroid hormone synthesis and endolymph ion balance. SLC26A4 biallelic mutations underlie autosomal recessive Pendred syndrome, a major cause of congenital deafness variably accompanied by euthyroid goiter.
Recent studies of transcriptional regulation of pendrin expression have revealed that SLC26A4 promoter binding sites for thyroid transcription factors 1 and 2 (TTF-1/2) and forkhead box transcription factor I1 (FOXI1) are essential for cell-specific promoter activity in thyroid and inner ear, respectively. Systemic pH, Cl− concentration, aldosterone, uroguanylin (UGN) and interleukins 4 (IL-4) and 13 (IL-13) each modulate pendrin transcription through distinct regulatory elements. The SLC26A4 promoter elements regulated by pH, Cl− and aldosterone remain undefined, but defined binding sites for heat shock factor 1 (HSF1) and signal transducer and activator of transcription protein 6 (STAT6), respectively, mediate transcriptional effects of UGN and IL-4/IL-13 on the SLC26A4 promoter. Recent findings also suggest epigenetic regulation of pendrin gene expression.
Although the detailed molecular mechanisms of pendrin transcriptional and epigenetic regulation remain incomplete, they will provide insight into pendrin roles in acid-base, electrolyte and water homeostasis, blood pressure regulation and airway function. They will, in addition, influence our understanding and potential treatment of hypertension, heart failure, hepatic failure and other fluid retention syndromes, and airway diseases such as asthma and chronic obstructive lung disease.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Adams KM, Abraham V, Cohen N, Kolls JK, Kreindler JL (2013) Pendrin is a participant in antimicrobial responses in the lung. Am J Respir Crit Care Med 187:A4741
Adams KM, Abraham V, Spielman D, Kolls JK, Rubenstein RC, Conner GE, Cohen NA, Kreindler JL (2014) IL-17A induces pendrin expression and chloride-bicarbonate exchange in human bronchial epithelial cells. PLoS One 9:e103263. doi:10.1371/journal.pone.01032632014
Adler L, Efrati E, Zelikovic I (2008) Molecular mechanisms of epithelial cell-specific expression and regulation of the human anion exchanger (pendrin) gene. Am J Physiol Cell Physiol 294:C1261–C1276
Alper SL, Sharma AK (2013) The SLC26 gene family of anion transporters and channels. Mol Aspects Med 34:494–515
Alpern RJ et al (2000) Renal acidification mechanisms. In: Brenner BM, Rector FC (eds) Brenner & Rector’s the kidney. Saunders, Philadelphia, pp 455–519
Alrefai WA, Tyagi S, Mansour F, Saksena S, Syed I, Ramaswamy K, Dudeja PK (2001) Sulfate and chloride transport in Caco-2 cells: differential regulation by thyroxine and the possible role of DRA gene. Am J Physiol Gastrointest Liver Physiol 280:G603–G613
Alrefai WA, Wen X, Jiang W, Katz JP, Steinbrecher KA, Cohen MB, Williams IR, Dudeja PK, Wu GD (2007) Molecular cloning and promoter analysis of downregulated in adenoma (DRA). Am J Physiol Gastrointest Liver Physiol 293:G923–G934
Anckar J, Sistonen L (2007) Heat shock factor 1 as a coordinator of stress and developmental pathways. Adv Exp Med Biol 594:78–88
Anwar S, Riazuddin S, Ahmed ZM, Tasneem S, Ateeq Ul J, Khan SY, Griffith AJ, Friedman TB, Riazuddin S (2009) SLC26A4 mutation spectrum associated with DFNB4 deafness and Pendred’s syndrome in Pakistanis. J Hum Genet 54:266–270
Arturi F, Russo D, Bidart JM, Scarpelli D, Schlumberger M, Filetti S (2001) Expression pattern of the pendrin and sodium/iodide symporter genes in human thyroid carcinoma cell lines and human thyroid tumors. Eur J Endocrinol 145:129–135
Bidart JM, Lacroix L, Evain-Brion D, Caillou B, Lazar V, Frydman R, Bellet D, Filetti S, Schlumberger M (2000a) Expression of Na+/I- symporter and Pendred syndrome genes in trophoblast cells. J Clin Endocrinol Metab 85:4367–4372
Bidart JM, Mian C, Lazar V, Russo D, Filetti S, Caillou B, Schlumberger M (2000b) Expression of pendrin and the Pendred syndrome (PDS) gene in human thyroid tissues. J Clin Endocrinol Metab 85:2028–2033
Blomqvist SR, Vidarsson H, Fitzgerald S, Johansson BR, Ollerstam A, Brown R, Persson AEG, Bergstrom G, Enerback S (2004) Distal renal tubular acidosis in mice that lack the forkhead transcription factor Foxi1. J Clin Invest 113:1560–1570
Campbell RM, Tummino PJ (2014) Cancer epigenetics drug discovery and development: the challenge of hitting the mark. J Clin Invest 124:64–69
Campbell C, Cucci RA, Prasad S, Green GE, Edeal JB, Galer CE, Karniski LP, Sheffield VC, Smith RJ (2001) Pendred syndrome, DFNB4, and PDS/SLC26A4 identification of eight novel mutations and possible genotype-phenotype correlations. Hum Mutat 17:403–411
Carlin RW, Sedlacek RL, Quesnell RR, Pierucci-Alves F, Grieger DM, Schultz BD (2006) PVD9902, a porcine vas deferens epithelial cell line that exhibits neurotransmitter-stimulated anion secretion and expresses numerous HCO3(-) transporters. Am J Physiol Cell Physiol 290:C1560–C1571
Carrithers SL, Ott CE, Hill MJ, Johnson BR, Cai W, Chang JJ, Shah RG, Sun C, Mann EA, Fonteles MC, Forte LR, Jackson BA, Giannella RA, Greenberg RN (2004) Guanylin and uroguanylin induce natriuresis in mice lacking guanylyl cyclase-C receptor. Kidney Int 65:40–53
Catalano MG, Fortunati N, Boccuzzi G (2012) Epigentics modifications and therapeutic prospects in human thyroid cancer. Front Endocrinol 3:1–8
Cetin Y, Kulaksiz H, Redecker P, Bargsten G, Adermann K, Grube D (1995) Bronchiolar nonciliated secretory (Clara) cells: source of guanylin in the mammalian lung. Proc Natl Acad Sci U S A 92:5925–5929
Choi BY, Stewart AK, Madeo AC, Pryor SP, Lenhard S, Kittles R, Eisenman D, Kim HJ, Niparko J, Thomsen J, Arnos KS, Nance WE, King KA, Zalewski CK, Brewer CC, Shawker T, Reynolds JC, Butman JA, Karniski LP, Alper SL, Griffith AJ (2009a) Hypo-functional SLC26A4 variants associated with nonsyndromic hearing loss and enlargement of the vestibular aqueduct: genotype-phenotype correlation or coincidental polymorphisms? Hum Mutat 30:599–608
Choi BY, Stewart AK, Nishimura KK, Cha WJ, Seong MW, Park SS, Kim SW, Chun YS, Chung JW, Park SN, Chang SO, Kim CS, Alper SL, Griffith AJ, Oh SH (2009b) Efficient molecular genetic diagnosis of enlarged vestibular aqueducts in East Asians. Genet Test Mol Biomarkers 13:679–687
Choi BY, Kim HM, Ito T, Lee KY, Li X, Monahan K, Wen Y, Wilson E, Kurima K, Saunders T, Petralia RS, Wangemann P, Friedman TB, Griffith A (2011) Mouse model of enlarged vestibular aqueducts defines temporal requirement of Slc26a4 expression for hearing acquisition. J Clin Invest 121:4516–4525
Comrie MM, Cutler CP, Cramb G (2001) Cloning and expression of guanylin from the European eel (Anguilla anguilla). Biochem Biophys Res Commun 281:1078–1085
Dai P, Stewart AK, Chebib F, Hsu A, Rozenfeld J, Huang D, Kang D, Lip V, Fang H, Shao H, Liu X, Shen Y, Yang W, Zelikovic I, Platt OS, Han D, Alper SL, Wu BL (2009) Distinct and novel SLC26A4/Pendrin mutations in Chinese and U.S. patients with non-syndromic hearing loss. Physiol Genomics 38:281–290
Das S, Bhattacharyya NP (2014) Transcription regulation of HYPK by heat shock factor 1. PLoS One 9(1):e85552. doi:10.1371/journal.pone.0085552
Dentice M, Luongo C, Elefante A, Ambrosio R, Salzano S, Zannini M, Nitsch R, Di Lauro R, Rossi G, Fenzi G, Salvatore D (2005) Pendrin is a novel in vivo downstream target gene of the TTF-1/Nkx-2.1 homeodomain transcription factor in differentiated thyroid cells. Mol Cell Biol 25:10171–10182
Dossena S, Rodighiero S, Vezzoli V, Nofziger C, Salvioni E, Boccazzi M, Grabmayer E, Botta G, Meyer G, Fugazzola L, Peccoz PB, Paulmichl M (2009) Functional characterization of wild-type and mutated pendrin (SLC26A4), the anion transporter involved in pendred syndrome. J Mol Endocrinol 43:93–103
Dou H, Xu J, Wang Z, Smith AN, Soleimani M, Karet FE, Greinwald JH Jr, Choo D (2004) Co-expression of pendrin, vacuolar H-ATPase alpha4- subunit and carbonic anhydrase II in epithelial cells of the murine endolymphatic sac. J Histochem Cytochem 52:1377–1384
Efrati E, Adler L, Tal O, Zelikovic I (2007) The pendrin gene, PDS, is transcriptionally regulated by ambient pH and chloride. J Am Soc Nephrol 8:6A
Ehret GB, Reichenbach P, Schindler U, Horvath CM, Fritz S, Nabholz M, Bucher P (2001) DNA binding specificity of different STAT proteins. Comparison of in vitro specificity with natural target sites. J Biol Chem 276:6675–6688
Eladari D, Chambrey R, Peti-Peterdi J (2012) A new look at electrolyte transport in the distal tubule. Annu Rev Physiol 74:325–349
Espiritu DJ, Bernardo AA, Robey RB, Arruda JA (2002) A central role for Pyk2-Src interaction in coupling diverse stimuli to increased epithelial NBC activity. Am J Physiol Renal Physiol 283:F663–F670
Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, Adawi F, Hazani E, Nassir E, Baxevanis AD, Sheffield VC, Green ED (1997) Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nat Genet 17:411–422
Everett LA, Morsli H, Wu DK, Green ED (1999) Expression pattern of the mouse ortholog of the Pendred’s syndrome gene (Pds) suggests a key role for pendrin in the inner ear. Proc Natl Acad Sci U S A 96:9727–9732
Fernandez-Lazo Y, Aguilera G, Pham TD, Park AY, Beierwalters WH, Sutliff RL, Verlander JW, Pacack K, Osunkoya AO, Ellis CL, Kim YH, Shipley GL, Wynne BM, Hoover RS, Sen SK, Plotsky PM, Wall SM (2015) Pendrin localizes to the adrenal medulla and modulates catecholamine release. Am J Physiol Endocrinol Metab 309(6):E534–E545. doi:10.1152/ajpendo.00035.2015 [Epub ahead of print]
Fonteles MC, Falcao do Nascimento NR (2011) Guanylin peptide family: history, interaction with ANP, and new pharmacological perspectives. Can J Physiol Pharmacol 89:575–585
Fonteles MC, Greenberg RN, Monteiro HS, Currie MG, Forte LR (1998) Natriuretic and kaliuretic activities of guanylin and uroguanylin in the isolated perfused rat kidney. Am J Physiol Renal Physiol 275:F191–F197
Forte LR (2003) A novel role for uroguanylin in the regulation of sodium balance. J Clin Invest 112:1138–1141
Forte LR (2004) Uroguanylin and guanylin peptides: pharmacology and experimental therapeutics. Pharmacol Ther 104:137–167
Forte LR, Fan X, Hamra FK (1996) Salt and water homeostasis: uroguanylin is a circulating peptide hormone with natriuretic activity. Am J Kidney Dis 28:296–304
Frische S, Kwon TH, Frokiaer J, Madsen KM, Nielsen S (2003) Regulated expression of pendrin in rat kidney in response to chronic NH4Cl or NaHCO3 loading. Am J Physiol Renal Physiol 284:F584–F593
Fruhbeck G (2012) Uroguanylin-a new gut-derived weapon against obesity? Nat Rev Endocrinol 8:5–6
Fukae H, Kinoshita H, Fujimoto S, Nakazato M, Eto T (2000) Plasma concentration of uroguanylin in patients on maintenance dialysis therapy. Nephron 84:206–210
Garnett JP, Hickman E, Burrows R, Hegyi P, Tiszlavicz L, Cuthbert AW, Fong P, Gray MA (2011) Novel role for pendrin in orchestrating bicarbonate secretion in cystic fibrosis transmembrane conductance regulator (CFTR)-expressing airway serous cells. J Biol Chem 286:41069–41082
Gennari FJ, Maddox DA (2000) Renal regulation of acid-base homeostasis integrated response. In: Seldin DW, Giebisch GH (eds) The kidney: physiology and pathophysiology, 3rd edn. Lippincott Williams & Wilkins, Philadelphia, pp 2015–2053
Gluck SL (2004) Acid sensing in renal epithelial cells. J Clin Invest 114:1696–1699
Gorbunov D, Sturlese M, Nies F, Kluge M, Bellanda M, Battistutta R, Oliver D (2014) Molecular architecture and the structural basis for anion interaction in prestin and SLC26 transporters. Nat Commun 5:3622. doi:10.1038/ncomms4622
Griffith AJ, Wangemann P (2011) Hearing loss associated with enlargement of the vestibular aqueduct: mechanistic insights from clinical phenotypes, genotypes, and mouse models. Hear Res 281:11–17
Grimm PR, Lazo-Fernandez Y, Delpire E, Wall SM, Dorsey SG, Weinman EJ, Coleman R, Wade JB, Welling PA (2015) Integrated compensatory network is activated in the absence of NCC phosphorylation. J Clin Invest 125:2136–2150
Haila S, Höglund P, Scherer SW, Lee JR, Kristo P, Coyle B, Trembath R, Holmberg C, de la Chapelle A, Kere J (1998) Genomic structure of the human congenital chloride diarrhea (CLD) gene. Gene 214:87–93
Hamm LL, Nakhoul NL (2008) Renal acidification. In: Brenner BM (ed) Brenner & Rector’s the kidney, 8th edn. Saunders, Philadelphia, pp 248–279
He L, Hannon GJ (2004) MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 5:522–531
Huang J, Shan J, Kim D, Liao J, Evagelidis A, Alper SL, Hanrahan JW (2012) Basolateral chloride loading by the anion exchanger type 2: role in fluid secretion by the human airway epithelial cell line calu-3. J Physiol 590:5299–5316
Hulander M, Kiernan AE, Blomqvist SR, Carlsson P, Samuelsson EJ, Johansson BR, Steel KP, Enerback S (2003) Lack of pendrin expression leads to deafness and expansion of the endolymphatic compartment in inner ears of Foxi1 null mutant mice. Development 130:2013–2025
Ishida A, Ohta N, Suzuki Y, Kakehata S, Okubo K, Ikeda H, Shiraishi H, Izuhara K (2012) Expression of pendrin and periostin in allergic rhinitis and chronic rhinosinusitis. Allergol Int 61:589–595
Jacques T, Picard N, Miller RL, Riemondy KA, Houillier P, Sohet F, Ramakrishnan SK, Busst CJ, Jayat M, Corniere N, Hassan H, Aronson PS, Hennings JC, Hubner CA, Nelson RD, Chambrey R, Eladari D (2013) Overexpression of pendrin in intercalated cells produces chloride-sensitive hypertension. J Am Soc Nephrol 24:1104–1113
Johnsen T, Larsen C, Friis J, Hougaard-Jensen F (1987) Pendred’s syndrome. Acoustic, vestibular and radiological findings in 17 unrelated patients. J Laryngol Otol 101:1187–1192
Kandasamy N, Fugazzola L, Evans M, Chatterjee K, Karet F (2011) Life-threatening alkalosis in Pendred syndrome. Eur J Endocrinol 165:167–170
Kikuchi M, Fujimoto S, Fukae H, Kinoshita H, Kita T, Nakazato M, Eto T (2005) Role of uroguanylin, a peptide with natriuretic activity, in rats with experimental nephrotic syndrome. J Am Soc Nephrol 16:392–397
Kim YH, Pech V, Spencer KB, Beierwaltes WH, Everett LA, Green ED, Shin W, Verlander JW, Sutliff RL, Wall SM (2007) Reduced ENaC protein abundance contributes to the lower blood pressure observed in pendrin-null mice. Am J Physiol Renal Physiol 293:F1314–F1324
Kim YH, Pham TD, Zheng W, Hong S, Baylis C, Pech V, Beierwaltes WH, Farley DB, Braverman LE, Verlander JW, Wall SM (2009) Role of pendrin in iodide balance: going with the flow. Am J Physiol Renal Physiol 297:F1069–F1079
Kim D, Kim J, Burghardt B, Best L, Steward MC (2014) Role of anion exchangers in Cl- and HCO3 - secretion by the human airway epithelial cell line Calu-3. Am J Physiol Cell Physiol 307:C208–C219
Kinoshita H, Fujimoto S, Nakazato M, Yokota N, Date Y, Yamaguchi H, Hisanaga S, Eto T (1997) Urine and plasma levels of uroguanylin and its molecular forms in renal diseases. Kidney Int 52:1028–1034
Kita T, Smith CE, Fok KF, Duffin KL, Moore WM, Karabatsos PJ, Kachur JF, Hamra FK, Pidhorodeckyj NV, Forte LR, Currie MG (1994) Characterization of human uroguanylin: a member of the guanylin peptide family. Am J Physiol Ren Fluid Electrolyte Physiol 266:F342–F348
Kita T, Kitamura K, Sakata J, Eto T (1999) Marked increase of guanylin secretion in response to salt loading in the rat small intestine. Am J Physiol Gastrointest Liver Physiol 277:G960–G966
Kitazono M, Robey R, Zhan Z, Sarlis NJ, Skarulis MC, Aikou T, Bates S, Fojo T (2001) Low concentrations of the histone deacetylase inhibitor, depsipeptide (FR901228), increase expression of the Na(+)/I(-) symporter and iodine accumulation in poorly differentiated thyroid carcinoma cells. J Clin Endocrinol Metab 86:3430–3435
Knepper MA (2015) Systems biology of diuretic resistance. J Clin Invest 125:1793–1795
Kobayashi T, Sugimoto T, Saijoh K, Fujii M, Chihara K (1997) Cloning and characterization of the 5’-flanking region of the mouse diastrophic dysplasia sulfate transporter gene. Biochem Biophys Res Commun 238(3):738–743
Kopp P (2000) Pendred’s syndrome and genetic defects in thyroid hormone synthesis. Rev Endocr Metab Disord 1:109–121
Krause WJ, Freeman RH, Forte LR (1990) Autoradiographic demonstration of specific binding sites for E. coli enterotoxin in various epithelia of the North American opossum. Cell Tissue Res 260:387–394
Kulaksiz H, Schmid A, Honscheid M, Ramaswamy A, Certin Y (2002) Clara cell impact in air-side activation of CFTR in small pulmonary airways. Proc Natl Acad Sci U S A 99:6796–6801
Kuperman DA, Lewis CC, Woodruff PG, Rodriguez MW, Yang YH, Dolganov GM, Fahy JV, Erle DJ (2005) Dissecting asthma using focused transgenic modeling and functional genomics. J Allergy Clin Immunol 116:305–311
Lacroix L, Michiels S, Mian C, Arturi F, Caillou B, Filetti S, Schlumberger M, Bidart JM (2006) HEX, PAX-8 and TTF-1 gene expression in human thyroid tissues: a comparative analysis with other genes involved in iodide metabolism. Clin Endocrinol (Oxf) 64:398–404
Lee A, Nofziger C, Dossena S, Vanoni S, Diasio R, Paulmichl M (2011) Methylation of the human pendrin promoter. Cell Physiol Biochem 28:397–406
Lee HJ, Yoo JE, Namkung W, Cho HJ, Kim K, Kang JW, Yoon JH, Choi JY (2015) Thick airway surface liquid volume and weak mucin expression in pendrin-deficient human airway epithelia. Physiol Rep 3(8):e12840. doi:10.14814/phy2.12480
Leviel F, Hubner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hatim H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D (2010) The Na + -dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest 120:1627–1635
Li W, Lee J, Vikis HG, Lee SH, Liu G, Aurandt J, Shen TL, Fearon ER, Guan JL, Han M, Rao Y, Hong K, Guan KL (2004) Activation of FAK and Src are receptor-proximal events required for netrin signaling. Nat Neurosci 7:1213–1221
Madeo AC, Manichaikul A, Pryor SP, Griffith AJ (2009) Do mutations of the pendred syndrome gene, SLC26A4, confer resistance to asthma and hypertension? J Med Genet 46:405–406
McCallum L, Lip S, Padmanabhan S (2015) The hidden hand of chloride in hypertension. Pflugers Arch Eur J Physiol 467:595–603
Mikita T, Campbell D, Wu P, Williamson K, Schindler U (1996) Requirements for interleukin-4-induced gene expression and functional characterization of Stat6. Mol Cell Biol 16:5811–5820
Mohebbi N, Perna A, van der Wijst J, Becker HM, Cappaso G, Wagner CA (2013) Regulation of two renal chloride transporters, AE1 and pendrin, by electrolytes and aldosterone. PLoS One 8(1):e55286. doi:10.1371/journal.pone.0055286
Morimoto RI (2008) Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev 22:1427–1438
Morimoto RI, Santoro MG (1998) Stress-inducible responses and heat shock proteins: new pharmacologic targets for cytoprotection. Nat Biotechnol 16:833–838
Moss NG, Fellner RC, Qian X, Yu SJ, Li Z, Nakazato M, Goy MF (2008) Uroguanylin, an intestinal natriuretic peptide, is delivered to the kidney as an unprocessed propeptide. Endocrinology 149:4486–4498
Mueller T, Dieplinger B (2012) The guanylin peptide family and proposed gastrointestinal-renal natriuretic signaling axis. Kidney Int 82:1253–1255
Muscella A, Verri SM, Urso L, Dimitri C, Botta G, Paulmichl M, Beck-Peccoz P, Fugazzola L, Storelli C (2008) PKC-ε-dependent cytosol-to-membrane translocation of pendrin in rat thyroid PC Cl3 cells. J Cell Physiol 217:103–112
Nakagami Y, Favoreto S Jr, Zhen G, Park SW, Nguyenvu LT, Kuperman DA, Dolganov GM, Huang X, Boushey HA, Avila PC, Erle DJ (2008) The epithelial anion transporter pendrin is induced by allergy and rhinovirus infection, regulates airway surface liquid, and increases airway reactivity and inflammation in an asthma model. J Immunol 181:2203–2210
Nakao I, Kanaji S, Ohta S, Matsushita H, Arima K, Yuyama N, Yamaya M, Nakayama K, Kubo H, Watanabe M, Sagara H, Sugiyama K, Tanaka H, Toda S, Hayashi H, Inoue H, Hoshino T, Shiraki A, Inoue M, Suzuki K, Aizawa H, Okinami S, Nagai H, Hasegawa M, Fukuda T, Green ED, Izuhara K (2008) Identification of pendrin as a common mediator for mucus production in bronchial asthma and chronic obstructive pulmonary disease. J Immunol 180:6262–6269
Nakaya K, Harbidge DG, Wangemann P, Schultz BD, Green ED, Wall SM, Marcus DC (2007) Lack of pendrin HCO3 - transport elevates vestibular endolymphatic [Ca2+] by inhibition of acid-sensitive TRPV5 and TRPV6 channels. Am J Physiol Renal Physiol 292:F1314–F1321
Nakazato M, Yamaguchi H, Shiomi K, Date Y, Fujimoto S, Kangawa K, Matsuo H, Matsukura S (1994) Identification of 10-kDa proguanylin as a major guanylin molecule in human intestine and plasma and its increase in renal insufficiency. Biochem Biophys Res Commun 205:1966–1975
Nofziger C, Vezzoli V, Dossena S, Schonherr T, Studnicka J, Nofziger J, Vanoni S, Stephan S, Silva ME, Meyer G, Paulmichl M (2011) STAT6 links IL-4/IL-13 stimulation with pendrin expression in asthma and chronic obstructive pulmonary disease. Clin Pharmacol Ther 90:399–405
Ohbayashi K, Yamaki KI (2000) Both inhalant and intravenous uroguanylin inhibit leukotriene C 4-induced airway changes. Peptides 21:1467–1472
Ohbayashi K, Yamaki K, Suzuki R, Takagi K (1998) Effects of uroguanylin and guanylin against antigen-induced bronchoconstriction and airway microvascular leakage in sensitized guinea pigs. Life Sci 62:1833–1844
Park HJ, Shaukat S, Liu XZ, Hahn SH, Naz S, Ghosh M, Kim HN, Moon SK, Abe S, Tukamoto K, Riazuddin S, Kabra M, Erdenetungalag R, Radnaabazar J, Khan S, Pandya A, Usami SI, Nance WE, Wilcox ER, Riazuddin S, Griffith AJ (2003) Origins and frequencies of SLC26A4 (PDS) mutations in east and south Asians: global implications for the epidemiology of deafness. J Med Genet 40:242–248
Pech V, Kim HY, Weinstein MA, Everett AL, Pham DT, Wall MS (2007) Angiotensin II increases chloride absorption in the cortical collecting duct in mice through a pendrin-dependent mechanism. Am J Physiol Renal Physiol 292:F914–F920
Pech V, Pham TD, Hong S, Weinstein AM, Spencer KB, Duke BJ, Walp E, Kim YH, Sutliff RL, Bao HF, Eaton DC, Wall SM (2010) Pendrin modulates ENaC function by changing luminal HCO3 -. J Am Soc Nephrol 21:1928–1941
Pedemonte N, Caci E, Sondo E, Caputo A, Rhoden K, Pfeffer U, Di Candia M, Bandettini R, Ravazzolo R, Zegarra-Moran O, Galietta LJ (2007) Thiocyanate transport in resting and IL-4- stimulated human bronchial epithelial cells: role of pendrin and anion channels. J Immunol 178:5144–5153
Pela I, Bigozzi M, Bianchi B (2008) Profound hypokalemia and hypochloremic metabolic alkalosis during thiazide therapy in a child with Pendred syndrome. Clin Nephrol 69:450–453
Petrovic S, Wang Z, Ma L, Soleimani M (2003) Regulation of the apical Cl-/HCO3- exchanger pendrin in rat cortical collecting duct in metabolic acidosis. Am J Physiol Renal Physiol 284:F103–F112
Piala AT, Moon TM, Akella R, He H, Cobb MH, Goldsmith EJ (2014) Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal 7(324):ra41. doi:10.1126/scisignal.2005050
Porra V, Bernier-Valentin F, Trouttet-Masson S, Berger-Dutrieux N, Peix JL, Perrin A, Selmi-Ruby S, Rousset B (2002) Characterization and semiquantitative analyses of pendrin expressed in normal and tumoral human thyroid tissues. J Clin Endocrinol Metab 87:1700–1707
Potthast R, Ehler E, Scheving LA, Sindic A, Schlatter E, Kuhn M (2001) High salt intake increases uroguanylin expression in mouse kidney. Endocrinology 142:3087–3097
Priyamvada S, Anbazhagan AN, Gujral T, Borthakur A, Saksena S, Gil RK, Alrefai WA, Dudjena PK (2015) All-trans-retinoic acid increases SLC26A3 DRA (down-regulated in adenoma) expression in intestinal epithelial cells via HNF-1β. J Biol Chem 290:15066–15077
Qian X, Moss NG, Fellner RC, Goy MF (2008) Circulating prouroguanylin is processed to its active natriuretic form exclusively within the renal tubules. Endocrinology 149:4499–4509
Qian X, Moss NC, Fellner RC, Taylor-Blake B, Goy MF (2011) The rat kidney contains high levels of prouroguanylin (the uroguanylin precursor) but does not express GC-C (the enteric uroguanylin receptor). Am J Physiol Renal Physiol 300:F561–F573
Quentin F, Chambrey R, Trinh-Trang-Tan MM, Fysekidis M, Cambillau M, Paillard M, Aronson PS, Eladari D (2004) The Cl-/HCO3- exchanger pendrin in the rat kidney is regulated in response to chronic alterations in chloride balance. Am J Physiol Renal Physiol 287:F1179–F1188
Raheja G, Singh V, Ma K, Boumendjel R, Borthakur A, Gill RK, Saksena S, Alrefai WA, Ramaswamy K, Dudeja PK (2010) Lactobacillus acidophilus stimulates the expression of SLC26A3 via a transcriptional mechanism. Am J Physiol Gastrointest Liver Physiol 298:G395–G401
Reardon W, Trembath RC (1996) Pendred syndrome. J Med Genet 33:1037–1040
Reimold FR, Heneghan JF, Stewart AK, Zelikovic I, Vandorpe DH, Shmukler BE, Alper SL (2011) Pendrin function and regulation in Xenopus oocytes. Cell Physiol Biochem 28:435–440
Rillema JA, Hill MA (2003) Prolactin regulation of the pendrin-iodide transporter in the mammary gland. Am J Physiol Endocrinol Metab 284:E25–E28
Rothbart SB, Strahl BD (2014) Interpreting the language of histone and DNA modifications. Biochim Biophys Acta 1839:627–643
Royaux IE, Suzuki K, Mori A, Katoh R, Everett LA, Kohn LD, Green ED (2000) Pendrin, the protein encoded by the Pendred syndrome gene (PDS), is an apical porter of iodide in the thyroid and is regulated by thyroglobulin in FRTL-5 cells. Endocrinology 141:839–845
Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED (2001) Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci U S A 98:4221–4226
Rozenfeld J, Efrati E, Adler L, Tal O, Carrithers SL, Alper SL, Zelikovic I (2011) Transcriptional regulation of the pendrin gene. Cell Physiol Biochem 28:385–396
Rozenfeld J, Tal O, Kladnitsky O, Adler L, Efrati E, Carrithers SL, Alper SL, Zelikovic I (2012) The pendrin anion exchanger gene is transcriptionally regulated by uroguanylin: a novel enterorenal link. Am J Physiol Renal Physiol 302:F614–F624
Rozenfeld J, Tal O, Kladnitsky O, Adler L, Efrati E, Carrithers SL, Alper SL, Zelikovic I (2013) Pendrin, a novel transcriptional target of the uroguanylin system. Cell Physiol Biochem 32(Suppl 1):221–237
Saksena S, Singla A, Goyal S, Katyal S, Bansal N, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK (2010) Mechanisms of transcriptional modulation of the human anion exchanger SLC26A3 gene expression by IFN-{gamma}. Am J Physiol Gastrointest Liver Physiol 298:G159–G166
Scanlon KM, Gau Y, Zhu J, Skerry C, Wall SM, Soleimani M, Carbonetti NH (2014) Epithelial anion transporter pendrin contributes to inflammatory lung pathology in mouse models of Bordetella pertussis infection. Infect Immun 82:4212–4221
Schwartz GJ (2001) Plasticity of intercalated cell polarity: effect of metabolic acidosis. Nephron 87:304–313
Scott DA, Wang R, Kreman TM, Sheffield VC, Karniski LP (1999) The Pendred syndrome gene encodes a chloride-iodide transport protein. Nat Genet 21:440–443
Shan J, Liao J, Robert R, Palmer ML, Fahrenkrug SC, O’Grady SM, Hanrahan JW (2012) Bicarbonate-dependent chloride transport drives fluid secretion by the human airway epithelial cell line Calu-3. J Physiol 590:5273–5297
Sharma S, Kelly TK, Jones PA (2010) Epigenetics in cancer. Carcinogenesis 31:27–36
Shcheynikov N, Yang D, Wang Y, Zeng W, Karniski LP, So I, Wall SM, Muallem S (2008) The Slc26a4 transporter functions as an electroneutral Cl-/I-/HCO3 - exchanger: role of Slc26a4 and Slc26a6 in I- and HCO3 - secretion and in regulation of CFTR in the parotid duct. J Physiol 586:3813–3824
Sheffield VC, Kraiem Z, Beck JC, Nishimura D, Stone EM, Salameh M, Sadeh O, Glaser B (1996) Pendred syndrome maps to chromosome 7q21-34 and is caused by an intrinsic defect in thyroid iodine organification. Nat Genet 12:424–426
Shelden MC, Howitt SM, Price GD (2010) Membrane topology of the cyanobacterial bicarbonate transporter, BicA, a member of the SulP (SLC26A) family. Mol Membr Biol 27:12–23
Sindic A, Schlatter E (2006) Cellular effects of guanylin and uroguanylin. J Am Soc Nephrol 17:607–616
Sindic A, Schlatter E (2007) Renal electrolyte effects of guanylin and uroguanylin. Curr Opin Nephrol Hypertens 16:10–15
Sindic A, Velic A, Basoglu C, Hirsch JR, Edemir B, Kuhn M, Schlatter E (2005) Uroguanylin and guanylin regulate transport of mouse cortical collecting duct independent of guanylate cyclase C. Kidney Int 68:1008–1017
Singla A, Kumar A, Priyamvada S, Tahniyath M, Saksena S, Gill RK, Alrefai WA, Dudeja K (2012) LPA stimulates intestinal DRA gene transcription via LPA2 receptor, PI3K/AKT, and c-Fos-dependent pathway. Am J Physiol Gastrointest Liver Physiol 302:G618–G627
Soleimani M, Xu J (2006) SLC26 chloride/base exchangers in the kidney in health and disease. Sem Nephrol 26:375–385
Soleimani M, Barone S, Xu J, Shull GF, Siddiqui F, Zahedi K, Amlal H (2012) Double knockout of pendrin and Na-Cl cotransporter (NCC) causes severe salt wasting, volume depletion, and renal failure. Proc Natl Acad Sci U S A 109:13368–13373
Suzuki K, Royaux IE, Everett LA, Mori-Aoki A, Suzuki S, Nakamura K, Sakai T, Katoh R, Toda S, Green ED, Kohn LD (2002) Expression of PDS/Pds, the Pendred syndrome gene, in endometrium. J Clin Endocrinol Metab 87:938–941
Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A (2009) Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324:930–935
Vallet M, Picard N, Loffing-Cueni D, Fysekidis M, Bloch-Faure M, Deschênes G, Breton S, Meneton P, Loffing J, Aronson PS, Chambrey R, Eladari D (2006) Pendrin regulation in mouse kidney primarily is chloride-dependent. J Am Soc Nephrol 17:2153–2163
Vanoni S, Nofziger C, Dossena S, Soyal SM, Patsch W, Plevani P, Duschl A, Paulmichl M (2013) The human pendrin promoter contains two N4GAS motifs with different functional relevance. Cell Physiol Biochem 32:238–248
Verlander JW, Hassell KA, Royaux IE, Glapion DM, Wang ME, Everett LA, Green ED, Wall SM (2003) Deoxycorticosterone upregulates PDS (Slc26a4) in mouse kidney: role of pendrin in mineralocorticoid-induced hypertension. Hypertension 42:356–362
Verlander JW, Kim YH, Shin W, Pham TD, Hassell KA, Beierwaltes WH, Green ED, Everett L, Matthews SW, Wall SM (2006) Dietary Cl- restriction upregulates pendrin expression within the apical plasma membrane of type B intercalated cells. Am J Physiol Renal Physiol 291:F833–F839
Verlander JW, Hong S, Pech V, Bailey JL, Agazatian D, Matthews SW, Coffman TM, Le T, Inagami T, Whitehill FM, Weiner ID, Farley DB, Kim YH, Wall SM (2011) Angiotensin II acts through the angiotensin 1a receptor to upregulate pendrin. Am J Physiol Renal Physiol 301:F1314–F1325
Vihervaara A, Sergelius C, Vasara J, Blom MA, Elsing AN, Roos-Mattjus P, Sistonen L (2013) Transcriptional response to stress in the dynamic chromatin environment of cycling and mitotic cells. Proc Natl Acad Sci U S A 110:E3388–E3397
Wagner CA, Finberg KE, Stehberger PA, Lifton RP, Giebisch GH, Aronson PS, Geibel JP (2002) Regulation of the expression of the Cl-/anion exchanger pendrin in mouse kidney by acid-base status. Kidney Int 62:2109–2117
Wall SM, Lazo-Fernandez Y (2015) The role of pendrin in renal physiology. Annu Rev Physiol 77:363–378
Wall SM, Hassell KA, Royaux IE, Green ED, Chang JY, Shipley GL, Verlander JW (2003) Localization of pendrin in mouse kidney. Am J Physiol Renal Physiol 284(1):F229–F241
Wangemann P (2011) The role of pendrin in the development of the murine inner ear. Cell Physiol Biochem 28:527–534
West AC, Johnstone RW (2014) New and emerging HDAC inhibitors for cancer treatment. J Clin Invest 124:30–39
Winter H, Braig C, Zimmermann U, Geisler HS, Fränzer JT, Weber T, Ley M, Engel J, Knirsch M, Bauer K, Christ S, Walsh EJ, McGee J, Köpschall I, Rohbock K, Knipper M (2006) Thyroid hormone receptors TRalpha1 and TRbeta differentially regulate gene expression of Kcnq4 and prestin during final differentiation of outer hair cells. J Cell Sci 119:2975–2984
Wu C, Morris JR (2001) Genes, genetics, and epigenetics: a correspondence. Science 293:1103–1105
Xing M, Tokumaru Y, Wu G, Westra WB, Ladenson PW, Sidransky D (2003) Hypermethylation of the Pendred syndrome gene SLC26A4 is an early event in thyroid tumorigenesis. Cancer Res 63:2312–2315
Yang T, Vidarsson H, Rodrigo-Blomqvist S, Rosengren SS, Enerback S, Smith RJ (2007) Transcriptional control of SLC26A4 is involved in Pendred syndrome and nonsyndromic enlargement of vestibular aqueduct (DFNB4). Am J Hum Genet 80:1055–1063
Yoshida A, Taniguchi S, Hisatome I, Royaux IE, Green ED, Kohn LD, Suzuki K (2002) Pendrin is an iodide-specific apical porter responsible for iodide efflux from thyroid cells. J Clin Endocrinol Metab 87:3356–3361
Yoshida A, Hisatome I, Taniguchi S, Sasaki N, Yamamoto Y, Miake J, Fukui H, Shimizu H, Okamura T, Okura T, Igawa O, Shigemasa C, Green ED, Kohn LD, Suzuki K (2004) Mechanism of iodide/chloride exchange by pendrin. Endocrinology 145:4301–4308
Yuge S, Inoue K, Hyodo S, Takei Y (2003) A novel guanylin family (guanylin, uroguanylin, and renoguanylin) in eels: possible osmoregulatory hormones in intestine and kidney. J Biol Chem 278:22726–22733
Yusa A, Miyazaki K, Kimura N, Izawa M, Kannagi R (2010) Epigenetic silencing of the sulfate transporter gene DTDST induces sialyl Lewisx expression and accelerates proliferation of colon cancer cells. Cancer Res 70:4064–4073
Zarnegar R, Brunaud L, Kanauchi H, Wong M, Fung M, Ginzinger D, Duh QY, Clark OH (2002) Increasing the effectiveness of radioactive iodine therapy in the treatment of thyroid cancer using Trichostatin A, a histone deacetylase inhibitor. Surgery 132:984–990; discussion 990
Zhang ZH, Jow F, Numann R, Hinson J (1998) The airway epithelium: a novel site of action by guanylin. Biochem Biophys Res Commun 244:50–56
Acknowledgments
J. Rozenfeld and O. Kladnitsky were supported by donations from Dora and Sidney Gabrel and Martin Kolinsky, respectively, London, UK, and by the L & A Kessler Family Foundation, Berkeley, CA, USA; SL Alper was supported by NIH grants DK43495 and DK34854 (Harvard Digestive Disease Center) and by the USA-Israel Binational Science Foundation; I. Zelikovic was supported by the USA-Israel Binational Science Foundation, by the Rappaport Institute for Research in the Medical Sciences and by the Dr. Y. Rabinovitz Research Fund, Technion – Israel Institute of Technology.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Rozenfeld, J., Nofziger, C., Kladnitsky, O., Alper, S.L., Zelikovic, I. (2017). Transcriptional Regulation and Epigenetics of Pendrin. In: Dossena, S., Paulmichl, M. (eds) The Role of Pendrin in Health and Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-43287-8_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-43287-8_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-43285-4
Online ISBN: 978-3-319-43287-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)