Abstract
Depression is a highly prevalent complex neuropsychiatric disorder, which ranks first among all mental and neurological disorders as a contributor to the global burden of disease. However, available treatments are still far from ideal, for their specificity as well as their efficacy. This situation can now be improved by the increasing availability of stem cells, which allows the development of in vitro human neural systems to model the brain. These models complement observations from animal models and patients with depression, allowing for a better understanding of the complexity of this psychiatric illness and potential treatments. Cells derived from the olfactory neuroepithelium, multipotent fetal hippocampal progenitor cells (HPCs) and human induced pluripotent stem cells (iPSCs) have shown promising leads. Using HPCs and iPSC-derived forebrain neurons, we managed to provide further insights into the action of drugs with antidepressant action as well as on molecular mechanisms underlying the effect of stress and inflammation, both linked to the pathophysiology of depression. Particular attention has been paid to the complex pathways by which the immune and stress systems differently determine the final developmental fate of HPCs and the synaptic plasticity of iPSCs. The combination of accessibility and validity of the available stem cells models will allow further work to increase our insights into the biology of depression and support the identification of novel therapeutic targets.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Neural Stem Cell
- Hippocampal Neurogenesis
- Antidepressant Action
- Immortalize Cell Line
- Behavioural Despair
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
How Can We Best Study Depression?
Depression ranks first among all mental and neurological disorders as a contributor to the global burden of disease and causes a heavy load on patients and their families. However, available treatments are far from ideal. Only a third of patients respond to the initial treatment, another third will get better only after several changes of medication and the rest will go on to be treatment resistant (Rapaport et al. 2003; Trivedi et al. 2006). Why is this? We still do not know why depression happens; neither do we clearly understand how antidepressants work. Much of the current understanding about the pathogenesis of major depression has come from animal models (Krishnan and Nestler 2011) as well as from peripheral (Felger et al. 2012) and central nervous system (CNS; Raison et al. 2010) circulating measurements from patients with depression. Due to the unique and complex features of human depression, the generation of valid and more insightful depression models has been less straightforward than modeling other disabling diseases (Krishnan and Nestler 2011). One possible approach is that of focusing on brain models, using neural cell lines. Undifferentiated or differentiated tumor-derived cells have been used as a translationally valid experimental model for several psychiatric disorders, including depression (Donnici et al. 2008; Alboni et al. 2013). However, such lines are limited in the cell types they can be made to resemble and may have major chromosomal abnormalities (Bray et al. 2012). Into this breach come new brain models of neural stem cells. Using multipotent fetal hippocampal progenitor cells (HPCs) and human induced pluripotent stem cells (iPSCs), we have mimicked clinically pertinent conditions to depressive disorders by combining depressogenic insults and antidepressant strategies (Anacker et al. 2011b, 2013a, b; Zunszain et al. 2012; Horowitz et al. 2015). Indeed, our outcomes provided evidence for the efficacy of such models in understanding the disorder as well as for giving more insights into antidepressants and their mechanisms of action. Particularly, we focused on neurogenesis as a potential candidate mechanism for the etiology of this condition as well as a substrate for antidepressant action.
The Neurogenesis Theory of Depression
A reduction in hippocampal neurogenesis, that is the birth of neurons from stem cells, has been suggested as one of the neurobiological alterations mediating the development of depressive-like behavior in animals, particularly under conditions of stress (David et al. 2009; Snyder et al. 2011; Surget et al. 2011). In the absence of effective neurogenesis, the depressive-like behavior elicited in animals by stress includes the hallmark abnormalities of clinical depression: increased hypothalamic-pituitary-adrenal (HPA) axis activity, glucocorticoid resistance (that is, impaired suppression of HPA axis activity by dexamethasone), anhedonia (assessed using the sucrose preference test), and behavioural despair (assessed using the forced swim test) (David et al. 2009; Snyder et al. 2011; Surget et al. 2011). Moreover, it has been suggested that an impaired neurogenesis may also precipitate depressive symptoms because of the lack of neurogenesis-dependent cognitive functions, such as the ability to enhance encoding of new memories and responding to contextual changes, which may be protective against behavioural despair in the face of repeated stressors (Sahay et al. 2011). Recent studies showing that the magnitude of adult neurogenesis in humans is probably larger than generally believed (Snyder and Cameron 2012; Spalding et al. 2013) provided even stronger support for the importance of neurogenesis and its proposed involvement in the association between stress and depression (Snyder et al. 2011).
Increased inflammation can also cause reductions in neurogenesis. Immune molecules, including interleukin-1beta (IL-1β), IL-6, interferon-alpha (IFN-α) and tumor necrosis factor-alpha (TNF-α) have been shown to be significantly upregulated in the peripheral blood of depressed patients (Howren et al. 2009; Dowlati et al. 2010). Particularly in the context of depression, IL-1β, IL6, IFN-α and IFN-γ have also been shown to easily move from the periphery into the brain (Dantzer et al. 2008; Najjar et al. 2013). Once they cross the brain-blood barrier, these molecules can alter distinct molecular and cellular mechanisms, including cell proliferation and neuronal maturation (Pickering and O’Connor 2007; Alboni et al. 2014) associated with complex cognitive processes, such as mood and learning functions (Makhija and Karunakaran 2013; Shigemoto-Mogami et al. 2014). In particular, using animal models, IL-1β, IL-18, IFN-α and TNF-α, have been shown to contribute to inhibition of synaptic plasticity and memory consolidation (Pickering and O’Connor 2007), causing similar impairments to those often reported in patients with major depressive disorder or in experimental models of depression (Pollak and Yirmiya 2002; Capuron and Miller 2004; Zunszain et al. 2013).
Furthermore, stress and inflammation interact. For example, in response to chronic IFN-α administration, patients with Hepatitis C Virus showed a hyper-reactivity of the HPA axis (Capuron et al. 2002; Raison et al. 2010). Most interestingly, around 30 % of those patients developed clinically significant depression (Raison et al. 2009), strengthening the notion that stress and inflammation might be among the crosstalk pathways leading to the pathogenesis of the depressive disorder. Indeed, it is of relevance in this context that stress and inflammation are among the different downstream molecular mechanisms that distinct antidepressants activate. Particularly, antidepressants from the selective serotonin reuptake inhibitor (SSRI) class, such as fluoxetine, have shown to normalize stress-induced HPA hyperactivity in rodents (Perera et al. 2011; Surget et al. 2011), whereas other serotonin-norepinephrine reuptake inhibitor (SNRI) antidepressants, such as venlafaxine, have normalized inflammatory alterations in cytokine-treated depressed patients (Capuron et al. 2002). However, irrespective of which distinct downstream molecular mechanisms specific antidepressants activate, those pathways may ultimately converge to stimulate neurogenesis, which is proposed as an essential substrate for antidepressant action (Schloesser et al. 2010).
Experimental Approaches
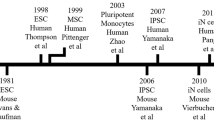
Among the approaches available to investigate the complexity of depressive disorders, immortalized cell lines and patient-derived stem cell lines have proved to be relevant in vitro human neural cell models (Fig. 1). Immortalized cell lines allow molecular, developmental and pathophysiological mechanisms to be studied with considerable reliability. Examples of immortalized cell lines are region-specific neural stem cells and tumour-derived cells. An alternative cell-based approach is to derive and compare neural cells from patients and control individuals, particularly using iPSCs or olfactory neuroepithelium-derived cell lines. Indeed, the use of cells from patients allows a more attentive analysis of the pathological processes arising from the whole range of genetic susceptibility variants characterizing each individual (Srikanth and Young-Pearse 2014). Evidence has shown the significant incidence of distinct genetic polymorphisms in patients with psychiatric conditions, including depression (Cao et al. 2015; Chen et al. 2015; Wang et al. 2015), suggesting the importance of using such models to investigate genetic differences, which may allow for a predictive diagnosis of this disorder (Pasca et al. 2014). For the purpose of this chapter we will focus on immortalized stem cells and iPSC models. We will subsequently report our findings, providing evidence for their efficacy in understanding depressive disorders and antidepressant mechanisms of action.
Immortalized Human Neural Cell Lines
Neural Stem Cell Lines
Stem cells derived from human fetal brain are multipotent (i.e., they can give rise to a range of neurons and glia) and allow developmental and physiological processes to be studied more faithfully. Particularly, clonal neural stem cell lines can be generated by conditional immortalization, whereby a regulated gene that drives cell division is introduced into the cell’s genome, allowing controlled expansion and differentiation (Pollock et al. 2006). Indeed, neural stem cell lines with normal chromosomes have been established from several human fetal brain regions, including cerebral cortex, hippocampus and striatum. This approach has several unique advantages. First, it delivers data from living human brain cells, not easily accessible in clinical samples; second, it can mimic a multitude of clinically relevant conditions within a tightly controlled experimental environment, providing a model system with which to explore the mechanisms of drug treatments for a variety of psychiatric disorders; and finally, it generates findings that are directly translatable in clinical samples (Bray et al. 2012). Using this approach, we have modeled “depression in a dish” using the cell line HPC0A07/03C (provided by ReNeuron Ltd, London), derived from the hippocampus, which allowed us to translate findings from bench-to-beside-and-back (Anacker et al. 2011b, 2013a, b; Zunszain et al. 2012; Horowitz et al. 2015). We will describe our observations in further detail.
Modeling the Role of Stress
As a first example of our translational approach, we managed to provide evidence for the detrimental role of stress on hippocampal neurogenesis. Impaired neurogenesis in rodents has recently been shown to contribute to the development of depressive-like behaviours, including anhedonia and behavioural despair in response to acute and chronic stressful insults (Zhu et al. 2014). Using our human hippocampal model, we showed that cortisol caused a reduction in the generation of new neurons via glucocorticoid receptor (GR)-dependent mechanisms, an effect which could be fully reverted by treatment with the SSRI sertraline. Indeed, subsequent stimulation with a GR-antagonist completely abolished the increase in neurogenesis induced by the antidepressant (Anacker et al. 2011b). Our model proved to be effective in providing further details from the complex interaction between stress and neuronal generation, proposing GR-dependent mechanisms as possible future targets of antidepressant drug treatment to overcome neurogenesis-related disturbances in depression (Anacker et al. 2011a).
Modeling the Role of Inflammation and Oxidative Stress
As a second example of the use of our model, we demonstrated the involvement of inflammation on hippocampal neurogenesis. Previous evidence had reported that in vitro stimulation with distinct cytokines, including IL-1β, IL-6, IFN-α and TNF-α, caused a significant alteration in both proliferation and neuronal maturation of human and animal cells (Borsini et al. 2015). Using our in vitro human neuronal model, we investigated the effect of two pro-inflammatory cytokines, IFN-α (our unpublished observation) and IL-1β (Zunszain et al. 2012), on neurogenesis. Findings showed that, upon treatment with both cytokines HPCs developed a “depressive phenotype” comprising reduced neurogenesis. Moreover, IL-1β was responsible for alterations in transcription pathways regulating the metabolism of tryptophan. Indeed, the inhibitory effects of IL-1β on neurogenesis were mediated, at least in part, by activation of the neurotoxic branch of the kynurenine pathway, one of the main pathways postulated to be involved in the development of depressive disorders (Baranyi et al. 2015).
A third example involved the use of tert-butylhydroxiperoxide (TBHP) to model oxidative stress. High levels of reactive oxygen species, shown in depressed patients, are known to affect cellular constituents, leading to neoepitopes and damage-associated molecular patterns that promote further immune responses (Bakunina et al. 2015). Cells treated with TBHP showed a dose-dependent increase in lipid peroxidation as well as reduced cell viability.
Studying Mechanism of Action of Antidepressants
Finally, we used this model to investigate the immunomodulatory properties of distinct compounds with antidepressant actions. We explored the effects of several conventional monoaminergic antidepressants and the omega-3 polyunsaturated fatty acids (n-3 PUFAs), eicosapentanoic acid (EPA) and docosahexanoic acid (DHA), on HPCs treated with the inflammatory and depressogenic IL-1β. In contrast to sertraline and DHA, which had pro-inflammatory properties, venlafaxine and EPA were shown to have anti-inflammatory effects via decreasing distinct cytokines, including IL-6, IL-8 and IP-10 (Horowitz et al. 2015). In addition, these compounds showed differential effects on neurogenesis. Again, the findings demonstrate the efficacy of this model for studying specific mechanisms of action of drugs with antidepressant action.
Tumour-Derived Cell Lines
These lines, with an ability to expand quite readily in culture, provide a standardized and potentially limitless alternative to study intracellular mechanisms of antidepressant action. Currently, the most commonly used human neural cell line is SH-SY5Y. This line displays neuronal properties, including neurite outgrowth, neurotransmitter synthesis and relevant receptor expression. The SH-SY5Y line has been widely used to study intracellular mechanisms of different antidepressant action, including the SSRI sertraline, the selective norepinephrine reuptake inhibitor (SNRI) desipramine and the norepinephrine–dopamine reuptake inhibitor (NDRI) bupropion (Lin 2015).
Patient-Derived Neural Cells
IPSCs
Among the patient-derived cell models, iPSC technology provides distinct cell types that are considered to be central to psychiatric disorders, such as those of the cortex and the hippocampus (Jaworska et al. 2015). Indeed, primary somatic cells, typically from skin, can be taken from an individual and reprogrammed into pluripotent stem cells that can give rise to all of the cell types that characterize the body, including those of the CNS. By capturing a patient's entire genome and any possible epigenetic variations, iPSCs constitute a unique source of material for studying neurodevelopmental features of psychiatric disorders in vitro.
Reports are now beginning to emerge in which this technology has been applied to cells taken from psychiatric patients. For example, human keratinocytes from healthy controls and patients with bipolar depression have been reprogrammed into cortical neurons. When compared with control cells, neurons derived from patients with bipolar depression showed an alteration in the expression of transcripts that regulate Hedgehog signaling (Cheung et al. 2009), as well as modulations in key components of the mTOR pathway (O’Shea and McInnis 2015), which have both been shown to be among the mechanisms involved in the development of depressive disorders (Rajendran et al. 2009; Ignacio et al. 2015). Using iPSC-derived forebrain neurons, we showed that ketamine, known to have fast-acting antidepressant efficacy in treatment-resistant patients, was able to rescue the detrimental effects produced by treatment with IL-1β and to increase the number of presynaptic and postsynaptic proteins.
Olfactory Neuroepithelium-Derived Stem Cells
Cells from the olfactory mucosa, which can be extracted through biopsy, can easily propagate, forming neurospheres of neural stem cells and differentiating neural progenitor cells. Although there are no studies using olfactory neuroepithelium-derived cells from depressed patients, they have been used to study schizophrenia (Matigian et al. 2010; Fan et al. 2012), suggesting the importance of using such models to investigate genetic differences, which may allow for a predictive diagnosis of depression in certain individuals.
Conclusions and Limitations of the Cell Models
Stem cell-based approaches to study psychiatric disorders are advancing on two main fronts. On one hand, clonal cell lines which accurately model the CNS are being used in controlled experiments to assess the mechanisms of antidepressant action for psychiatric disorders, which might in the short term lead to advancements in therapeutic strategies for these conditions. On the other hand, patient-derived cells and cells from control patients allow the study of pathological processes deriving from the multiple genetic susceptibility variants, which can now be investigated with more accuracy. However, both cell models have some limitations that need to be pointed out. Although human neural cell lines can be used to investigate the molecular and cellular functions of individual susceptibility genes, they do not capture the many genetic variables that contribute to the development of psychiatric disorders (Bray et al. 2012). Patient-derived cell lines offer the advantage of capturing each individual's whole genome, but there is limited knowledge as to which cell types are most relevant to study specific psychiatric conditions (Sandoe and Eggan 2013). In addition, while iPSC technology can model the effects of medications, these cells may lose the effects of environmental influences that may contribute to the development of the psychiatric illnesses, such as stressors or negative life events (Okano and Yamanaka 2014).
Although cell-based models are still unable to elucidate the molecular complexity of psychiatric illnesses, the enormous progress in stem cell technologies has revolutionized the field of “in vitro disease modeling,” providing not only a window into the mechanisms underlying the depressive disorder but also a platform for screening novel therapeutic strategies for the prevention and treatment of this condition.
References
Alboni S, Gibellini L, Montanari C, Benatti C, Benatti S, Tascedda F, Brunello N, Cossarizza A, Pariante CM (2013) N-acetyl-cysteine prevents toxic oxidative effects induced by IFN-alpha in human neurons. Int J Neuropsychopharmacol 16:1849–1865
Alboni S, Montanari C, Benatti C, Sanchez-Alavez M, Rigillo G, Blom JM, Brunello N, Conti B, Pariante MC, Tascedda F (2014) Interleukin 18 activates MAPKs and STAT3 but not NF-kappaB in hippocampal HT-22 cells. Brain Behav Immun 40:85–94
Anacker C, Zunszain PA, Carvalho LA, Pariante CM (2011a) The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology 36:415–425
Anacker C, Zunszain PA, Cattaneo A, Carvalho LA, Garabedian MJ, Thuret S, Price J, Pariante CM (2011b) Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol Psychiatry 16:738–750
Anacker C, Cattaneo A, Luoni A, Musaelyan K, Zunszain PA, Milanesi E, Rybka J, Berry A, Cirulli F, Thuret S, Price J, Riva MA, Gennarelli M, Pariante CM (2013a) Glucocorticoid-related molecular signaling pathways regulating hippocampal neurogenesis. Neuropsychopharmacology 38:872–883
Anacker C, Cattaneo A, Musaelyan K, Zunszain PA, Horowitz M, Molteni R, Luoni A, Calabrese F, Tansey K, Gennarelli M, Thuret S, Price J, Uher R, Riva MA, Pariante CM (2013b) Role for the kinase SGK1 in stress, depression, and glucocorticoid effects on hippocampal neurogenesis. Proc Natl Acad Sci USA 110:8708–8713
Bakunina N, Pariante CM, Zunszain PA (2015) Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology. doi:10.1111/imm.12443
Baranyi A, Meinitzer A, Breitenecker RJ, Amouzadeh-Ghadikolai O, Stauber R, Rothenhausler HB (2015) Quinolinic acid responses during interferon-alpha-induced depressive symptomatology in patients with chronic hepatitis C infection—a novel aspect for depression and inflammatory hypothesis. PLoS One 10:e137022
Borsini A, Zunszain PA, Thuret S, Pariante CM (2015) The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci 38:145–157
Bray NJ, Kapur S, Price J (2012) Investigating schizophrenia in a “dish”: possibilities, potential and limitations. World Psychiatry 11:153–155
Cao S, Li H, Lou L, Xie Z, Zhao X, Pang J, Sui J, Xie G (2015) Association study between 5-HT2A and NET gene polymorphisms and recurrent major depression disorder in Chinese Han population. Pak J Pharm Sci 28:1101–1108
Capuron L, Miller AH (2004) Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry 56:819–824
Capuron L, Hauser P, Hinze-Selch D, Miller AH, Neveu PJ (2002) Treatment of cytokine-induced depression. Brain Behav Immun 16:575–580
Chen J, Wang M, Waheed Khan RA, He K, Wang Q, Li Z, Shen J, Song Z, Li W, Wen Z, Jiang Y, Xu Y, Shi Y, Ji W (2015) The GSK3B gene confers risk for both major depressive disorder and schizophrenia in the Han Chinese population. J Affect Disord 185:149–155
Cheung HO, Zhang X, Ribeiro A, Mo R, Makino S, Puviindran V, Law KK, Briscoe J, Hui CC (2009) The kinesin protein Kif7 is a critical regulator of Gli transcription factors in mammalian hedgehog signaling. Sci Signal 2:ra29
Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Neurosci 9:46–56
David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R (2009) Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62:479–493
Donnici L, Tiraboschi E, Tardito D, Musazzi L, Racagni G, Popoli M (2008) Time-dependent biphasic modulation of human BDNF by antidepressants in neuroblastoma cells. BMC Neurosci 9:61
Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL (2010) A meta-analysis of cytokines in major depression. Biol Psychiatry 67:446–457
Fan Y, Abrahamsen G, McGrath JJ, Mackay-Sim A (2012) Altered cell cycle dynamics in schizophrenia. Biol Psychiatry 71:129–135
Felger JC, Cole SW, Pace TW, Hu F, Woolwine BJ, Doho GH, Raison CL, Miller AH (2012) Molecular signatures of peripheral blood mononuclear cells during chronic interferon-alpha treatment: relationship with depression and fatigue. Psychol Med 42:1591–1603
Horowitz MA, Wertz J, Zhu D, Cattaneo A, Musaelyan K, Nikkheslat N, Thuret S, Pariante CM, Zunszain PA (2015) Antidepressant compounds can be both pro- and anti-inflammatory in human hippocampal cells. Int J Neuropsychopharmacol 18(3). doi:10.1093/ijnp/pyu076
Howren MB, Lamkin DM, Suls J (2009) Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 71:171–186
Ignacio ZM, Reus GZ, Arent CO, Abelaira HM, Pitcher MR, Quevedo J (2015) New perspectives on the involvement of mTOR in depression as well as in the action of antidepressant drugs. Brit J Clin Phamacol. doi:10.1111/bcp.12845
Jaworska N, Yucel K, Courtright A, MacMaster FP, Sembo M, MacQueen G (2015) Subgenual anterior cingulate cortex and hippocampal volumes in depressed youth: the role of comorbidity and age. J Affect Disord 190:726–732
Krishnan V, Nestler EJ (2011) Animal models of depression: molecular perspectives. Curr Top Behav Neurosci 7:121–147
Lin PY (2015) Regulation of proteolytic cleavage of brain-derived neurotrophic factor precursor by antidepressants in human neuroblastoma cells. Neuropsychiatr Dis Treat 11:2529–2532
Makhija K, Karunakaran S (2013) The role of inflammatory cytokines on the aetiopathogenesis of depression. Aust N Z J Psychiatry 47:828–839
Matigian N, Matigian N, Abrahamsen G, Sutharsan R, Cook AL, Vitale AM, Nouwens A, Bellette B, An J, Anderson M, Beckhouse AG, Bennebroek M, Cecil R, Chalk AM, Cochrane J, Fan Y, Féron F, McCurdy R, McGrath JJ, Murrell W, Perry C, Raju J, Ravishankar S, Silburn PA, Sutherland GT, Mahler S, Mellick GD, Wood SA, Sue CM, Wells CA, Mackay-Sim A (2010) Disease-specific, neurosphere-derived cells as models for brain disorders. Dis Model Mech 3:785–798
Najjar S, Pearlman DM, Devinsky O, Najjar A, Zagzag D (2013) Neurovascular unit dysfunction with blood-brain barrier hyperpermeability contributes to major depressive disorder: a review of clinical and experimental evidence. J Neuroinflammation 10:142
Okano H, Yamanaka S (2014) iPS cell technologies: significance and applications to CNS regeneration and disease. Mol Brain 7:22. doi:10.1186/1756-6606-7-22
O’Shea KS, McInnis MG (2015) Induced pluripotent stem cell (iPSC) models of bipolar disorder. Neuropsychopharmacology 40:248–249
Pasca SP, Panagiotakos G, Dolmetsch RE (2014) Generating human neurons in vitro and using them to understand neuropsychiatric disease. Ann Rev Neurosci 37:479–501
Perera TD, Dwork AJ, Keegan KA, Thirumangalakudi L, Lipira CM, Joyce N, Lange C, Higley JD, Rosoklija G, Hen R, Sackeim HA, Coplan JD (2011) Necessity of hippocampal neurogenesis for the therapeutic action of antidepressants in adult nonhuman primates. PLoS One 6:e17600
Pickering M, O’Connor JJ (2007) Pro-inflammatory cytokines and their effects in the dentate gyrus. Prog Brain Res 163:339–354
Pollak Y, Yirmiya R (2002) Cytokine-induced changes in mood and behaviour: implications for ‘depression due to a general medical condition’, immunotherapy and antidepressive treatment. Int J Neuropsychopharmacol 5:389–399
Pollock K, Stroemer P, Patel S, Stevanato L, Hope A, Miljan E, Dong Z, Hodges H, Price J, Sinden JD (2006) A conditionally immortal clonal stem cell line from human cortical neuroepithelium for the treatment of ischemic stroke. Exp Neurol 199:143–155
Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, Vogt GJ, Massung B, Miller AH (2009) Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry 65:296–303
Raison CL, Borisov AS, Woolwine BJ, Massung B, Vogt G, Miller AH (2010) Interferon-alpha effects on diurnal hypothalamic-pituitary-adrenal axis activity: relationship with proinflammatory cytokines and behavior. Mol Psychiatry 15:535–547
Rajendran R, Jha S, Fernandes KA, Banerjee SB, Mohammad F, Dias BG, Vaidya VA (2009) Monoaminergic regulation of Sonic hedgehog signaling cascade expression in the adult rat hippocampus. Neurosci Lett 453:190–194
Rapaport MH, Schneider LS, Dunner DL, Davies JT, Pitts CD (2003) Efficacy of controlled-release paroxetine in the treatment of late-life depression. J Clin Psychiatry 64:1065–1074
Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R (2011) Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472:466–470
Sandoe J, Eggan K (2013) Opportunities and challenges of pluripotent stem cell neurodegenerative disease models. Nat Neurosci 16:780–789
Schloesser RJ, Lehmann M, Martinowich K, Manji HK, Herkenham M (2010) Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Mol Psychiatry 15:1152–1163
Shigemoto-Mogami Y, Hoshikawa K, Goldman JE, Sekino Y, Sato K (2014) Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J Neurosci 34:2231–2243
Snyder JS, Cameron HA (2012) Could adult hippocampal neurogenesis be relevant for human behavior? Behav Brain Res 227:384–390
Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA (2011) Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476:458–461
Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, Possnert G, Mash DC, Druid H, Frisen J (2013) Dynamics of hippocampal neurogenesis in adult humans. Cell 153:1219–1227
Srikanth P, Young-Pearse TL (2014) Stem cells on the brain: modeling neurodevelopmental and neurodegenerative diseases using human induced pluripotent stem cells. J Neurogenet 28:5–29
Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, Palme R, Griebel G, Ibarguen-Vargas Y, Hen R, Belzung C (2011) Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry 16:1177–1188
Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M, Team SS (2006) Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. Am J Psychiatry 163:28–40
Wang Y, Sun N, Li S, Du Q, Xu Y, Liu Z, Zhang K (2015) A genetic susceptibility mechanism for major depression: combinations of polymorphisms defined the risk of major depression and subpopulations. Medicine (Baltimore) 94:e778
Zhu S, Wang J, Zhang Y, Li V, Kong J, He J, Li XM (2014) Unpredictable chronic mild stress induces anxiety and depression-like behaviors and inactivates AMP-activated protein kinase in mice. Brain Res 1576:81–90
Zunszain PA, Anacker C, Cattaneo A, Choudhury S, Musaelyan K, Myint AM, Thuret S, Price J, Pariante CM (2012) Interleukin-1beta: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology 37:939–949
Zunszain PA, Hepgul N, Pariante CM (2013) Inflammation and depression. Curr Top Behav Neurosci 14:135–151
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, a link is provided to the Creative Commons license and any changes made are indicated.
The images or other third party material in this chapter are included in the work's Creative Commons license, unless indicated otherwise in the credit line; if such material is not included in the work's Creative Commons license and the respective action is not permitted by statutory regulation, users will need to obtain permission from the license holder to duplicate, adapt or reproduce the material.
Copyright information
© 2016 The Author(s)
About this chapter
Cite this chapter
Borsini, A., Zunszain, P.A. (2016). Advances in Stem Cells Biology: New Approaches to Understand Depression. In: Pfaff, D., Christen, Y. (eds) Stem Cells in Neuroendocrinology. Research and Perspectives in Endocrine Interactions. Springer, Cham. https://doi.org/10.1007/978-3-319-41603-8_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-41603-8_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-41602-1
Online ISBN: 978-3-319-41603-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)