Summary
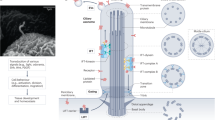
Primary cilia are nonmotile, tubular organelles extending from the cell plasma membrane. They are important in maintaining normal cell physiology and in many clinical disorders (i.e., cilia-related diseases or ciliopathies) associated with abnormalities in ciliary structure and/or function.
In the intrahepatic bile ducts, primary cilia extend from the cholangiocyte apical plasma membrane into the ductal lumen. Cholangiocyte cilia are sensory organelles that recognize the current state of bile, i.e., its flow, chemical composition, and osmolality, and transmit the related information into intracellular signaling, i.e., function as mechano-, chemo-, and osmosensors. In contrast, in ciliopathies, the structural and/or functional abnormalities of these organelles in cholangiocytes lining liver cysts are associated with activation or down-regulation of several intracellular signaling pathways, in particular, cAMP and [Ca2+]i, leading to hepatic cystogenesis.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Christensen ST, Pedersen LB, Schneider L, Satir P. Sensory cilia and integration of signal transduction in human health and disease. Traffic 2007;8, 97–109.
Pan J. Cilia and ciliopathies: From Chlamydomonas and beyond. Sci China C Life Sci 2008;51, 479–486.
Marshall WF, Nonaka S. Cilia: Tuning in to the cell’s antenna. Curr Biol 2006;16, R604–R614.
Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol 2007;69, 377–400.
Dawe HR, Farr H, Gull K. Centriole/basal body morphogenesis and migration during ciliogenesis in animal cells. J Cell Sci 2007;120(Pt 1), 7–15.
Marshall WF. The cell biological basis of ciliary disease. J Cell Biol 2008;180, 17–21.
Reiter JF. A cilium is not a cilium is not a cilium: Signaling contributes to ciliary morphological diversity. Dev Cell 2008;14, 635–636.
Singla V, Reiter JF. The primary cilium as the cell’s antenna: Signaling at a sensory organelle. Science 2006;313, 629–633.
Bisgrove BW, Yost HJ. The roles of cilia in developmental disorders and disease. Development 2006;133, 4131–4143.
Menco BP. Ultrastructural aspects of olfactory transduction and perireceptor events. Semin Cell Biol 1994;5, 11–24.
Nonaka S, Tanaka Y, Okada Y, et al. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 1998;95, 829–837.
Davenport JR, Yoder BK. An incredible decade for the primary cilium: A look at a once-forgotten organelle. Am J Physiol Renal Physiol 2005;289, F1159–F1169.
Sorokin SP. Centriole formation and ciliogenesis. Aspen Emphysema Conf 1968;11, 213–216.
Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: An emerging class of human genetic disorders. Annu Rev Genomics Hum Genet 2006;7, 125–148.
Fliegauf M, Benzing T, Omran H. When cilia go bad: Cilia defects and ciliopathies. Nat Rev Mol Cell Biol 2007;8, 880–893.
Yoder BK. Role of primary cilia in the pathogenesis of polycystic kidney disease. J Am Soc Nephrol 2007;18, 1381–1388.
Siroky BJ, Guay-Woodford LM. Renal cystic disease: The role of the primary cilium/centrosome complex in pathogenesis. Adv Chronic Kidney Dis 2006;13, 131–137.
Adams M, Smith UM, Logan CV, Johnson CA. Recent advances in the molecular pathology, cell biology and genetics of ciliopathies. J Med Genet 2008;45, 257–267.
Masyuk AI, Masyuk TV, LaRusso NF. Cholangiocyte primary cilia in liver health and disease. Dev Dyn 2008;237, 2007–2012.
Hagiwara H, Ohwada N, Takata K. Cell biology of normal and abnormal ciliogenesis in the ciliated epithelium. Int Rev Cytol 2004;234, 101–141.
Jensen CG, Poole CA, McGlashan SR, et al. Ultrastructural, tomographic and confocal imaging of the chondrocyte primary cilium in situ. Cell Biol Int 2004;28, 101–110.
Haimo LT, Rosenbaum JL. Cilia, flagella, and microtubules. J Cell Biol 1981;91(3 Pt 2), 125s–130s.
Eley L, Yates LM, Goodship JA. Cilia and disease. Curr Opin Genet Dev 2005;15, 308–314.
Raychowdhury MK, McLaughlin M, Ramos AJ, et al. Characterization of single channel currents from primary cilia of renal epithelial cells. J Biol Chem 2005;280, 34718–34722.
Gradilone SA, Masyuk AI, Splinter PL, et al. Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proc Natl Acad Sci USA 2007;104, 19138–19143.
Teilmann SC, Byskov AG, Pedersen PA, Wheatley DN, Pazour GJ, Christensen ST. Localization of transient receptor potential ion channels in primary and motile cilia of the female murine reproductive organs. Mol Reprod Dev 2005;71, 444–452.
Masyuk AI, Masyuk TV, Splinter PL, Huang BQ, Stroope AJ, LaRusso NF. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology 2006;131, 911–920.
Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 2002;13, 2508–2516.
Masyuk TV, Huang BQ, Ward CJ, et al. Defects in cholangiocyte fibrocystin expression and ciliary structure in the PCK rat. Gastroenterology 2003;125, 1303–1310.
Handel M, Schulz S, Stanarius A, et al. Selective targeting of somatostatin receptor 3 to neuronal cilia. Neuroscience 1999;89, 909–926.
Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate smoothened functions at the primary cilium. Nature 2005;437, 1018–1021.
Masyuk AI, Gradilone SA, Banales JM, et al. Cholangiocyte primary cilia are chemosensory organelles that detect biliary nucleotides via P2Y12 purinergic receptors. Am J Physiol Gastrointest Liver Physiol 2008;295, G725–G734.
Michaud EJ, Yoder BK. The primary cilium in cell signaling and cancer. Cancer Res 2006;66, 6463–6467.
Sloboda RD, Rosenbaum JL. Making sense of cilia and flagella. J Cell Biol 2007;179, 575–582.
Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol 2002;3, 813–825.
Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 2000;1, 31–39.
Jenkins PM, Hurd TW, Zhang L, et al. Ciliary targeting of olfactory CNG channels requires the CNGB1b subunit and the kinesin-2 motor protein, KIF17. Curr Biol 2006;16, 1211–1216.
Geng L, Okuhara D, Yu Z, et al. Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J Cell Sci 2006;119(Pt 7), 1383–1395.
Schermer B, Hopker K, Omran H, et al. Phosphorylation by casein kinase 2 induces PACS-1 binding of nephrocystin and targeting to cilia. EMBO J 2005;24, 4415–4424.
Grisham JW. Ciliated epithelial cells in normal murine intrahepatic bile ducts. Proc Soc Exp Biol Med 1963;114, 318–320.
Tobe K, Tsuchiya T, Itoshima T, Nagashima H, Kobayashi T. Electron microscopy of fat-storing cells in liver diseases with special reference to cilia and cytoplasmic cholesterol crystals. Arch Histol Jpn 1985;48, 435–441.
Huang BQ, Masyuk TV, Muff MA, Tietz PS, Masyuk AI, Larusso NF. Isolation and characterization of cholangiocyte primary cilia. Am J Physiol Gastrointest Liver Physiol 2006;291, G500–G509.
Strazzabosco M, Fabris L, Spirli C. Pathophysiology of cholangiopathies. J Clin Gastroenterol 2005;39(4 Suppl 2), S90–S102.
Masyuk T, Masyuk A, LaRusso N. Cholangiociliopathies: Mechanisms of development and therapeutic targets. Kerala, India: Transworld Research Network; 2008.
Salisbury JL, D’Assoro AB, Lingle WL. Centrosome amplification and the origin of chromosomal instability in breast cancer. J Mammary Gland Biol Neoplasia 2004;9, 275–283.
Doxsey S. Re-evaluating centrosome function. Nat Rev Mol Cell Biol 2001;2, 688–698.
Rieder CL, Faruki S, Khodjakov A. The centrosome in vertebrates: More than a microtubule-organizing center. Trends Cell Biol 2001;11, 413–419.
Sorokin S. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Biol 1962;15, 363–377.
Fukasawa K. Centrosome amplification, chromosome instability and cancer development. Cancer Lett 2005;230, 6–19.
Pelletier L. Centrioles: Duplicating precariously. Curr Biol 2007;17, R770–R773.
Ishikawa H, Kubo A, Tsukita S. Odf2-deficient mother centrioles lack distal/subdistal appendages and the ability to generate primary cilia. Nat Cell Biol 2005;7, 517–524.
Doxsey S, McCollum D, Theurkauf W. Centrosomes in cellular regulation. Annu Rev Cell Dev Biol 2005;21, 411–434.
Doxsey S, Zimmerman W, Mikule K. Centrosome control of the cell cycle. Trends Cell Biol 2005;15, 303–311.
Badano JL, Teslovich TM, Katsanis N. The centrosome in human genetic disease. Nat Rev Genet 2005;6, 194–205.
Burakov A, Nadezhdina E, Slepchenko B, Rodionov V. Centrosome positioning in interphase cells. J Cell Biol 2003;162, 963–969.
Marshall WF. Centriole assembly: The origin of nine-ness. Curr Biol 2007;17, R1057–R1059.
Basto R, Lau J, Vinogradova T, et al. Flies without centrioles. Cell 2006;125, 1375–1386.
Pelletier L. Centrosomes: Keeping tumors in check. Curr Biol 2008;18, R702–R704.
Dammermann A, Muller-Reichert T, Pelletier L, Habermann B, Desai A, Oegema K. Centriole assembly requires both centriolar and pericentriolar material proteins. Dev Cell 2004;7, 815–829.
Jensen CG, Jensen LC, Rieder CL. The occurrence and structure of primary cilia in a subline of Potorous tridactylus. Exp Cell Res 1979;123, 444–449.
Rieder CL, Jensen CG, Jensen LC. The resorption of primary cilia during mitosis in a vertebrate (PtK1) cell line. J Ultrastruct Res 1979;68, 173–185.
Collier S, Gubb D. Drosophila tissue polarity requires the cell-autonomous activity of the fuzzy gene, which encodes a novel transmembrane protein. Development 1997;124, 4029–4037.
Blatt EN, Yan XH, Wuerffel MK, Hamilos DL, Brody SL. Forkhead transcription factor HFH-4 expression is temporally related to ciliogenesis. Am J Respir Cell Mol Biol 1999;21, 168–176.
Brody SL, Yan XH, Wuerffel MK, Song SK, Shapiro SD. Ciliogenesis and left-right axis defects in forkhead factor HFH-4-null mice. Am J Respir Cell Mol Biol 2000;23, 45–51.
Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet 2006;38, 303–311.
Li G, Vega R, Nelms K, et al. A role for Alstrom syndrome protein, alms1, in kidney ciliogenesis and cellular quiescence. PLoS Genet 2007;3, e8.
Torkko JM, Manninen A, Schuck S, Simons K. Depletion of apical transport proteins perturbs epithelial cyst formation and ciliogenesis. J Cell Sci 2008;121(Pt 8), 1193–1203.
Yoshimura S, Egerer J, Fuchs E, Haas AK, Barr FA. Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol 2007;178, 363–369.
Town T, Breunig JJ, Sarkisian MR, et al. The stumpy gene is required for mammalian ciliogenesis. Proc Natl Acad Sci USA 2008;105, 2853–2858.
Lutz MS, Burk RD. Primary cilium formation requires von Hippel-Lindau gene function in renal-derived cells. Cancer Res 2006;66, 6903–6907.
Vieira OV, Gaus K, Verkade P, Fullekrug J, Vaz WL, Simons K. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc Natl Acad Sci USA 2006;103, 18556–18561.
Tamakoshi T, Itakura T, Chandra A, et al. Roles of the Foxj1 and Inv genes in the left-right determination of internal organs in mice. Biochem Biophys Res Commun 2006;339, 932–938.
Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci USA 1993;90, 5519–5523.
Pazour GJ, Dickert BL, Vucica Y, et al. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol 2000;151, 709–718.
Scholey JM. Intraflagellar transport. Annu Rev Cell Dev Biol 2003;19, 423–443.
Cole DG. Kinesin-II, the heteromeric kinesin. Cell Mol Life Sci 1999;56, 217–226.
Bossinger O, Bachmann A. Ciliogenesis: Polarity proteins on the move. Curr Biol 2004;14, R844–R846.
Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol 1998;141, 993–1008.
Pazour GJ, Rosenbaum JL. Intraflagellar transport and cilia-dependent diseases. Trends Cell Biol 2002;12, 551–555.
Jurczyk A, Gromley A, Redick S, et al. Pericentrin forms a complex with intraflagellar transport proteins and polycystin-2 and is required for primary cilia assembly. J Cell Biol 2004;166, 637–643.
Masyuk TV, Huang BQ, Masyuk AI, et al. Biliary dysgenesis in the PCK rat, an orthologous model of autosomal recessive polycystic kidney disease. Am J Pathol 2004;165, 1719–1730.
Battini L, Macip S, Fedorova E, et al. Loss of polycystin-1 causes centrosome amplification and genomic instability. Hum Mol Genet 2008;17, 2819–2833.
Simons M, Walz G. Polycystic kidney disease: Cell division without a c(l)ue? Kidney Int 2006;70, 854–864.
de Anda FC, Pollarolo G, Da Silva JS, Camoletto PG, Feiguin F, Dotti CG. Centrosome localization determines neuronal polarity. Nature 2005;436, 704–708.
Schwartz EA, Leonard ML, Bizios R, Bowser SS. Analysis and modeling of the primary cilium bending response to fluid shear. Am J Physiol 1997;272(1 Pt 2), F132–F138.
Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol 2001;184, 71–79.
Praetorius HA, Spring KR. Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol 2003;191, 69–76.
Nauli SM, Alenghat FJ, Luo Y, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 2003;33, 129–137.
Nauli SM, Zhou J. Polycystins and mechanosensation in renal and nodal cilia. Bioessays 2004;26, 844–856.
Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol 2003;285, F998–F1012.
Liu W, Murcia NS, Duan Y, et al. Mechanoregulation of intracellular Ca2+ concentration is attenuated in collecting duct of monocilium-impaired orpk mice. Am J Physiol Renal Physiol 2005;289, F978–F988.
Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, Germino GG. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet 1997;16, 179–183.
Xu GM, Gonzalez-Perrett S, Essafi M, et al. Polycystin-1 activates and stabilizes the polycystin-2 channel. J Biol Chem 2003;278, 1457–1462.
Cooper DM. Molecular and cellular requirements for the regulation of adenylate cyclases by calcium. Biochem Soc Trans 2003;31(Pt 5), 912–915.
Chabardes D, Imbert-Teboul M, Elalouf JM. Functional properties of Ca2+-inhibitable type 5 and type 6 adenylyl cyclases and role of Ca2+ increase in the inhibition of intracellular cAMP content. Cell Signal 1999;11, 651–663.
Brailov I, Bancila M, Brisorgueil MJ, Miquel MC, Hamon M, Verge D. Localization of 5-HT(6) receptors at the plasma membrane of neuronal cilia in the rat brain. Brain Res 2000;872, 271–275.
Bargmann CI. Chemosensation in C. elegans. WormBook 2006, 1–29.
Tobin D, Madsen D, Kahn-Kirby A, et al. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron 2002;35, 307–318.
Liedtke W, Choe Y, Marti-Renom MA, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 2000;103, 525–535.
Liedtke W. Role of TRPV ion channels in sensory transduction of osmotic stimuli in mammals. Exp Physiol 2007;92, 507–512.
Liedtke W. Transient receptor potential vanilloid channels functioning in transduction of osmotic stimuli. J Endocrinol 2006;191, 515–523.
Plant TD, Strotmann R. Trpv4. Handb Exp Pharmacol 2007, 189–205.
Andrade YN, Fernandes J, Vazquez E, et al. TRPV4 channel is involved in the coupling of fluid viscosity changes to epithelial ciliary activity. J Cell Biol 2005;168, 869–874.
Yoder BK, Tousson A, Millican L, et al. Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am J Physiol Renal Physiol 2002;282, F541–F552.
Murcia NS, Richards WG, Yoder BK, Mucenski ML, Dunlap JR, Woychik RP. The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development 2000;127, 2347–2355.
Lin F, Hiesberger T, Cordes K, et al. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci USA 2003;100, 5286–5291.
Torres VE, Wang X, Qian Q, Somlo S, Harris PC, Gattone VH, 2nd. Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med 2004;10, 363–364.
Gattone VH, 2nd, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 2003;9, 1323–1326.
Yamaguchi T, Nagao S, Wallace DP, et al. Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int 2003;63, 1983–1994.
Belibi FA, Reif G, Wallace DP, et al. Cyclic AMP promotes growth and secretion in human polycystic kidney epithelial cells. Kidney Int 2004;66, 964–973.
Hanaoka K, Guggino WB. cAMP regulates cell proliferation and cyst formation in autosomal polycystic kidney disease cells. J Am Soc Nephrol 2000;11, 1179–1187.
Yamaguchi T, Wallace DP, Magenheimer BS, Hempson SJ, Grantham JJ, Calvet JP. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem 2004;279, 40419–40430.
Yamaguchi T, Hempson SJ, Reif GA, Hedge AM, Wallace DP. Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. J Am Soc Nephrol 2006;17, 178–187.
Masyuk TV, Masyuk AI, Torres VE, Harris PC, Larusso NF. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3′,5′-cyclic monophosphate. Gastroenterology 2007;132, 1104–1116.
Banales JM, Masyuk TV, Gradilone SA, Masyuk AI, Medina JF, LaRusso NF. The cAMP effectors, Epac and PKA, are involved in the benign hyperproliferation of cholangiocytes from an animal model of ARPKD. Hepatology 2009;49(1):160–174.
Acknowledgment
This work was supported by the National Institutes of Health (N.F. LaRusso: grant DK 24031), by the PKD Foundation (T.V. Masyuk), and by the Mayo Foundation.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Masyuk, T.V., Masyuk, A.I., LaRusso, N.F. (2010). Cholangiocyte Cilia and Basal Bodies. In: Murray, K., Larson, A. (eds) Fibrocystic Diseases of the Liver. Clinical Gastroenterology. Humana Press. https://doi.org/10.1007/978-1-60327-524-8_3
Download citation
DOI: https://doi.org/10.1007/978-1-60327-524-8_3
Published:
Publisher Name: Humana Press
Print ISBN: 978-1-60327-523-1
Online ISBN: 978-1-60327-524-8
eBook Packages: MedicineMedicine (R0)