Abstract
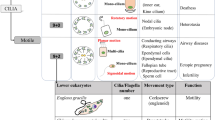
The biological function of motile cilia/flagella has long been recognized. The non-motile primary cilium, once regarded as a vestigial organelle, however, has been found recently to play unexpected roles in mammalian physiology and development. Defects in cilia have profound impact on human health. Diseases related to cilia, collectively called ciliopathies include male infertility, primary cilia dyskinesia, renal cyst formation, blindness, polydactyly, obesity, hypertension, and even mental retardation. Our current understanding of cilia and ciliopathies has been fueled by basic research employing various model organisms including Chlamydomonas, a unicellular green alga. This review article provides a general introduction to the cell biology of cilia and an overview of various cilia-related diseases.
Similar content being viewed by others
References
Silflow C D, Lefebvre P A. Assembly and motility of eukaryotic cilia and flagella. Lessons from Chlamydomonas reinhardtii. Plant Physiol, 2001, 127: 1500–1507, 11743094, 10.1104/pp.127.4.1500, 1:CAS:528:DC%2BD38XjtVWgtQ%3D%3D
Brokaw C J, Kamiya R. Bending patterns of Chlamydomonas flagella: IV. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function. Cell Motil Cytoskeleton, 1987, 8: 68–75, 2958145, 10.1002/cm.970080110, 1:STN:280:BieD3c3jvVw%3D
Porter M E, Sale W S. The 9 + 2 axoneme anchors multiple inner arm dyneins and a network of kinases and phosphatases that control motility. J Cell Biol, 2000, 151: 37–42, 10.1083/jcb.151.5.F37
Dentler W L. Structures linking the tips of ciliary and flagellar microtubules to the membrane. J Cell Sci, 1980, 42: 207–220, 6772653, 1:STN:280:Bi%2BB2cbntVA%3D
Sloboda R D. Intraflagellar transport and the flagellar tip complex. J Cell Biochem, 2005, 94: 266–272, 15558569, 10.1002/jcb.20323, 1:CAS:528:DC%2BD2MXpsFegsA%3D%3D
Rosenbaum J L, Witman G B. Intraflagellar transport. Nat Rev Mol Cell Biol, 2002, 3: 813–825, 12415299, 10.1038/nrm952, 1:CAS:528:DC%2BD38Xotlerur0%3D
Sanders M A, Salisbury J L. Centrin-mediated microtubule severing during flagellar excision in Chlamydomonas reinhardtii. J Cell Biol, 1989, 108: 1751–1760, 2654141, 10.1083/jcb.108.5.1751, 1:CAS:528:DyaL1MXhvV2gs74%3D
Dutcher S K. Elucidation of basal body and centriole functions in Chlamydomonas reinhardtii. Traffic, 2003, 4: 443–451, 12795689, 10.1034/j.1600-0854.2003.00104.x, 1:CAS:528:DC%2BD3sXltFektLY%3D
Zariwala M A, Knowles M R, Omran H. Genetic defects in ciliary structure and function. Annu Rev Physiol, 2007, 69: 423–450, 17059358, 10.1146/annurev.physiol.69.040705.141301, 1:CAS:528:DC%2BD2sXltVaksLo%3D
Afzelius B A. The immotile-cilia syndrome and other ciliary diseases. Int Rev Exp Pathol, 1979, 19: 1–43, 156703, 1:STN:280:CSaB3MrlsVQ%3D
Nonaka S, Tanaka Y, Okada Y, et al. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell, 1998, 95: 829–837, 9865700, 10.1016/S0092-8674(00)81705-5, 1:CAS:528:DyaK1MXivVKn
Marszalek J R, Liu X, Roberts E A, et al. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell, 2000, 102: 175–187, 10943838, 10.1016/S0092-8674(00)00023-4, 1:CAS:528:DC%2BD3cXltl2hsro%3D
Menco B P. Ultrastructural aspects of olfactory signaling. Chem Senses, 1997, 22: 295–311, 9218142, 10.1093/chemse/22.3.295, 1:STN:280:ByiA287isVw%3D
Sobkowicz H M, Slapnick S M, August B K. The kinocilium of auditory hair cells and evidence for its morphogenetic role during the regeneration of stereocilia and cuticular plates. J Neurocytol, 1995, 24: 633–653, 7500120, 10.1007/BF01179815, 1:STN:280:BymC3c%2Fis1M%3D
Axelrod J D. Basal bodies, kinocilia and planar cell polarity. Nat Genet, 2008, 40: 10–11, 18163128, 10.1038/ng0108-10, 1:CAS:528:DC%2BD1cXhtVyktg%3D%3D
Schneider L, Clement C A, Teilmann S C, et al. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol, 2005, 15: 1861–1866, 16243034, 10.1016/j.cub.2005.09.012, 1:CAS:528:DC%2BD2MXhtFCjsL%2FJ
Rohatgi R, Milenkovic L, Scott M P. Patched1 regulates hedgehog signaling at the primary cilium. Science, 2007, 317: 372–376, 17641202, 10.1126/science.1139740, 1:CAS:528:DC%2BD2sXnslGrs70%3D
Huangfu D, Liu A, Rakeman A S, et al. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature, 2003, 426: 83–87, 14603322, 10.1038/nature02061, 1:CAS:528:DC%2BD3sXoslSjtbw%3D
Liu A, Wang B, Niswander L A. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development, 2005, 132: 3103–3111, 15930098, 10.1242/dev.01894, 1:CAS:528:DC%2BD2MXnvVWmt7Y%3D
Simons M, Gloy J, Ganner A, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet, 2005, 37: 537–543, 15852005, 10.1038/ng1552, 1:CAS:528:DC%2BD2MXjsF2ks7s%3D
Ross A J, May-Simera H, Eichers E R, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet, 2005, 37: 1135–1140, 16170314, 10.1038/ng1644, 1:CAS:528:DC%2BD2MXhtVCntL%2FK
Jones C, Roper V C, Foucher I, et al. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat Genet, 2008, 40: 69–77, 18066062, 10.1038/ng.2007.54, 1:CAS:528:DC%2BD1cXhtVykuw%3D%3D
Nauli S M, Alenghat F J, Luo Y, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet, 2003, 33: 129–137, 12514735, 10.1038/ng1076, 1:CAS:528:DC%2BD3sXnsFSksw%3D%3D
Pazour G J, Witman G B. The vertebrate primary cilium is a sensory organelle. Curr Opin Cell Biol, 2003, 15: 105–110, 12517711, 10.1016/S0955-0674(02)00012-1, 1:CAS:528:DC%2BD3sXotVOg
Sorokin S. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Biol, 1962, 15: 363–377, 13978319, 10.1083/jcb.15.2.363, 1:STN:280:CC2C2s%2Fht1M%3D
Sorokin S P. Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J Cell Sci, 1968, 3: 207–230, 5661997, 1:STN:280:CCeA38ngsF0%3D
Hagiwara H, Ohwada N, Aoki T, et al. Ciliogenesis and ciliary abnormalities. Med Electron Microsc, 2000, 33: 109–114, 11810467, 10.1007/s007950000009, 1:STN:280:DC%2BD38%2Foslynug%3D%3D
Dawe H R, Farr H, Gull K. Centriole/basal body morphogenesis and migration during ciliogenesis in animal cells. J Cell Sci, 2007, 120: 7–15, 17182899, 10.1242/jcs.03305, 1:CAS:528:DC%2BD2sXht1Gmsrk%3D
Pan J, Snell W. The primary cilium: Keeper of the key to cell division. Cell, 2007, 129: 1255–1257, 17604715, 10.1016/j.cell.2007.06.018, 1:CAS:528:DC%2BD2sXotV2hs7o%3D
Pan J, Wang Q, Snell W J. An aurora kinase is essential for flagellar disassembly in Chlamydomonas. Dev Cell, 2004, 6: 445–451, 15030766, 10.1016/S1534-5807(04)00064-4, 1:CAS:528:DC%2BD2cXis1KmsLs%3D
Pan J, Snell W J. Chlamydomonas shortens its flagella by activating axonemal disassembly, stimulating IFT particle trafficking, and blocking anterograde cargo loading. Dev Cell, 2005, 9: 431–438, 16139231, 10.1016/j.devcel.2005.07.010, 1:CAS:528:DC%2BD2MXhtVert7%2FN
Wloga D, Camba A, Rogowski K, et al. Members of the NIMA-related kinase family promote disassembly of cilia by multiple mechanisms. Mol Biol Cell, 2006, 17: 2799–2810, 16611747, 10.1091/mbc.E05-05-0450, 1:CAS:528:DC%2BD28XlvVCisL4%3D
Pugacheva E N, Jablonski S A, Hartman T R, et al. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell, 2007, 129: 1351–1363, 17604723, 10.1016/j.cell.2007.04.035, 1:CAS:528:DC%2BD2sXotV2hsL0%3D
Kozminski K G, Johnson K A, Forscher P, et al. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci USA, 1993, 90: 5519–5523, 8516294, 10.1073/pnas.90.12.5519, 1:STN:280:ByyB1Mnhs10%3D
Iomini C, Babaev-Khaimov V, Sassaroli M, et al. Protein particles in Chlamydomonas flagella undergo a transport cycle consisting of four phases. J Cell Biol, 2001, 153: 13–24, 11285270, 10.1083/jcb.153.1.13, 1:CAS:528:DC%2BD3MXisVyhtL4%3D
Mueller J, Perrone C A, Bower R, et al. The FLA3 KAP subunit is required for localization of kinesin-2 to the site of flagellar assembly and processive anterograde intraflagellar transport. Mol Biol Cell, 2005, 16: 1341–1354, 15616187, 10.1091/mbc.E04-10-0931, 1:CAS:528:DC%2BD2MXit1Srt7Y%3D
Snow J J, Ou G, Gunnarson A L, et al. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat Cell Biol, 2004, 6: 1109–1113, 15489852, 10.1038/ncb1186, 1:CAS:528:DC%2BD2cXptFentLo%3D
Follit J A, Tuft R A, Fogarty K E, et al. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell, 2006, 17: 3781–3792, 16775004, 10.1091/mbc.E06-02-0133, 1:CAS:528:DC%2BD28Xpt1alur0%3D
Cole D G, Diener D R, Himelblau A L, et al. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol, 1998, 141: 993–1008, 9585417, 10.1083/jcb.141.4.993, 1:CAS:528:DyaK1cXjt1Kntb4%3D
Piperno G, Mead K. Transport of a novel complex in the cytoplasmic matrix of Chlamydomonas flagella. Proc Natl Acad Sci USA, 1997, 94: 4457–4462, 9114011, 10.1073/pnas.94.9.4457, 1:CAS:528:DyaK2sXjtVyrtbk%3D
Cole D G. The intraflagellar transport machinery of Chlamydomonas reinhardtii. Traffic, 2003, 4: 435–442, 12795688, 10.1034/j.1600-0854.2003.t01-1-00103.x, 1:CAS:528:DC%2BD3sXltFektLk%3D
Qin H, Diener D R, Geimer S, et al. Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J Cell Biol, 2004, 164: 255–266, 14718520, 10.1083/jcb.200308132, 1:CAS:528:DC%2BD2cXmsV2isg%3D%3D
Pazour G J, Dickert B L, Witman G B. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J Cell Biol, 1999, 144: 473–481, 9971742, 10.1083/jcb.144.3.473, 1:CAS:528:DyaK1MXhtFGru7s%3D
Pazour G J, Dickert B L, Vucica Y, et al. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol, 2000, 151: 709–718, 11062270, 10.1083/jcb.151.3.709, 1:CAS:528:DC%2BD3cXnsl2lsbg%3D
Davidge J A, Chambers E, Dickinson H A, et al. Trypanosome IFT mutants provide insight into the motor location for mobility of the flagella connector and flagellar membrane formation. J Cell Sci, 2006, 119: 3935–3943, 16954145, 10.1242/jcs.03203, 1:CAS:528:DC%2BD2sXit1Cgtw%3D%3D
Tsao C C, Gorovsky M A. Different effects of Tetrahymena IFT172 domains on anterograde and retrograde intraflagellar transport. Mol Biol Cell, 2008, 19: 1450–1461, 18199688, 10.1091/mbc.E07-05-0403
Scholey J M. Intraflagellar transport. Annu Rev Cell Dev Biol, 2003, 19: 423–443, 14570576, 10.1146/annurev.cellbio.19.111401.091318, 1:CAS:528:DC%2BD3sXpsFamt7g%3D
Kramer-Zucker A G, Olale F, Haycraft C J, et al. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer’s vesicle is required for normal organogenesis. Development, 2005, 132: 1907–1921, 15790966, 10.1242/dev.01772, 1:CAS:528:DC%2BD2MXktlShsLs%3D
Tsujikawa M, Malicki J. Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron, 2004, 42: 703–716, 15182712, 10.1016/S0896-6273(04)00268-5, 1:CAS:528:DC%2BD2cXlslWhtbg%3D
Pazour G J, Baker S A, Deane J A, et al. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol, 2002, 157: 103–113, 11916979, 10.1083/jcb.200107108, 1:CAS:528:DC%2BD38Xis1KnsLY%3D
Pan J, Snell W J. Signal transduction during fertilization in the unicellular green alga, Chlamydomonas. Curr Opin Microbiol, 2000, 3: 596–602, 11121779, 10.1016/S1369-5274(00)00146-6, 1:CAS:528:DC%2BD3MXis1amsA%3D%3D
Pazour G J, Agrin N, Leszyk J, et al. Proteomic analysis of a eukaryotic cilium. J Cell Biol, 2005, 170: 103–113, 15998802, 10.1083/jcb.200504008, 1:CAS:528:DC%2BD2MXlvFCnsrg%3D
Tam L W, Lefebvre P A. Cloning of flagellar genes in Chlamydomonas reinhardtii by DNA insertional mutagenesis. Genetics, 1993, 135: 375–384, 8244002, 1:CAS:528:DyaK2cXlt1Khur8%3D
Pennarun G, Escudier E, Chapelin C, et al. Loss-of-function mutations in a human gene related to Chlamydomonas reinhardtii dynein IC78 result in primary ciliary dyskinesia. Am J Hum Genet, 1999, 65: 1508–1519, 10577904, 10.1086/302683, 1:CAS:528:DC%2BD3cXjvFentQ%3D%3D
Wheatley D N, Wang A M, Strugnell G E. Expression of primary cilia in mammalian cells. Cell Biol Int, 1996, 20: 73–81, 8936410, 10.1006/cbir.1996.0011, 1:STN:280:ByiD1M%2FltVY%3D
Storm van’s Gravesande K, Omran H. Primary ciliary dyskinesia: Clinical presentation, diagnosis and genetics. Ann Med, 2005, 37: 439–449, 16203616, 10.1080/07853890510011985
Cowan M J, Gladwin M T, Shelhamer J H. Disorders of ciliary motility. Am J Med Sci, 2001, 321: 3–10, 11202477, 10.1097/00000441-200101000-00002, 1:STN:280:DC%2BD3M7hvVyqsQ%3D%3D
Ibanez-Tallon I, Pagenstecher A, Fliegauf M, et al. Dysfunction of axonemal dynein heavy chain Mdnah5 inhibits ependymal flow and reveals a novel mechanism for hydrocephalus formation. Hum Mol Genet, 2004, 13: 2133–2141, 15269178, 10.1093/hmg/ddh219, 1:CAS:528:DC%2BD2cXnsVyisrc%3D
Adams N A, Awadein A, Toma H S. The retinal ciliopathies. Ophthalmic Genet, 2007, 28: 113–125, 17896309, 10.1080/13816810701537424, 1:CAS:528:DC%2BD2sXht1amtLrP
Afzelius B A. Cilia-related diseases. J Pathol, 2004, 204: 470–477, 15495266, 10.1002/path.1652, 1:CAS:528:DC%2BD2cXhtVCgsrjP
Kulaga H M, Leitch C C, Eichers E R, et al. Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nat Genet, 2004, 36: 994–998, 15322545, 10.1038/ng1418, 1:CAS:528:DC%2BD2cXntFSku7o%3D
Williams D S. Usher syndrome: Animal models, retinal function of Usher proteins, and prospects for gene therapy. Vision Res, 2008, 48: 433–441, 17936325, 10.1016/j.visres.2007.08.015, 1:CAS:528:DC%2BD1cXhtlOmtL0%3D
Witzgall R. New developments in the field of cystic kidney diseases. Curr Mol Med, 2005, 5: 455–465, 16101475, 10.2174/1566524054553496, 1:CAS:528:DC%2BD2MXmvVyktLk%3D
Hildebrandt F, Zhou W. Nephronophthisis-associated ciliopathies. J Am Soc Nephrol, 2007, 18: 1855–1871, 17513324, 10.1681/ASN.2006121344, 1:CAS:528:DC%2BD2sXnt1Wjs7g%3D
Beales P L. Lifting the lid on Pandora’s box: The Bardet-Biedl syndrome. Curr Opin Genet Dev, 2005, 15: 315–323, 15917208, 10.1016/j.gde.2005.04.006, 1:CAS:528:DC%2BD2MXks1Gjsb8%3D
Ou G, Blacque O E, Snow J J, et al. Functional coordination of intraflagellar transport motors. Nature, 2005, 436: 583–587, 16049494, 10.1038/nature03818, 1:CAS:528:DC%2BD2MXmsFCgsL4%3D
Nachury M V, Loktev A V, Zhang Q, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell, 2007, 129: 1201–1213, 17574030, 10.1016/j.cell.2007.03.053, 1:CAS:528:DC%2BD2sXntVOnt7Y%3D
Li G, Vega R, Nelms K, et al. A role for Alstrom syndrome protein, alms1, in kidney ciliogenesis and cellular quiescence. PLoS Genet, 2007, 3: e8, 17206865, 10.1371/journal.pgen.0030008
Dawe H R, Smith U M, Cullinane A R, et al. The Meckel-Gruber Syndrome proteins MKS1 and meckelin interact and are required for primary cilium formation. Hum Mol Genet, 2007, 16: 173–186, 17185389, 10.1093/hmg/ddl459, 1:CAS:528:DC%2BD2sXhtVKktrc%3D
Ferrante M I, Zullo A, Barra A, et al. Oral-facial-digital type I protein is required for primary cilia formation and left-right axis specification. Nat Genet, 2006, 38: 112–117, 16311594, 10.1038/ng1684, 1:CAS:528:DC%2BD2MXhtlCmtr7E
Arts H H, Doherty D, van Beersum S E, et al. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nat Genet, 2007, 39: 882–888, 17558407, 10.1038/ng2069, 1:CAS:528:DC%2BD2sXmvFKlsLs%3D
Otto E A, Loeys B, Khanna H, et al. Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior-Loken syndrome and interacts with RPGR and calmodulin. Nat Genet, 2005, 37: 282–288, 15723066, 10.1038/ng1520, 1:CAS:528:DC%2BD2MXhsFOqu7k%3D
Sayer J A, Otto E A, O’Toole J F, et al. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet, 2006, 38: 674–681, 16682973, 10.1038/ng1786, 1:CAS:528:DC%2BD28XltVOhtb0%3D
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (Grant Nos. 30671090 and 30771084), and National Basic Research Program of China (“973” Program) (Grant No. 2007CB914401)
Rights and permissions
About this article
Cite this article
Pan, J. Cilia and ciliopathies: From Chlamydomonas and beyond. SCI CHINA SER C 51, 479–486 (2008). https://doi.org/10.1007/s11427-008-0071-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-008-0071-3