Abstract
Recent models on cognitive aging consider the ability to maintain and update context information to be a key source of age-related impairments in various cognitive tasks (Braver & Barch in Neuroscience & Biobehavioral Reviews, 26: 809–817, 2002). Context updating has been investigated with a modified AX-continuous-performance task by comparing performance and brain activity between context-dependent trials (i.e., correct responses require updating of the preceding cue information) and context-independent trials (i.e., correct responses are independent of cue information). We used an event-related potential (ERP) approach to identify sources of age differences in context processing in the early and late processing of cue information. Our behavioral data showed longer latencies and higher error rates on context-dependent than on context-independent trials for older than for younger adults, suggesting age-related impairments in context updating. The ERP data revealed larger P3b amplitudes for context-dependent than for context-independent trials only in younger adults. In contrast, in older adults, P3b amplitudes were more evenly distributed across the scalp and did not differ between context conditions. Interestingly, older but not younger adults were sensitive to changes of cue identity, as indicated by larger P3b amplitudes on cue-change than on cue-repeat trials, irrespective of the actual context condition. We also found a larger CNV on context-dependent than on context-independent trials, reflecting active maintenance of context information and response preparation. The age-differential effects in the P3b suggest that both younger and older adults were engaged in updating task-relevant information, but relied on different information: Whereas younger participants indeed relied on context cues to update and reconfigure the task settings, older adults relied on changes in cue identity, irrespective of context information.
Similar content being viewed by others
Introduction
It is generally assumed that successful behavioral adaptation requires cognitive control mechanisms that guide actions and thoughts according to internal and external goals (see Braver & West, 2008; Miller & Cohen, 2001). Cognitive control refers to a set of processes including the active representation, maintenance, and updating of goal information (Braver & Cohen, 2000). In daily life, for example, one may intend to go to the gym in the evening. This goal needs to be maintained and protected against incoming events (e.g., an unexpected dinner invitation). However, cognitive control is also seen as a flexible mechanism allowing the updating of representations even after goal failure—that is, when confronted with salient trigger information (e.g., sport shoes). Such cues can help to update goal representations and ensure a reorientation toward previously specified goals.
One model that describes the neural basis of cognitive control, the guided activation theory of prefrontal cortex (PFC) function, has been suggested by Miller and Cohen (2001). In this model, frontal dopamine (DA) projections are thought to carry new information into PFC, whereas persistent activity of PFC neurons is associated with the representation of goals as well as the means to achieve them. This representation serves as a top-down signal for posterior (sub)cortical areas that are responsible for implementing stimulus–response mappings and output processes. Meanwhile, a large body of evidence suggests that the PFC (Raz, 2000; West, 1996) and the DA system (Bäckman & Farde, 2005; Bäckman, Ginovart, Dixon, Wahlin, Wahlin, Halldin, & Farde, 2000; Rinne, 1987; Suhara, Fukuda, Inoue, Itoh, Suzuki, Yamasaki, & Tateno, 1991) are particularly sensitive to aging, because negative changes are stronger and occur at an earlier age than in posterior cortical structures and other neurotransmitter systems. Such age-related changes are found at the neuronal level—for example, in significant gray and white matter loss, neurofibrillary tangles, and decreased cerebral blood flow and glucose metabolism in the PFC (Bäckman & Farde, 2005; Raz, 2000; Suhara et al., 1991). Moreover, at the behavioral level, performance deficits in older adults have been shown in a range of cognitive control tasks thought to be mediated by PFC and DA functions—for instance, in working memory tasks (Hale, Myerson, Emery, Lawrence, & Dufault, 2007; Reuter-Lorenz & Sylvester, 2005), switching tasks (for a meta-analysis, see Wasylyshyn, Verhaeghen, & Sliwinski, 2011; for a review, see Kray & Ferdinand, 2014), or performance-monitoring tasks (Eppinger & Kray, 2011; Ferdinand & Kray, 2013; Hämmerer & Eppinger, 2012; Nieuwenhius, Ridderinkhof, Talsma, Coles,Holroyd, Kok, & van der Molen, 2002).
In the dual-mechanisms-of-control theory (DMC), Braver and colleagues (Braver, 2012; Braver & Barch, 2002; Braver, Satpute, Rush, Racine, & Barch, 2005; Braver & West, 2008) suggested that age differences in these cognitive control tasks can be explained by one common underlying mechanism, referring to the ability to represent, update, and maintain context information. By context, they meant all kinds of representations about the task instructions, rules, and internal or external goals that guide behavior. The active online maintenance of context information is thought to rely on sustained activity of dorsolateral PFC (DL-PFC) neurons, whereas DA projections to the DL-PFC serve as a “gating mechanism.” This “gating mechanism” insulates and refreshes context representations by regulating the access of new information into PFC. Given the known negative age-related changes in the PFC and the DA system, the theory posits that older adults show impairments in the processing of contextual information.
To measure context processing Braver and colleagues often used a modified version of the AX-continuous-performance task (AX-CPT). In the AX-CPT, letters are consecutively presented in cue–probe pairs and correct responses to probes are dependent on the context provided by the preceding cue. Interestingly, they found evidence for age-related changes in the dynamic control of behavior during this task (Braver et al., 2005; Rush, Barch, & Braver, 2006). Whereas younger adults showed a tendency to be strongly engaged in updating and maintaining context information to prepare for the upcoming response execution (proactive control), older adults typically engaged less in advance preparation and needed to reactivate the context information when confronted with the probe (reactive control; for a review, see Braver & Barch, 2002). Initial evidence from studies using functional magnetic resonance imaging (fMRI; Braver & Barch, 2002; Braver, Paxton, Locke, & Barch, 2009; Jimura & Braver, 2009; MacDonald & Carter, 2003; Paxton, Barch, Racine, & Braver, 2008) has suggested that these age-related changes in the control of behavior are linked to differential activation patterns within PFC. For younger adults who usually show a rather proactive, cue-based control style, an increase in lateral PFC activation was found after cue presentation that persisted during the cue–probe delay (Braver & Bongiolatti, 2002; Jimura & Braver, 2009; Perlstein, Dixit, Carter, Noll, & Cohen, 2003). In contrast, for older adults who often show a reactive, probe-based control style, larger lateral PFC activation was found with the onset of the probe presentation (Jimura & Braver, 2009; Paxton et al., 2008).
Moreover, age-related differences in context processing have been shown to be susceptible to various experimental manipulations: Extended practice and directed strategy training toward processing of the cue reduced context processing deficits and induced a proactive shift to larger cue-based activations in the elderly (Braver et al., 2009; Paxton, Barch, Storandt, & Braver, 2006). In turn, penalty-based monetary incentives reduced the proactive bias in younger adults and changed the temporal activation in PFC toward a reactive control style (Braver et al., 2009; Locke & Braver, 2008). On the basis of these findings, Braver and colleagues concluded that the neural mechanisms underlying different cognitive control processes are anatomically organized within the same brain areas. However, contrasting temporal activation within these brain areas account for different cognitive control modes. Thus, age differences in context updating should be visible in temporal activations of PFC regions during cue and probe presentation. So far, evidence for this view is primarily based on behavioral and fMRI methods that are less suitable to capture age differences in the more fine-grained temporal resolution of information processing during cue and probe intervals. The main goal of our study was to use an electroencephalogram (EEG) approach to examine whether age-related changes are reflected in neuronal correlates of context processing by using a modified AX-CPT.
EEG correlates of cue-related processing in cognitive control tasks
One study so far has applied an EEG approach to a modified version of the AX-CPT in a student sample, to determine the neuronal correlates of context processing (Lenartowicz, Escobedo-Quiroz, & Cohen, 2010). Updating of context information was manipulated on a trial-by-trial basis, and participants responded to cue–probe pairs that differed in their dependency on the preceding cue. Four cue–probe combinations were context-dependent, and four combinations were context-independent pairs (see Fig. 1a). On context-independent (c-indep) trials, the correct response to probes was independent of the preceding context cue, and thus only relied on the assignment to one of two response buttons. On context-dependent (c-dep) trials, correct responses to probes were exactly reversed for the cue–probe combinations, and therefore required the processing of the cue information as well as the reconfiguration of cognitive processes as stimulus–response (S–R) mappings were reversed. C-dep and c-indep trials had a 25 % probability each and were intermixed with 50 % control trials, which did not require any response. The results of this study showed a context effect at the behavioral level—that is, better performance on c-indep than on c-dep trials. At the electrophysiological level, they revealed a larger frontally distributed P2 component after context cue presentation on c-dep trials than on c-indep and control trials, which was interpreted as early updating of the context cue information. A second context effect was observed in a parietally distributed P3b, with a larger amplitude for c-dep trials than for c-indep and control trials that was linked to task reconfiguration processes. Finally, the results indicated a context effect in the centrally focused negative slow wave. It was more negative for c-dep than for c-indep and control trials, lasting from 700 ms to the end of the cue interval; this was attributed to task maintenance processes. Altogether, three EEG components—the P2, the P3b, and the negative slow wave—have been identified to be sensitive to cue-related processing in the AX-CPT, which has been applied to measure context updating. We used a similar approach in the present study, to determine whether age differences in cue-related processing would be reflected in all three components, or whether differential age effects would be obtained in these components.
(a) Example of the assignment of cue and probe pictures to correct response keys on c-dep and c-indep trials. On c-dep trials, the correct responses to probes (represented by the pictures of the bird and the cat) after presentation of the cues (represented by the pictures of the younger woman and the older man) are exactly reversed for the two cues; for example, here participants have to press the left response key if the bird follows the younger woman, whereas they have to press the right key if the bird follows the older man. Thus, the correct responses to probes depend on information about the cue presentation. On c-indep trials, correct responses to the probes (represented by the pictures of the fish and the rabbit) are exactly the same for both cues (represented by the pictures of the younger man and the older woman); for example, here participants always have to press the left key if they see the picture of the fish and the right key if they see the picture of the rabbit. Thus, correct responses are independent of the cue presentation. (b) Trial procedure and presentation times of the stimuli in the modified AX-CPT. The probe was presented for 5,000 ms or until a response was made, and the intertrial interval was set to 200 ms
Given that context processing contains the updating of all types of information, it also includes the updating of task sets, frequently investigated with the task-switching paradigm. In cued task-switching paradigms, the to-be-executed task is also announced by a preceding cue. If enough time is provided, the cue information can be used to configure and reconfigure the task set in advance. The term task set refers to the configuration of the cognitive processes required for selection, adoption, and execution of an appropriate response according to the prior task-goal instructions, and thus task switching measures the efficient reconfiguration of two or more task-set instructions (Monsell, 2003; Rogers & Monsell, 1995). Costs of switching between tasks can be derived at a more local level by calculating the difference between performance on task-switch trials and task-repetition trials within mixed blocks (see Rogers & Monsell, 1995); these are termed specific switch costs (Kray & Lindenberger, 2000). Moreover, the costs of switching between tasks have also been measured by determining the differences between performance on mixed blocks and single-task blocks involving only performing one task (see Allport, Styles, & Hsieh, 1994); these are termed general switch costs (Kray & Lindenberger, 2000). Importantly, age differences are usually found in general switch costs and explained as age-related impairments in dealing with dual-task demands, since mixed blocks require the selection and maintenance of different task sets in working memory (for a meta-analysis, see Wasylyshyn, Verhaeghen, & Sliwinski, 2011; for a review, see Kray & Ferdinand, 2014). Age effects on specific switch costs have revealed mixed results and seem to be smaller than age differences in general switch costs (Kray, Eber, & Karbach, 2008; Kray, Eber, & Lindenberger, 2004). This pattern of findings has been explained by, for instance, the assumption that older adults have a tendency to update the task set even when it is not required—that is, even on repeat trials within mixed blocks (see Mayr, 2001)—in this way reducing the difference between repeat and switch trials (see also Kray & Ferdinand, 2014).
ERP studies on age differences in task switching have examined psychophysiological processes associated with cue- and target-related processes (e.g., Eppinger, Kray, Mecklinger, & John, 2007). Of specific interest of the present study are ERP findings that have been reported on age differences in updating and reconfiguration of task sets in cue-related time intervals. The results of these studies indicated that younger adults typically show a posteriorly distributed positive component approximately 300 ms after task cue presentation, which is larger in mixed relative to single-task blocks. This “mixing cost positivity” (Karayanidis, Provost, Brown, Paton, & Heathcote, 2011a; Karayanidis, Whitson, Heathcote, & Michie, 2011b) bears resemblance to the P3b subcomponent of the P300 event-related potential. Although the functional meaning of the P3b is still a matter of debate (Verleger, Jaśkowski, & Wascher, 2005), one model by Donchin and Coles (1988) associates the amplitude of the P3b to the amount of resource allocation that is available for revising working memory content to incoming stimuli. Given that the mixing cost positivity in task switching has a striking resemblance to the P3b usually investigated with the oddball paradigm, it is frequently labeled as P3b. Accordingly, the larger amplitude on mixed relative to single-task blocks in younger adults was interpreted as reflecting the updating of task sets after task cue presentation, which is essential on mixed but not on single-task blocks (Eppinger et al., 2007; Kray, Eppinger, & Mecklinger, 2005; West, 2004). In older adults, the majority of cued task-switching studies have found the P3b to be delayed, but have not found amplitude differences as compared to younger adults (but see West & Moore, 2005, for an attenuated P3b in older adults that they associated with impaired WM updating). This delay in P3b latency indicates a slowing of updating processes (Karayanidis et al., 2011b). However, the delayed and/or prolonged P3b amplitudes may also reflect older adults’ tendency to use the whole cue–target interval (CTI) to prepare for the upcoming response (Karayanidis et al., 2011b). Accordingly, shortening the CTI leads to an earlier onset of the P3b in older adults (Czernochowski, 2011). However, age differences have also been found in the distribution of the P3b: Although the P3b amplitude typically increases from frontal to posterior electrodes in younger adults, older adults exhibit a more evenly distributed P3b—that is, a flattened anterior–posterior gradient, associated with a compensatory recruitment of frontal regions during task cue updating (Eppinger et al., 2007; Friedman, Kazmerski, & Fabiani, 1997). Younger adults also show a larger positive component on switch relative to repeat trials within mixed blocks (Karayanidis et al., 2011b), and this “switch cost positivity” has been interpreted as reflecting anticipatory reconfiguration processes on mixed blocks. In older adults this P3b-like switch cost positivity is small or absent due to a larger amplitude on repeat trials. This suggests that older adults may pursue a specific control strategy and update task sets on both switch and repeat trials. This finding may account for the lack of behavioral age effects on specific switch costs (Eppinger et al., 2007; Karayanidis et al., 2011b). At the end of the cue interval, age effects in ERPs have been obtained in a negative slow wave that was linked to the maintenance of task representations over time. For instance, Kray et al. (2005) found a centrally focused contingent negative variation (CNV) that was larger on mixed than on single blocks in older but not in younger adults, suggesting a deficit in maintaining the updated task set until presentation of the executive stimulus in the elderly. ERP task-switching studies are a basis for expectations of the present study, because the context cue in the modified AX-CPT and the task cue in cued switching paradigms serve the same purpose—that is, informing participants about the upcoming task and providing information required for task preparation in advance. Nevertheless, it is essential to note that there are important differences between the AX-CPT and cued switching tasks. First, although c-indep trials bear a resemblance to single-task blocks, because the context cue is not necessary to be able to respond correctly to the subsequent probe, no genuine single block is actually included in the AX-CPT. Second, cued task-switching paradigms usually include two different task cues that announce which of the two tasks should be performed on the upcoming trial. In contrast, the AX-CPT consists of two context conditions (c-dep, c-indep) and each one is represented by two context cues, yielding a total of four different context cues. Given the task structure, a change in the context cue identity from one to the other therefore has a differential impact on context updating and task-reconfiguration processes on c-dep and c-indep trials. On two consecutive c-dep trials, a change in the context cue (e.g., a trial including the cue of the younger woman followed by a trial including the cue of the older man in Fig. 1a) should involve context updating and task-set reconfiguration, because response assignments are directly reversed for both cues. In contrast, a change in the context cue identity on two consecutive c-indep trials (i.e., a trial including the cue of the younger man followed by a cue–probe combination including the older-woman cue in Fig. 1a) should not require context updating and task reconfiguration, because the response assignments are the same for the two cues. Since the response assignments are the same as in the previous (n – 1) trial, an exact repetition of the context cue should not require context updating and task reconfiguration on both c-dep and c-indep trials. In order to ensure that context effects in the ERPs were actually due to differential context-updating requirements on c-dep and c-indep trials, and did not reflect a perceptual change in the context-cue identity, irrespective of the context condition, the study by Lenartowicz et al. (2010) included a control analysis. This analysis focused on sequence effects; that is, they investigated ERPs time-locked to perceptual changes in the context-cue identity across c-dep and c-indep trials. As we outlined above, only changes in context-cue identity on c-dep trials should require context updating and task reconfiguration, but not changes in cue identity on c-indep trials. In contrast, an exact context cue repetition should not require context-updating processes on either c-dep or c-indep trials. The authors found a larger P2 amplitude for context-cue changes on c-dep than on c-indep trials, signaling that different processes were involved in changes of cue identity and changes of context. However, c-dep trials in which the context cue was exactly repeated had the largest P2 amplitude, indicating that participants updated context information even when the cue signaled no change in task-set reconfiguration. Lenartowicz et al. further found that the P3b and the negative slow wave were sensitive to changes in context-cue identity independent of the context manipulation, and concluded that this result might indicate an effect of cue priming on the P3b and the negative slow wave.
The present study
The main goal of this study was to examine age differences in the neuronal correlates of processing context information by means of an ERP approach on the modified AX-CPT. Specifically, we were interested in whether age-related differences could be found already in early cue-related potentials (P2), or only in the late cue-related potentials (P3b, CNV) that have previously been identified and linked to context processing in the study by Lenartowicz et al. (2010). Therefore, we adapted the AX-CPT used in the previous study. First, given that context-specific effects and context-unspecific effects had been clearly separated in this preceding study, we did not include control trials (also in order to increase the number of trials for EEG analysis). Second, we used different stimulus materials—that is, pictures of neutral faces as cues, and pictures of animals as probes. This was done to make sure that older adults could separate cue from probe events and to help them keep track of the response assignments.
On the basis of previous findings, we expected to reveal a context effect—that is, a difference between c-dep and c-indep trials—in the behavioral and ERP data (Karayanidis et al., 2011b; Kray et al., 2005; Kray & Ferdinand, 2014). Latencies and error rates should be larger on c-dep than on c-indep trials, because c-dep trials, but not c-indep trials, require updating of cue information in order to respond correctly. Moreover, we also expected to replicate (1) a larger P2 amplitude for c-dep than for c-indep trials, an effect that has been specifically linked to early context updating; (2) a larger P3b for c-dep than for c-indep trials, associated with subsequent task reconfiguration; and (3) a larger CNV on c-dep than on c-indep trials, linked to task-maintenance processes (Kray et al., 2005; Lenartowicz et al., 2010). We also expected to see age differences in context processing—that is, larger context effects for younger than for older adults—at the behavioral level (Braver & Barch, 2002; Kray et al., 2005; Lenartowicz et al., 2010). At the neuronal level, it was an open question whether age differences would be found in all or only in the late ERP components. According to the DMC theory (Braver, 2012; Braver & Barch, 2002; Braver et al., 2001), we should expect to find age differences already in the P2 if this component is specifically related to context updating (Lenartowicz et al., 2010). On the basis of a number of studies from the task-switching literature, we further expected effects of age and context manipulation to show up in the later P3b. Here, older adults should show a smaller P3b context effect and a more evenly distributed P3b topography (Friedman et al., 1997) than should younger adults. At the end of the cue interval, we expected to find effects of age and the context manipulation in the CNV, linked to the maintenance of task rules (Kray et al., 2005; West, 2004). Finally, to make sure that the behavioral and ERP differences between c-dep and c-indep trials were not simply due to perceptual changes in the context cue identity, without any changes in the context condition, we also examined the effects of cue changes in the present study (Lenartowicz et al., 2010).
Method
Participants
Groups of 23 younger and 26 older adults participated in the study. The older adults were recruited from a participant pool and the younger adults were students at Saarland University. They were paid €24 or received course credit for their participation. Informed consent was obtained from all participants. Five younger and eight older adults had to be excludedFootnote 1 because their error rates were more than three standard deviations above the corresponding group mean, technical problems during EEG recording occurred, less than 15 artifact-free trials were obtained for EEG analysis, or due to psychoactive drug use. The final sample included 18 younger adults (mean age = 22.4, range = 19–27 years, 44 % females) and 18 older adults (mean age = 75.1 years, range = 68–82 years, 44 % females). According to self-report, all participants were German native speakers, had normal or corrected-to-normal vision, reported no signs of color-blindness, and were free of neurological or psychological disorders.
Participants performed three psychometric tests in order to show the representativeness of the age groups on fluid and crystallized intelligence performance measures. The digit symbol substitution test (DSST; adapted from Wechsler, 1955, 2008) measured speed of processing, the backward digit span task (BDST; adapted from Wechsler, 1955, 2008) served as a working memory span measure, and the spot-a-word test (Lehrl, 1977; Lindenberger, Mayr, & Kliegl, 1993) as an indicator of vocabulary.Footnote 2 In line with the existing literature and the two-process model of intellectual development (Baltes, Lindenberger, & Staudinger, 1998), we found age differences in the fluid domain of intelligence: Younger adults performed better than older adults in the DSST, F(1, 34) = 60.4, p < .001, η p 2 = .64, and the BDST, F(1, 34) = 5.7, p < .05, η p 2 = .14. In the spot-a-word test, an indicator of crystallized intelligence, older adults reached higher scores than did younger adults, F(1, 34) = 29.8, p < .001, η p 2 = .47. The results on the three intellectual control variables and the characteristics of the samples are displayed in Table 1.
Apparatus, tasks, and stimuli
Similar to the paradigm used by Lenartowicz et al. (2010), participants performed a modified version of the AX-CPT, here consisting of pictures of animals and faces instead of letters. Participants watched a series of cue–probe combinations and had to respond to the probe with a left or a right keypress. To especially help older adults separate cue from probe events, we used pictures of faces as the cues and pictures of animals as the probes. Performance was measured in two different trial types. On c-dep trials, the correct responses were dependent on the preceding cue, since correct responses to probes were exactly reversed for both cue–probe combinations, and therefore were dependent on context information (see Fig. 1a). For example, participants had to respond with a right keypress to the picture of the cat and a left buttonpress to the picture of the bird if they followed the picture of the young woman, and vice versa if the probe pictures followed the picture of the old man. On c-indep trials, the correct response was independent of the preceding cue; that is, the correct response to probes could be given without information about the preceding cue. As is shown in Fig. 1a, the correct responses to the pictures of the fish and the rabbit were the same for both facial-context cues on c-indep trials.
The stimuli were presented in a 3.5 × 5.5 cm black frame at the center of a 24-in. monitor on a white background (see Fig. 1b). We presented four colored pictures of animals as probes (i.e., rabbit, bird, cat, and fish, taken from the databases of Snodgrass & Vanderwart, 1980, and Rossion & Pourtois, 2004) and four colored pictures of young and old faces as cues (Minear & Park, 2004).
Procedure
All participants were individually tested in one session, which took approximately 3 h. Participants first filled in an informed consent, a demographic questionnaire, and a handedness rating (Oldfield, 1971, German version). Then they were tested in the three psychometric tests described above and the AX-CPT. Before starting the experimental task, all participants initially performed three practice blocks of the AX-CPT, with the first block consisting of 24 c-indep trials, and the second block of 24 c-dep trials. The third block was a mixed block, consisting of 24 c-dep and c-indep trials. If participants did not understand the task during the first practice run, practice blocks were repeated. Inspection of the experimental protocol showed that none of the participants had more than two repetitions of any practice block. The experimental phase was composed of eight blocks with 104 trials each, yielding a total of 832 trials per subject. Between blocks, a short rest period was mandatory.
The blocks consisted of equal numbers of c-dep and c-indep trials, which were randomly presented. The assignments of cues and probes to response keys and trial types were pseudorandom across participants, with the only constraint being that a young and an old facial picture were presented in both trial types for each participant. This led to four cue–probe conditions, which were equally assigned to male and female participants separately in each age group.
The trial procedure is displayed in Fig. 1b. Each trial started with a fixation cross (250 ms) followed by the cue (750 ms). After a blank interstimulus interval (750 ms), the probe was presented for 5,000 ms or until the participant responded (see Lenartowicz et al., 2010). If the response was not given within 5,000 ms after probe presentation, the trial was considered a timeout. The probe was followed by another blank screen (500 ms). Afterward, feedback (“correct,” “incorrect,” or “too slow”) was given for 300 ms. The intertrial interval was 200 ms. All participants were instructed to respond as quickly and as accurately as possible.
EEG recording
Participants were seated in a dimly lit, electrically shielded, and sound-attenuated chamber. During the task, EEG and electro-ocular (EOG) activity were recorded simultaneously by Brain Vision Recorder (Brain Products, Munich, Germany) with 59 Ag–AgCl active electrodes (extended international 10–20 system; Jasper, 1958) in an elastic cap (Acticap, Brain Products, Munich, Germany). The left mastoid served as a reference, and the ground electrode was placed at AFz. Impedances were kept below 20 kΩ. The EOG measured vertical eye movements from two electrodes above and below the right eye, and horizontal eye movements from the outer canthi of both eyes. EEG and EOG were low-pass filtered online (250 Hz) and analog-to-digital converted (500 Hz SR). The data were band-pass filtered offline from 0.01–30 Hz and re-referenced to linked mastoids prior to statistical analysis. Recording epochs including eye movements were corrected by using a linear regression approach (Gratton, Coles, & Donchin, 1983), and epochs with other recording artifacts were rejected before averaging whenever the standard deviation in a 200-ms time interval exceeded 30 μV in ocular electrodes, or 20 μV in the representative electrode Cz. After ocular correction, data preprocessing also included a visual screening for artifacts in all electrodes. Additional artifacts in this screening procedure were rarely found, and trials containing technical and muscular artifacts were removed before averaging.
Data analysis
Behavioral data
Practice blocks and trials with reaction times (RTs) faster than 100 ms were excluded from analysis (<1 % of trials). The analysis of latencies was based on correct responses. The analysis of error rates included incorrect responses without timeoutsFootnote 3 that exceeded the maximum response time of 5,000 ms. To analyze effects of context manipulation and to control for perceptual changes in cue identify, a repeated measure analysis of variance (ANOVA) with a significance criterion of α = .05 containing the factors Age Group (younger and older adults), Trial Type (c-dep and c-indep trials), and Cue Change Type (cue-repeat and cue-change trials), was computed for latencies and error rates.
ERP data
To analyze cue-locked ERPs, trials in the interval lasting from 200 ms preceding the cue onset to 1,500 ms thereafter were averaged. The selection of the time interval and the electrodes involved in statistical analyses for the three EEG components and for both age groups described in the following were based on the literature (Karayanidis, Coltheart, Michie, & Murphy, 2003) and on visual inspection. The P2 peaked postcue at about 180 ms in the younger age group, and at about 200 ms in the older age group. Because the age difference in P2 latencies was statistically significant, F(1, 35) = 22.0, p < .001, and in line with previous studies (Lenartowicz et al., 2010), we defined the mean P2 amplitudes as the 80-ms time interval around the peak latency in each age group. Since the P2 had been analyzed at frontal, fronto-central, and central electrodes in the study by Lenartowicz et al. (2010), we also restricted the analysis to these areas. The electrodes of interest (F3, FZ, F4, FC3, FCZ, FC4, C3, CZ, and C4) were organized according to the factors Anterior–Posterior (frontal, fronto-central, and central areas) and Laterality (left, midline, and right lateral electrodes), resulting in three electrodes in each lateral position. The inspection of P3b peak latencies revealed a difference in peak latencies between the age groups (at about 520 ms for younger and 590 ms for older adults) that was statistically significant, F(1, 35) = 26.5, p < .001. Thus, we defined the time window in which the P3b amplitude was measured as ranging from 420 to 620 ms for the younger adults, and from 490 to 690 ms for the older adults. The P3b analysis included 15 electrodes at three laterality sites over frontal (F3, FZ, F4), fronto-central (FC3, FCZ, FC4), central (C3, CZ, C4), centro-parietal (CP3, CPZ, CP4), and parietal (P3, PZ, P4) regions. The CNV component was analyzed in a time window from 1,200 to 1,500 ms at six electrodes (F3, FZ, F4, FC3, FCZ, FC4) at three laterality sites over the frontal (F3, FZ, F4) and fronto-central (FC3, FCZ, FC4) areas in both age groups.
For each component, a repeated measures ANOVA was conducted including the factors Age Group (younger and older adults), Laterality (left lateral, midline, and right lateral electrodes), Anterior–Posterior (frontal, fronto-central, central, centro-parietal, and parietal electrodes), and the experimentally manipulated factors Trial Type (c-dep and c-indep trials) and Cue Change Type (cue-repeat and cue-change trials). In line with previous literature (Karayanidis et al., 2011b; Kray et al., 2005; Lenartowicz et al., 2010), the factor Anterior–Posterior included frontal, fronto-central, and central electrodes for the P2 analysis; frontal, fronto-central, central, centro-parietal, and parietal electrodes for the P3b analysis; and frontal and fronto-central electrodes for analysis of the CNV. To control for age differences in scalp distributions, ANOVAs were conducted using vector-normalized amplitude data (McCarthy & Wood, 1985). If necessary, Greenhouse–Geisser corrections for nonsphericity (Keselman & Rogan, 1980) were applied for behavioral and ERP data, and epsilon-corrected p values are reported, together with the epsilon values (ε) and uncorrected degrees of freedom. Post-hoc analyses of significant interactions were only applied if they contained the factor Age Group or the experimentally manipulated factors Trial Type or Cue Change Type.
Results
Behavioral data
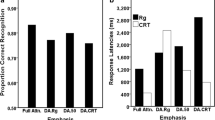
The means and standard errors of the means (SEMs) of latencies and error rates for c-dep and c-indep trials are displayed in Fig. 2, for both age groups and separately for changes and repetitions of cue identity. An ANOVA with the factors Age Group (younger, older adults), Trial Type (c-dep, c-indep trials), and Cue Change Type (cue-repeat, cue-change trials) on error rates showed significant main effects of the factors Age Group, F(1, 34) = 8.0, p < .01, η p 2 = .19, Trial Type, F(1, 34) = 88.0, p < .001, η p 2 = .72, and Cue Change Type, F(1, 34) = 28.5, p < .001, η p 2 = .46. Moreover, we obtained significant interactions between age group and trial type, F(1, 34) = 6.6, p < .05, η p 2 = .16, and between trial type and cue change type, F(1, 34) = 24.1, p < .001, η p 2 = .41. To understand the nature of the two-way interactions, post-hoc comparisons were computed. The interaction between trial type and age group was due to higher error rates on c-dep than on c-indep trials for younger adults, F(1, 17) = 77.4, p < .001, η p 2 = .82, as well as for older adults, F(1, 17) = 42.0, p < .001, η p 2 = .71. Higher error rates for older than for younger adults were only found on c-dep trials, F(1, 34) = 8.7, p < .01, η p 2 = .20 (see Fig. 2a). In order to analyze the significant interaction between trial type and cue change type, separate analyses were computed for each level of the factors Cue Change Type and Trial Type. Error rates were higher on cue-change than on cue-repeat trials for c-dep trials only, F(1, 35) = 35.9, p < .001, η p 2 = .51. Moreover, error rates were higher on c-dep than on c-indep trials for both cue-change and cue-repeat trials [cue-change trials, F(1, 35) = 83.4, p < .001, η p 2 = .71; cue-repeat trials, F(1, 35) = 26.6, p < .001, η p 2 = .43], thus confirming that a context effect occurred, irrespective of changes in cue identity.
(a) Mean error rates and standard errors of the means for c-dep and c-indep trials, shown separately for cue-change and cue-repeat trials for younger and older adults. (b) Mean reaction times (RTs) and standard errors of the means for c-dep and c-indep trials, shown separately for cue-change and cue-repeat trials for younger and older adults
The ANOVA with the factors Age Group (younger, older adults), Trial Type (c-dep, c-indep trials), and Cue Change Type (cue-repeat, cue-change trials) on latenciesFootnote 4 revealed significant main effects of age group, F(1, 34) = 41.9, p < .001, η p 2 = .55, trial type, F(1, 34) = 107.2, p < .001, η p 2 = .76, and cue change type, F(1, 34) = 42.6, p < .001, η p 2 = .56. Significant higher-order interactions were found between age group and trial type, F(1, 34) = 15.9, p < .001, η p 2 = .32, between trial type and cue change type, F(1, 34) = 31.5, p < . 001, η p 2 = .48, and marginally between age group, cue change type, and trial type, F(1, 34) = 4.1, p = .051, η p 2 = .11. Given that the three-way interaction was only marginally significant, and no longer significant when analyzing the natural logarithm of raw RTs (see note 4), post-hoc comparisons were computed for the two-way interactions. Again, separate analyses for each level of the factors Age Group and Trial Type revealed longer latencies on c-dep than on c-indep trials for younger adults, F(1, 17) = 41.8, p < .001, η p 2 = .71, and for older adults, F(1, 17) = 67.9, p < .001, η p 2 = .80. Longer latencies for older than for younger adults were obtained on c-dep trials, F(1, 34) = 38.7, p < .001, η p 2 = .53, and on c-indep trials, F(1, 34) = 35.6, p < .001, η p 2 = .51, but were more pronounced on c-dep trials (see Fig. 2b). The analysis on the interaction between trial type and cue-change type showed longer RTs for c-dep than for c-indep trials on both cue-change, F(1, 35) = 66.2, p < .001, η p 2 = .66, and cue-repeat trials, F(1, 35) = 51.2, p < .001, η p 2 = .59. Latencies were also longer on cue-change than on cue-repeat trials for both levels of the factor Trial Type [c-dep, F(1, 35) = 36.7, p < .001, η p 2 = .51; c-indep, F(1, 35) = 7.9, p < .01, η p 2 = .16].
ERP data
Figure 3 displays grand average waveforms in the cue interval at five midline electrodes for c-dep and c-indep trials, shown separately for cue-change and cue-repeat trials and for older and younger adults (see Fig. 3a and b, respectively). The order of analysis of the specific ERP components reflects their temporal appearance within the postcue interval.
Grand average waveforms elicited by cues during c-dep (black lines) and c-indep (gray lines) trials, shown separately for cue changes (solid lines) and cue repetitions (dotted lines) at five midline electrodes over frontal (FZ), fronto-central (FCZ), central (CZ), centro-parietal (CPZ), and parietal (PZ) regions for younger (a) and older (b) adults. C-dep and c-indep trials elicited comparable P2 amplitudes in younger and older adults, but larger parietal P3b amplitudes were found for c-dep than for c-indep trials in younger, but not in older, adults. The contingent negative variation (CNV) was more negative-going for c-dep than for c-indep trials, and this effect was most pronounced at midline electrodes in both age groups. For visual presentation, the waveforms were low-pass filtered at 12 Hz
The P2 component
The ANOVA with the factors Age Group (younger, older adults), Trial Type (c-dep, c-indep trials), Cue Change Type (cue-repeat, cue-change trials), Laterality (left, midline, right lateralized electrodes), and Anterior–Posterior (frontal, fronto-central, central electrodes) showed only a main effect of age group, F(1, 34) = 73.4, p < .001, η p 2 = .68. No significant interactions including the factor Age Group, Trial Type, or Cue Change Type were found (see Fig. 3).
The P3b component
The ANOVA with the factors Age Group (younger, older adults), Trial Type (c-dep, c-indep trials), Cue Change Type (cue-repeat, cue-change trials), Laterality (left, midline, right lateralized electrodes), and Anterior–Posterior (frontal, fronto-central, central, centro-parietal, parietal electrodes) showed significant main effects of age group, F(1, 34) = 20.0, p < .001, η p 2 = .37, cue change type, F(1, 34) = 12.3, p < .01, η p 2 = .27, laterality, F(2, 68) = 3.4, p < .05, η p 2 = .09, and anterior–posterior, F(4, 136) = 33.0, p < .001, η p 2 = .49, ε = .355. We further obtained significant higher-order interactions between trial type and age group, F(1, 34) = 8.0, p < .01, η p 2 = .19, between anterior–posterior and age group, F(4, 136) = 34.3, p < .001, η p 2 = .50, and between cue change type and age group, F(1, 34) = 6.5, p < .05, η p 2 = .16. The factor Cue Change Type further interacted with anterior–posterior, F(4, 136) = 7.1, p < .01, η p 2 = .17, ε = .407, and with trial type, F(1, 34) = 4.2, p < .05, η p 2 = .11. Furthermore, significant three-way interactions were found between age group, trial type, and anterior–posterior, F(4, 136) = 4.6, p < .05, η p 2 = .06, and between trial type, cue change type, and laterality, F(2, 68) = 3.8, p < .05, η p 2 = .10, ε = .881. To understand the nature of the two- and three-way interaction, we computed post-hoc analyses.
In younger adults, P3b amplitudes increased from frontal to posterior electrodes, F(4, 68) = 47.2, p < .001, η p 2 = .74. P3b amplitudes on c-dep trials were larger than those on c-indep trials (Fig. 3a) at central, F(1, 17) = 7.4, p < .05, η p 2 = .30, centro-parietal, F(1, 17) = 15.4, p < .01, η p 2 = .48, and parietal, F(1, 17) = 21.2, p < .001, η p 2 = .56, electrodes. The P3b was largest at parietal electrodes, as can be inferred from the effect sizes (see Fig. 4). In older adults, we also found a significant interaction between trial type and anterior–posterior, F(1, 17) = 4.7, p < .05, η p 2 = .21, but P3b amplitudes on c-dep and c-indep trials did not differ at any electrode position from frontal to parietal electrodes (all ps > .219; see Figs. 3b and 4).
Mean P3b amplitudes of vector-normalized data in younger and older adults. In younger adults, P3b amplitudes increase from frontal to parietal electrodes, and this effect is more pronounced in c-dep (black bars) than in c-indep (gray bars) trials at central, centro-parietal, and parietal electrodes. In older adults, P3b amplitudes are comparable on c-dep and c-indep trials at all electrode positions from frontal to posterior
The interaction between cue change type and age group showed significant age differences in P3b amplitudes on both cue-change trials, F(1, 34) = 25.3, p < .001, η p 2 = .43, and cue-repeat trials, F(1, 34) = 9.4, p < .01, η p 2 = .22. However, cue-change trials had larger P3b amplitudes than did cue-repeat trials in older adults only, F(1, 17) = 19.5, p < .001, η p 2 = .53.
Finally, the factor Cue Change Type was also involved in a two-way interaction with the factor Trial Type, and in a three-way interaction with the factors Trial Type and Laterality. The two-way interaction was due to larger P3b amplitudes for cue-change than for cue-repeat trials on c-dep trials, F(1, 35) = 12.0, p < .01, η p 2 = .26, and larger P3b amplitudes for cue-change trials on c-dep than on c-indep trials, F(1, 35) = 8.0, p < .01, η p 2 = .19. The three-way interaction showed that P3b amplitudes were larger for cue changes than for cue repetitions on c-dep trials at left lateral, F(1, 35) = 14.8, p < .001, η p 2 = .30, and midline, F(1, 35) = 10.6, p < .01, η p 2 = .23, electrode positions, whereas no difference between cue-change and cue-repeat trials was found on c-indep trials (all p values > .292). On cue-change trials, c-dep and c-indep trials differed at left lateral, F(1, 35) = 9.4, p < .01, η p 2 = .21, and central, F(1, 35) = 10.6, p < .01, η p 2 = .23, electrodes, but not on cue-repeat trials (all p values > .512).
CNV component
The ANOVA with the factors Age Group (younger, older adults), Trial Type (c-dep, c-indep trials), Cue Change Type (cue-repeat, cue-change trials), Laterality (left, midline, right lateralized electrodes), and Anterior–Posterior (frontal, fronto-central electrodes) showed significant main effects of anterior–posterior, F(1, 34) = 43.6, p < .001, η p 2 = .56, laterality, F(2, 68) = 11.6, p < .001, η p 2 = .26, cue change type, F(1, 34) = 9.2, p < .01, η p 2 = .21, and trial type, F(1, 34) = 13.2, p < .01, η p 2 = .28, along with and a significant interaction between trial type and laterality, F(2, 68) = 6.4, p < .01, η p 2 = .16, and a marginally significant interaction between cue change type, anterior–posterior, and age group, F(1, 34) = 3.9, p = .056, η p 2 = .10.
The interaction between trial type and laterality was due to larger negative-going amplitudes at midline electrodes for both c-dep [left lateral vs. midline electrodes, F(1, 35) = 28.0, p < .001, η p 2 = .45; right lateral vs. midline electrodes, F(1, 35) = 25.2, p < .001, η p 2 = .42] and c-indep [left lateral vs. midline electrodes, F(1, 35) = 8.6, p < .01, η p 2 = .20; right lateral vs. midline electrodes, F(1, 35) = 11.8, p < .01, η p 2 = .25] trials. CNV amplitudes were also more negative-going on c-dep than on c-indep trials for each level of the factor Laterality [left lateral, F(1, 35) = 7.8, p < .01, η p 2 = .18; midline, F(1, 35) = 20.4, p < .001, η p 2 = .37; right lateral, F(1, 35) = 7.8, p < .01, η p 2 = .18].
The three-way interaction between cue change type, anterior–posterior, and age group showed that in younger adults, CNV amplitudes were more negative-going for cue-repeat than for cue-change trials at frontal, F(1, 17) = 9.3, p < .01, η p 2 = .35, and fronto-central, F(1, 17) = 10.1, p < .01, η p 2 = .38, electrodes. CNV amplitudes were also more negative at fronto-central than at frontal electrodes for both cue-change trials, F(1, 17) = 10.9, p < .01, η p 2 = .39, and cue-repeat trials, F(1, 17) = 12.3, p < .01, η p 2 = .42. In older adults, we only found larger negative-going amplitudes at fronto-central than at midline electrodes for cue-change trials, F(1, 17) = 36.0, p < .001, η p 2 = .68, and cue-repeat trials, F(1, 17) = 26.9, p < .001, η p 2 = .61.
Discussion
The main goal of this study was to investigate age differences in cue-related ERP correlates of cognitive control that have been attributed to the processing of context information (see Lenartowicz et al., 2010). Therefore, we modified the AX-CPT so that it was suitable for EEG measurement as well as for comparing task performance and ERP correlates between younger and older adults. In this task, participants performed c-dep and c-indep trial types. On c-dep trials, the configuration of S–R rules depending on the preceding cue was required, whereas on c-indep trials, the configuration of S–R rules was independent of the cue. In order to separate the effects of context processing from those of processing a change in cue identity, we also examined the effects of cue changes on c-dep and c-indep trials.
At the behavioral level, our results show the expected effect of the context manipulation—that is, longer latencies and higher error rates on c-dep than on c-indep trials (Lenartowicz et al., 2010). Age differences for both performance measures were more pronounced on c-dep than on c-indep trials, indicating that older adults exhibit difficulties in processing context information. This is generally in line with previous research and theoretical considerations, suggesting that the updating and maintaining of context information is vulnerable to increasing age (e.g., Braver & Barch, 2002; Braver et al., 2001).
We also investigated whether the effects of changes in cue identity can be separated from cue-related context updating. Important to note is that changes in cue identity in the present study reflected a change in S–R assignments only in c-dep conditions, but not in c-indep conditions. Our results indicated that the effects of changes in cue identity can be separated from the context effect. Importantly, context effects were found for both cue-change and cue-repeat trials for younger, as well as for older, adults (see Fig. 2). This suggests that the context effect can be obtained even when the reconfiguration of task rules is not required. We also found effects of cue change on c-dep trials, in which the S–R assignment needed to be changed, for both error rates and latencies. In contrast, on c-indep trials, in which the reconfiguration of S–R assignments was not required, we did not find a reliable effect of cue change for the error data. For latencies, the effect of cue change was larger for c-dep than for c-indep trials.
Taken together, our behavioral data suggest that context updating can be separated from processing cue changes, and that task performance was mostly impaired on trials requiring high demands on cognitive control—namely, the updating of context information as well as the reconfiguration of S–R rules. The results also indicate that age differences were only found for the context-updating effect, but not for the processing of cue changes.
The primary aim of this study was to examine age differences in cue-related ERPs in order to identify whether early and/or late preparatory activity contributes to age differences in context processing. For this reason, we focused our analysis on three ERP components that had been identified in a previous study: the P2, the P3b, and the CNV. For the P2, Lenartowicz et al. (2010) reported larger amplitudes on c-dep than on c-indep trials at frontal electrode locations, independent of changes in cue identity. In accordance with this study, we did not find an effect of changes in cue identity on P2 amplitudes. However, we were not able to replicate the P2 context effect, for either younger or older adults. One explanation for this discrepancy is that the two studies differed in their composition of trial types and stimulus characteristics. In the study by Lenartowicz et al., c-dep and c-indep trials had a 25 % probability each and were intermixed with 50 % control trials, consisting of a single cue that did not require any response. As a consequence, the most difficult c-dep trials occurred rarely and were very salient, probably leading to a different processing mode. In contrast, in the present study, c-dep and c-indep trials both had likelihoods of 50 %, forcing participants to regularly switch between trial types, and this may have diminished the effects of the context manipulation. Hence, differences in cue frequency and stimulus repetitions could explain the different outcomes across studies. This idea is also in line with studies investigating the role of the P2 in visual and auditory processing (Luck & Hillyard, 1994). In these studies, a larger P2 has been shown for task-relevant stimuli, in particular for infrequent targets (Potts, 2004) and in tasks that require occasional effort (Falkenstein, Hoormann, Hohnsbein, & Kleinsorge, 2003). Since control trials in the previous study (Lenartowicz et al., 2010) did not require any response and occurred more often than either c-dep or c-indep trials, the difference between P2 amplitudes on context and control trials may have reflected an effect of processing effort, rather than context processing per se (Potts, 2004). In the present study, P2 amplitudes were comparable on c-dep and c-indep trials, probably because both trial types had the same frequency and were equally relevant for further processing.
Differences in cue-related activities on c-dep and c-indep trials were clearly present in the two later ERP components, the P3b and CNV (see Figs. 3a and b). In line with the results of the study by Lenartowicz et al. (2010), P3b amplitudes were larger on c-dep than on c-indep trials in younger adults. We interpreted this finding in accord with those of oddball experiments (Donchin & Coles, 1988) and task-switching studies (Kray et al., 2005; Kray & Ferdinand, 2014), in which the P3b has been assumed to reflect the updating of task context in working memory. Furthermore, we found age differences in the P3b amplitudes and topographies. Whereas in younger adults, P3b amplitude increased from frontal to parietal electrodes to a larger extent on c-dep than on c-indep trials and had a clear parietal focus, older adults showed no context effects in the parietal P3b and a more flattened anterior–posterior gradient of the P3b (see Fig. 4). Consistent with previous results from task-switching studies (Friedman, Nessler, Johnson, Ritter, & Bersick, 2008) that reported reduced P3b differentiation within mixed blocks in the elderly, the comparable P3b amplitudes on c-dep and c-indep trials may suggest that older adults were equally engaged in updating the task context in both trial types, even if it was not required (as in c-indep trials). This finding fits the view that older adults have a tendency to update task settings on every trial (e.g., Czernochowski, 2011; Karayanidis et al., 2011b; Kray & Ferdinand, 2014; Mayr, 2001; Whitson, Karayanidis, & Michie, 2012).
However, to fully understand the age differences in cue-related processing in the AX-CPT, the results of the changes in cue identity have to be taken into account. Interestingly, younger adults showed no effect of cue identity changes on P3b amplitudes. Thus, younger adults only seemed to update context information and task rules on c-dep trials, but not on c-indep trials, and they did not further distinguish between cue changes and cue repetitions within c-dep trials. In contrast, older adults showed a larger P3b amplitude on cue-change than on cue-repeat trials, irrespective of the context condition. Hence, with regard to P3b amplitudes, older adults not only seemed to update context information to the same extent on c-dep and c-indep trials, but they differentiated between conditions in which the cue identity changed versus stayed the same. This could reflect older adults’ tendency to update task representations whenever there is a change in the environment (here, the cue identity), independently of whether the cue indicates a change of response settings (as was the case on c-dep trials) or not (as was the case on c-indep trials). This tendency constitutes a control mode that could be described as “Be aware—if there is a change in cue identity, there is probably also a change in the to-be-updated S–R rules.” In sum, this study provides evidence that whereas younger adults update context information and task rules particularly on c-dep trials, older adults tend to update the context information—irrespective of the actual context condition—whenever the cue changes, to minimize response interference.
The CNV is thought to reflect maintenance of task sets, in line with previous task-switching experiments (Kray et al., 2005; West, 2004). Consistent with the preceding study (Lenartowicz et al., 2010) and in accordance with our hypothesis that more maintenance is necessary on c-dep than on c-indep trials, the CNV was larger on c-dep- than on c-indep trials. Unlike in previous research (Kray et al., 2005; Wild-Wall, Hohnsbein, & Falkenstein, 2007), age differences in CNVs were not obtained, indicating that older adults maintained cue information to the same extent as younger adults. However, the presence or absence of age effects in maintenance abilities, as reflected in the CNV, seems to go along with differences in task and group characteristics between studies (Wild-Wall et al., 2007). For example, Braver et al. (2005) manipulated the maintenance demands of context information in the AX-CPT by varying the delay between cue and probe (either 1 or 5 s) and investigated behavioral data in a young (aged 18–24 years), a young-old (<75 years of age), and an old-old (>75 years of age) age group. Young-old adults did not differ in maintenance capabilities from young adults, even when the demands of context maintenance were increased. Impairments in context maintenance were only present in old-old adults in the long-interval condition. In a similar vein, Kray et al. (2005) investigated age differences in maintenance during task switching using an ERP approach. In this study, with a time interval of 2,500 ms between the onset of the cue and the probe, differences in CNV amplitudes between younger and older adults were obtained and were interpreted as age-related difficulties in maintaining the task cue over a longer period of time. Concerning our study, these results are important for two reasons: First, we used a sample of high-performing and rather young-old (mean age = 75.1 years; see Table 1) adults. Second, as in the low-maintenance condition of Braver et al. (2005), we used a short (1,500-ms) cue–probe interval. Both of these factors probably reduced the chance to detect age differences in maintenance.
The only further effect on the CNV data was a larger amplitude on cue-repeat than on cue-change trials in younger adults. Although this effect was only marginally significant and was smaller than the effect of the context manipulation on the CNV amplitude, this finding is difficult to explain, given the absence of an effect of cue-identity change in the P3b data for younger adults. We can only speculate that the larger amplitude on repeat trials was due to a repetition effect of S–R rules from the previous trial. Following the small effects of context updating and task-set reconfiguration in cue-repeat trials on the P3b, younger adults may have pursued a strategy to maintain the context information on cue-repeat trials from the previous trial. If so, the CNV was larger on repeat trials because S–R rules had to be maintained from the previous trial—that is, over a longer period of time. Taken together, future studies will be necessary to dissociate the effects of maintaining context information from cue repetitions on CNV amplitudes.
Considering the assumptions of the DMC theory (Braver, 2012; Braver & Barch, 2002), age differences in context updating are postulated to underlie age differences in a variety of cognitive tasks (Braver & Barch, 2002; Braver & West, 2008). A number of previous aging studies investigated age differences in context processing with a more common version of the AX-CPT that allows for the distinction between two types of control modes, pro- and reactive control (Braver, 2012; Braver & Barch, 2002; Braver et al., 2009; Braver et al., 2005; Paxton et al., 2008; Paxton et al., 2006; Rush et al., 2006). These studies indicated that younger adults primarily adopt a proactive control mode in this task, which means a strong engagement in updating and maintenance of the context cue in advance of target presentation. In contrast, older adults show a reactive control mode, which means less engagement in updating and maintaining the context cue, as well as reactivation of the cue information during target presentation. Although our version of the AX-CPT does not allow for a direct comparison of these control styles, and our behavioral findings clearly show age differences in the context-updating effect, the analysis of cue-related ERP components is not consistent with the view that older adults engage less in advance preparation. Instead, both younger and older adults update relevant cue information in the AX-CPT, but in a different way. Younger adults indeed differentiate between different kinds of context cues—those indicating the need to reconfigure task settings, and thus that require context updating (c-dep trials), and those that are not informative as to relevant S–R rules (c-indep trials); hence, younger adults build up a higher-order rule or task representation to perform the underlying task in which changes of cue identity are irrelevant. Older adults may also build up such a higher-order representation, but nonetheless they update whenever the context cue identity changes from trial to trial, independently of whether or not the cue is informative—that is, irrespective of the actual context condition. Interestingly, older adults exactly followed the task instructions, in that participants were instructed on four different S–R rules for the four different cues, without explicitly pointing out the mappings onto the two context conditions. This is one major difference from the task-switching paradigm, in which participants are usually instructed to perform a switch between two tasks and their corresponding S–R rules. Hence, one interesting question for future studies will be to investigate whether age differences in the P3b effects disappear with a change in task instructions highlighting the cue dependency.
Apart from updating, the DMC theory further stresses the ability to maintain context information as a source of age differences in cognitive control. Studies investigating age differences in maintenance abilities so far have revealed mixed results, with maintenance capabilities predominantly being assessed using working memory tasks (Dennis & Cabeza, 2008). Age differences in these studies have usually been more pronounced in processing aspects as compared with simple storage aspects and have increased with the rising complexity of the paradigm (Reuter-Lorenz & Sylvester, 2005). In the AX-CPT, age effects have been shown to occur only in the oldest adults when maintenance demands were increased (Braver et al., 2005), indicating that context updating and context maintenance seem to be dissociable components, with age-related declines occurring at an earlier age with the former. Given that we did not find age differences in maintenance abilities, as reflected in the CNV, this supports the assumption of the DMC theory by confirming the trade-off between age deficits in context updating and maintenance on the basis of ERP evidence: Effects of context updating and age differences were apparent in the P3b, whereas maintenance processes, reflected in the CNV, showed no age effects.
Limitations
The present study also has several limitations and raises questions that will need to be addressed in future research. First, the DMC theory explicitly highlights the role of a prefrontal dopamine-guided gating mechanism at the time that context cues are detected (Braver & Cohen, 2000). However, focusing on behavioral and ERP data, no conclusions can be drawn as to the involvement of prefrontal circuits or DA in context updating. Therefore, molecular imaging studies will be necessary to link DA loss to deficits in context processing (Nyberg, Lövdén, Riklund, Lindenberger, & Bäckman, 2012). In this regard, it would be interesting to focus on longitudinal work investigating the interaction between increasing age, changing DA levels, and context maintenance deficits. Second, the CNV is also assumed to be modulated by DA levels (Linssen et al., 2011). However, we did not find substantial age effects in CNV amplitudes. Therefore, one can only speculate how increasing age and changing DA levels are reflected in the CNV or in maintaining performance in daily life. Moreover, future research will also need to specify conditions in which age differences in maintenance abilities are likely to occur—for example, by systematically varying the delay between cue and probe presentation and measuring age differences in related ERP components.
Conclusions
In the present study, we applied an ERP approach to identify age differences in the processing of context information. Our behavioral data clearly show age differences in context processing; that is, older adults were slower and produced more errors than did younger adults on trials requiring the updating of context information. The analysis of ERP correlates enables the identification of processes underlying these age-related differences. Although effects of context updating and context maintenance were reflected in the ERP data, age differences were only found in context updating and in conditions of changes in the cue identity. One important new insight from our study is that younger adults were able to build up a higher-order rule of task representations and that they updated context information only whenever task demands had to be revised. This effect was reflected in larger parietal P3b amplitudes on c-dep than on c-indep trials, irrespective of changes in cue identity. In contrast, older adults constantly updated context information, particularly when the cue identity changed. This was the case irrespective of the actual context condition, as indexed by age differences in P3b amplitudes and scalp topographies. The active maintenance of contextual information reflected in the CNV showed no age differences, suggesting an absence of maintenance deficits in the elderly.
Notes
In the younger age group, two participants were excluded due to error rates higher than three standard deviations above the corresponding group mean in the AX-CPT. Second, one participant was excluded because of psychoactive use, and two were excluded due to a large number of artifacts in the EEG files. In the older age group, one participant produced a very high error rate (above three SDs from the corresponding group mean) on context-dependent trials in the AX-CPT. Second, two older adults had to be excluded because of health reasons and psychoactive drug use that could have affected the EEG recording. Third, five older adults were excluded due to a large number of artifacts in the EEG-files—for example, caused by motor artifacts (e.g., restless-leg syndrome).
In the DSST, participants were presented with a sheet containing three lines of numbers from 1 to 9 in a random order. A template on the top showed which number belonged to each of nine different symbols. Participants had to insert the correct symbol below each number as quickly and as accurately as possible. The score was the number of correctly combined symbols within 90s (maximum value 93). The BDST was conducted to assess working memory. Participants were orally presented with sequences of digits ranging from 3 to 8, and had to repeat aloud each sequence in the reversed order of presentation. Three sequences for each span were given, and participants’ scores were the numbers of correctly repeated sequences. The spot-a-word test consisted of 35 items presented successively on a computer screen. Each item consisted of one word and four nonwords, and participants had to select the genuine word. Three practice examples were given in advance. The test score was the total number of correctly identified words (maximum value 35).
Because of the long presentation time of the probe, timeouts were generally rare, occurring on less than 0.01 % of trials. Timeouts were produced only by three older adults and by one younger adult, who together generated only 15 timeouts (out of 832 trials per participant).
To take into account that interactions including the factor Age Group may have been due to age differences in baseline performance, we also used the natural logarithm of raw RTs (Kray & Lindenberger, 2000) for the ANOVA. This analysis confirmed the analysis of the RT data, in that significant main effects of age group, F(1, 34) = 50.3, p < .001, η p 2 = .60, trial type, F(1, 34) = 121.9, p < .001, η p 2 = .78, and cue change type, F(1, 34) = 71.3, p < .001, η p 2 = .68, occurred. We also found higher-order interactions involving the factors Age Group and Trial Type, F(1, 34) = 10.1 p < .01, η p 2 = .23, and Trial Type and Cue Change Type, F(1, 34) = 47.9 p < .001, η p 2 = .59.
References
Allport, D. A., Styles, E. A., & Hsieh, S. (1994). Shifting intentional set: Exploring the dynamic control of tasks. In C. Umiltà & M. Moscovitch (Eds.), Attention and performance XV: Conscious and nonconscious information processing (pp. 421–452). Cambridge: MIT Press.
Bäckman, L., & Farde, L. (2005). The role of dopamine systems in cognitive aging. In R. Cabeza, L. Nysberg, & D. Park (Eds.), Cognitive neuroscience of aging (pp. 58–84). Oxford: Oxford University Press.
Bäckman, L., Ginovart, N., Dixon, R. A., Robins Wahlin, T.-B., Wahlin, Ä., Halldin, C., & Farde, L. (2000). Age-related cognitive deficits mediated by changes in the striatal dopamine system. American Journal of Psychiatry, 157, 635–637. doi:10.1176/appi.ajp.157.4.635
Baltes, P. B., Lindenberger, U., & Staudinger, U. M. (1998). Life-span theory in developmental psychology. In W. Damon & R. Lerner (Eds.), Handbook of child psychology (Theoretical models of human development, Vol. 1, pp. 1029–1143). New York: Wiley.
Braver, T. S. (2012). The variable nature of cognitive control: A dual mechanisms framework. Trends in Cognitive Sciences, 16, 106–113. doi:10.1016/j.tics.2011.12.010
Braver, T. S., & Barch, D. M. (2002). A theory of cognitive control, aging cognition, and neuromodulation. Neuroscience & Biobehavioral Reviews, 26, 809–817. doi:10.1016/S0149-7634(02)00067-2
Braver, T. S., & Bongiolatti, S. R. (2002). The role of frontopolar cortex in subgoal processing during working memory. NeuroImage, 15, 523–536.
Braver, T. S., & Cohen, J. D. (2000). On the control of control: The role of dopamine in regulating prefrontal function and working memory. In S. Monsell & J. Driver (Eds.), Control of cognitive processes: Attention and performance XVIII (pp. 713–738). Cambridge: MIT Press.
Braver, T. S., & West, R. (2008). Working memory, executive control, and aging. In F. I. M. Craik & T. A. Salthouse (Eds.), The handbook of aging and cognition (pp. 311–372). New York: Psychology Press.
Braver, T. S., Barch, D. M., Keys, B. A., Carter, C. S., Cohen, J. D., Kaye, J. A., Janowsky, J. S., . . . Reed, B. R. (2001). Context processing in older adults: Evidence for a theory relating cognitive control to neurobiology in healthy aging. Journal of Experimental Psychology: General, 130, 746–763. doi:10.1037/0096-3445.130.4.746
Braver, T. S., Satpute, A. B., Rush, B. K., Racine, C. A., & Barch, D. M. (2005). Context processing and context maintenance in healthy aging and early stage dementia of the Alzheimer’s type. Psychology and Aging, 20, 33–46. doi:10.1037/0882-7974.20.1.33
Braver, T. S., Paxton, J. L., Locke, H. S., & Barch, D. M. (2009). Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proceedings of the National Academy of Sciences, 106, 7351–7356.
Czernochowski, D. (2011). ERP evidence for scarce rule representation in older adults following short, but not long preparatory intervals. Frontiers in Psychology, 2(221), 1–13. doi:10.3389/fpsyg.2011.00221
Dennis, N. A., & Cabeza, R. (2008). Neuroimaging of healthy cognitive aging. In F. I. M. Craik & T. A. Salthouse (Eds.), The handbook of aging and cognition (pp. 1–54). New York: Psychology Press.
Donchin, E., & Coles, M. (1988). Is the P300 component a manifestation of context updating? Behavioral and Brain Sciences, 11, 357–427.
Eppinger, B., & Kray, J. (2011). To choose or to avoid: Age differences in learning from positive and negative feedback. Journal of Cognitive Neuroscience, 23, 41–52. doi:10.1162/jocn.2009.21364
Eppinger, B., Kray, J., Mecklinger, A., & John, O. (2007). Age differences in task-switching and response monitoring: Evidence from ERPs. Biological Psychology, 75, 52–67.
Falkenstein, M., Hoormann, J., Hohnsbein, J., & Kleinsorge, T. (2003). Short-term mobilization of processing resources is revealed in the event-related potential. Psychophysiology, 40, 914–923.
Ferdinand, N. K., & Kray, J. (2013). Age-related changes in processing positive and negative feedback: Is there a positivity effect in older adults? Biological Psychology, 94, 235–241.
Friedman, D., Kazmerski, V., & Fabiani, M. (1997). An overview of age-related changes in the scalp distribution of P3b. Electroencephalography and Clinical Neurophysiology, 104, 498–513.
Friedman, D., Nessler, D., Johnson, R., Jr., Ritter, W., & Bersick, M. (2008). Age-related changes in executive function: An event-related potential (ERP) investigation of task-switching. Aging, Neuropsychology, and Cognition, 15, 95–128. doi:10.1080/13825580701533769
Gratton, G., Coles, M. G., & Donchin, E. (1983). A new method for off-line removal of ocular artifact. Electroencephalography and Neurophysiology, 55, 468–484.
Hale, S., Myerson, J., Emery, L., Lawrence, B. M., & Dufault, C. (2007). Variation in working memory across the life span. In A. A. Conway, C. Jarrold, M. J. Kane, A. Miyake, & J. N. Towse (Eds.), Variation in working memory (pp. 194–226). Oxford: Oxford University Press.
Hämmerer, D., & Eppinger, B. (2012). Dopaminergic and prefrontal contributions to reward-based learning and outcome monitoring during child development and aging. Developmental Psychology, 48, 862–874.
Jasper, H. H. (1958). The ten–twenty electrode system of the International Federation. Electroencephalography and Clinical Neurophysiology, 10, 370–375.
Jimura, K., & Braver, T. S. (2009). Age-related shifts in brain activity dynamics during task switching. Cerebral Cortex, 20, 1420–1431. doi:10.1093/cercor/bhp206
Karayanidis, F., Coltheart, M., Michie, P. T., & Murphy, K. (2003). Electrophysiological correlates of anticipatory and poststimulus components of task switching. Psychophysiology, 40, 329–348.
Karayanidis, F., Provost, A., Brown, S., Paton, B., & Heathcote, A. (2011a). Switch-specific and general preparation map onto different ERP components in a task-switching paradigm. Psychophysiology, 48, 559–568. doi:10.1111/j.1469-8986.2010.01115.x
Karayanidis, F., Whitson, L. R., Heathcote, A., & Michie, P. T. (2011b). Variability in proactive and reactive control processes across the adult lifespan. Frontiers in Psychology, 2(318), 1–19. doi:10.3389/fpsyg.2011.00318
Keselman, H. J., & Rogan, J. C. (1980). Repeated measures F tests and psychophysiological research: Controlling the number of false positives. Psychophysiology, 17, 499–503.
Kray, J., & Ferdinand, N. K. (2014). Task switching and aging. In J. Grange & G. Houghton (Eds.), Task switching and cognitive control. Oxford: Oxford University Press.
Kray, J., & Lindenberger, U. (2000). Adult age differences in task switching. Psychology and Aging, 15, 126–147.
Kray, J., Eber, J., & Lindenberger, U. (2004). Age differences in executive functioning across the lifespan: The role of verbalization in task preparation. Acta Psychologica, 115, 143–165.
Kray, J., Eppinger, B., & Mecklinger, A. (2005). Age differences in attentional control: An event-related potential approach. Psychophysiology, 42, 407–416.
Kray, J., Eber, J., & Karbach, J. (2008). Verbal self-instructions in task switching: A compensatory tool for action-control deficits in childhood and old age? Developmental Science, 11, 223–236.
Lehrl, S. (1977). Mehrfachwahl-Wortschatz-Test Form B. Erlangen: Straube.
Lenartowicz, A., Escobedo-Quiroz, R., & Cohen, J. D. (2010). Updating of context in working memory: An event-related potential study. Cognitive, Affective, & Behavioral Neuroscience, 10, 298–315. doi:10.3758/CABN.10.2.298
Lindenberger, U., Mayr, U., & Kliegl, R. (1993). Speed and intelligence in old age. Psychology and Aging, 8, 207–220.
Linssen, A. M., Vuurman, E. F., Sambeth, A., Nave, S., Spooren, W., Vargas, G., & Riedel, W. J. (2011). Contingent negative variation as a dopaminergic biomarker: Evidence from dose-related effects of methylphenidate. Psychopharmacology, 218, 533–542. doi:10.1007/s00213-011-2345-x
Locke, H. S., & Braver, T. S. (2008). Motivational influences on cognitive control: Behavior, brain activation, and individual differences. Cognitive, Affective, & Behavioral Neuroscience, 8, 99–112. doi:10.3758/CABN.8.1.99
Luck, S. J., & Hillyard, S. A. (1994). Electrophysiological correlates of feature analysis during visual search. Psychophysiology, 31, 209–308.
MacDonald, A. W., III, & Carter, C. S. (2003). Event-related fMRI study of context processing in dorsolateral prefrontal cortex of patients with schizophrenia. Journal of Abnormal Psychology, 112, 689–697.
Mayr, U. (2001). Age differences in the selection of mental sets: The role of inhibition, stimulus ambiguity, and response-set overlap. Psychology and Aging, 16, 96–109.
McCarthy, G., & Wood, C. C. (1985). Scalp distributions of event-related potentials: An ambiguity associated with analysis of variance models. Electroencephalography and Clinical Neurophysiology, 62, 203–208.
Miller, E. K., & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. doi:10.1146/annurev.neuro.24.1.167
Minear, M., & Park, D. C. (2004). A lifespan database of adult facial stimuli. Behavior Research Methods, Instruments, & Computers, 36, 630–633. doi:10.3758/BF03206543
Monsell, S. (2003). Task switching. Trends in Cognitive Sciences, 7, 134–140. doi:10.1016/S1364-6613(03)00028-7
Nieuwenhius, S., Ridderinkhof, K. R., Talsma, D., Coles, M. G. H., Holroyd, C. B., Kok, A., & van der Molen, M. W. (2002). A computational account of altered error processing in older age: Dopamine and the error-related negativity. Cognitive, Affective, & Behavioral Neuroscience, 2, 19–36. doi:10.3758/CABN.2.1.19
Nyberg, L., Lövdén, M., Riklund, K., Lindenberger, U., & Bäckman, L. (2012). Memory aging and brain maintenance. Trends in Cognitive Sciences, 16, 292–305.
Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9, 97–113. doi:10.1016/0028-3932(71)90067-4
Paxton, J. L., Barch, D. M., Storandt, M., & Braver, T. S. (2006). Effects of environmental support and strategy training on older adults’ use of context. Psychology and Aging, 21, 499–509. doi:10.1037/0882-7974.21.3.499
Paxton, J. L., Barch, D. M., Racine, C. A., & Braver, T. S. (2008). Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cerebral Cortex, 18, 1010–1028. doi:10.1093/cercor/bhm135
Perlstein, W. M., Dixit, N. K., Carter, C. S., Noll, D. C., & Cohen, J. D. (2003). Prefrontal cortex dysfunction mediates deficits in working memory and prepotent responding in schizophrenia. Society of Biological Psychiatry, 53, 25–38.
Potts, G. F. (2004). An ERP index of task relevance evaluation of visual stimuli. Brain and Cognition, 56, 5–13.
Raz, N. (2000). Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In F. I. M. Craik & T. A. Salthouse (Eds.), The handbook of aging and cognition (pp. 1–91). Mahwah: Erlbaum.
Reuter-Lorenz, P. A., & Sylvester, C. Y. C. (2005). The cognitive neuroscience of working memory and aging. In R. Cabeza, L. Nyberg, & D. Park (Eds.), Cognitive neuroscience of aging (pp. 186–217). Oxford: Oxford University Press.
Rinne, J. O. (1987). Muscarine and dopaminergic receptors in the aging human brain. Brain Research, 404, 162–168.
Rogers, R. D., & Monsell, S. (1995). Costs of a predictable switch between simple cognitive tasks. Journal of Experimental Psychology: General, 124, 207–231. doi:10.1037/0096-3445.124.2.207
Rossion, B., & Pourtois, G. (2004). Revisiting Snodgrass and Vanderwart’s object pictorial set: The role of surface detail in basic-level object recognition. Perception, 33, 217–236. doi:10.1068/p5117
Rush, B. K., Barch, D. M., & Braver, T. S. (2006). Accounting for cognitive aging: Context processing, inhibition or processing speed? Aging, Neuropsychology, and Cognition, 13, 588–610. doi:10.1080/13825580600680703
Snodgrass, J. G., & Vanderwart, M. (1980). A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning and Memory, 6, 174–215. doi:10.1037/0278-7393.6.2.174
Suhara, T., Fukuda, H., Inoue, O., Itoh, T., Suzuki, K., Yamasaki, T., & Tateno, Y. (1991). Age-related changes in human D1 dopamine receptors measured by positron emission tomography. Psychopharmacology, 103, 41–45.
Verleger, R., Jaśkowski, P., & Wascher, E. (2005). Evidence for an integrative role of P3b in linking reaction to perception. Journal of Psychophysiology, 19, 165–181.
Wasylyshyn, C., Verhaeghen, P., & Sliwinski, M. J. (2011). Aging and task switching: A meta-analysis. Psychology and Aging, 26, 15–20.
Wechsler, D. (1955). WAIS: Wechsler Adult Intelligence Scale. New York: Psychological Corp.
Wechsler, D., Corp, P., & Education, P. (2008). WAIS-IV: Wechsler Adult Intelligence Scale–Fourth Edition. San Antonio: Pearson.
West, R. (1996). An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin, 120, 272–292. doi:10.1037/0033-2909.120.2.272
West, R. (2004). The effects of aging on controlled attention and conflict processing in the Stroop task. Journal of Cognitive Neuroscience, 16, 103–113.
West, R., & Moore, K. (2005). Adjustments of cognitive control in younger and older adults. Cortex, 41, 570–581.
Whitson, L. R., Karayanidis, F., & Michie, P. T. (2012). Task practice differentially modulates task-switching performance across the adult life-span. Acta Psychologica, 139, 124–136. doi:10.1016/j.actpsy.2011.09.004
Wild-Wall, N., Hohnsbein, J., & Falkenstein, M. (2007). Effects of ageing on cognitive task preparation as reflected by event-related potentials. Clinical Neurophysiology, 118, 558–569.
Author note
This research was supported by the German Research Foundation (Grant No. IRTG-1457). We thank Maren Wolff, Bianca Schulz, and Aline Becker for data collection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmitt, H., Ferdinand, N.K. & Kray, J. Age-differential effects on updating cue information: Evidence from event-related potentials. Cogn Affect Behav Neurosci 14, 1115–1131 (2014). https://doi.org/10.3758/s13415-014-0268-9
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-014-0268-9