Abstract
Switching between tasks requires individuals to inhibit mental representations of the previous task demands and to activate representations of the new demands. To date, investigators have identified only one way to measure task set inhibition—that is, through a backward inhibition (BI) paradigm. In this paradigm, participants take more time to return to a task set that was recently abandoned (e.g., “A” in an ABA task sequence) than to a nonrecently abandoned task set (e.g., CBA), and investigators have demonstrated that this time cost reflects time needed to overcome the inhibition of the recently abandoned task set. To date, however, investigators have not been able to use this paradigm, or any other, to isolate brain activity related to task set inhibition. For example, contrasting the brain activity elicited by ABA and CBA trials will not isolate activity related to task set inhibition, because inhibition occurs during the initial switch away from task A (i.e., ABA). Given that there is currently no way to directly isolate the brain activity related to task set inhibition, we decided instead to examine how brain activity during task switching varies in individuals who are better than others at inhibiting the previous task set. We found that participants who were good at inhibiting previous task sets, as measured with the BI paradigm, exhibited more activity in the basal ganglia and supplementary motor area/premotor area when task switching, as measured via functional magnetic resonance imaging. These findings suggest that activity in these regions plays a role in task set inhibition.
Similar content being viewed by others
To engage in goal-driven behavior, individuals must maintain in working memory higher-order mental representations of the ongoing goal and of the procedures needed to implement that goal. These higher-order representations, which together form a “task set,” will configure cognitive resources so that lower-level systems (e.g., the visual system) will be biased toward task-relevant information and the motoric system will implement the correct sequence of responses to such information. For example, to correct all of the misspelled words in a document, an individual must maintain a task set maintained in working memory that will bias the visual system to search for and identify misspelled words and to then enable the motoric system to enact the appropriate set of responses on the keyboard to correct those words when they are found.
Investigators have demonstrated that although individuals must exert intentional “executive” control to implement a task set, even more control is needed if a change in demands (e.g., the doorbell rings during editing) requires individuals to switch tasks (see, e.g., Monsell, 2003). Task switching requires individuals to activate the new task set and also to deactivate the previous task set so that the new task set can gain dominance over behavior. Although the deactivation of no-longer-relevant task sets can occur from the passive decay of activity over time, investigators have postulated that an active inhibitory process is also exerted upon the previous task set to speed up the rate of deactivation, allowing for a faster and smoother transition to the new task set (e.g., Mayr & Keele, 2000).
Evidence of inhibition during task switching has been difficult to establish because noninhibitory mechanisms, such as a priming of former stimulus–response associations, can be shown to account for many of the phenomena related to task switching (for a review, see Koch, Gade, Schuch, & Philipp, 2010). However, Mayr and Keele (2000) provided evidence of an inhibitory process in task switching with their “backward inhibition” (BI) paradigm. These researchers hypothesized that if task set A is inhibited when switching to task B, then it should take longer to immediately return to task set A (e.g., “A” in an ABA task sequence), as compared to a task set that has not been recently inhibited (e.g., “A” in an CBA task sequence), because extra time will be needed to overcome the inhibition of task set A. Consistent with their hypothesis, Mayr and Keele found that reaction times (RTs) are longer at the end of ABA sequences than at the end of CBA sequences; they labeled this effect backward inhibition. Further research has ruled out noninhibitory explanations of BI, such as an episodic-conflict account, which posits that the memory of not responding to task A interferes with the current need to respond to task A (for reviews, see Mayr, 2007, and Koch et al., 2010).
Although the BI paradigm is a method for behaviorally measuring inhibition during task switching, investigators have yet to isolate the brain activity related to the inhibition of previous task sets. Investigators have previously noted the difficulties of isolating brain activity related to BI (Koch et al., 2010; Mayr, 2007). For example, because the ABA-to-CBA comparison is an indirect measure of inhibition, comparing brain activity on ABA to that on CBA trials does not isolate activity related to inhibition, but instead isolates the brain activity involved in overcoming the inhibition of task set A (see Dreher & Berman, 2002, and Sinai, Goffaux, & Phillips, 2007, for studies that have examined this contrast). The actual inhibition of task set A occurs a trial earlier, during the switch to task set B (ABA). Unfortunately, the brain activity involved in inhibitory processes also cannot be isolated by comparing the switch to task B in ABA versus CBA sequences, because inhibition is equally exerted in both cases over the initial task set.
Brain systems involved in task set inhibition can only be isolated by comparing a trial in which inhibition is exerted to one in which it is not, with the trials otherwise being identical. The one trial type that does not involve the inhibition of a previous task set is a repeat trial (e.g., AA or BB). However, the comparison of switch to repeat trials (AB > AA) elicits activity related to both inhibitory mechanisms (i.e., inhibition of the prior task set) and noninhibitory mechanisms (e.g., activation of the new task set) involved in task switching (Koch et al., 2010). As a result, none of the many brain-imaging studies that have examined this contrast (e.g., Crone, Wendelken, Donohue, & Bunge, 2006; Rushworth, Hadland, Paus, & Sipila, 2002; Wager, Jonides, Smith, & Nichols, 2005; for a meta-analysis, see Wager, Reading, & Jonides, 2004) have identified activity specific to the inhibition of previous task sets.
Other investigators have also tried to elicit brain activity specific to the inhibition of previous task sets by examining how the brain responds to “switch-away” cues that inform participants of the need to switch task sets without identifying the upcoming task (Karayanidis et al., 2009; Nicholson, Karayanidis, Davies, & Michie, 2006). Because participants did not know which task set to activate, Nicholson et al. argued that brain activity in response to this cue reflects cognitive processes involved in the inhibition of the no-longer-relevant task set. It is difficult, however, to rule out alternative accounts. For example, increased brain activity after a switch-away cue as compared to a repeat cue could reflect increased conflict monitoring or cognitive alertness in the participants, which would be more expected after a switch-away cue than a repeat cue.
Although investigators have not been able to isolate brain activity specific to BI with imaging approaches, some researchers have examined how damage to select regions of the brain affects task set inhibition. For example, Mayr, Diedrichsen, Ivry, and Keele (2006) found that participants with circumscribed damage to the right, but not the left, lateral prefrontal cortex (PFC) exhibited reduced BI, suggesting that the right lateral prefrontal cortex plays a role in the inhibition of no-longer-relevant task sets. However, the sample size used in this study was small (n = 4), and two out of the four participants with right lateral prefrontal damage still numerically exhibited a BI effect. Therefore, the reliability of this effect is unclear. Moreover, damage to the right lateral PFC in these individuals was extensive, and it is not clear which subregion of right lateral PFC was involved in BI. Mayr and others have postulated that the right inferior frontal gyrus (rIFG) may be the critical region (Aron, Robbins, & Poldrack, 2004; Koch et al., 2010; Mayr, 2007), because this region is known to be involved in other forms of inhibition, such as response inhibition and the inhibition of long-term memory retrieval (e.g., Aron, Monsell, Sahakian, & Robbins, 2004; Depue, Curran, & Banich, 2007). However, direct support for this postulation has yet to be provided.
Investigators have also found evidence that the basal ganglia (BG) may be involved in task set inhibition. For example, Fales, Vanek, and Knowlton (2006) found that individuals with Parkinson’s disease, which is primarily associated with BG dysfunction, exhibit aberrant BI, and Markett, Montag, Walter, Plieger, and Reuter (2011) found that individual differences in BI scores are predicted by a polymorphism in the DRD2 gene, which affects D2 receptor density in the BG (see also Stelzel, Basten, Montag, Reuter, & Fiebach, 2010). Nonetheless, because investigators have often reported difficulties replicating behavioral genetic associations (e.g., Risch et al., 2009), and because Parkinson’s disease affects the functioning of a number of brain regions besides the BG, converging evidence is still needed.
The goal of the present study was to use functional magnetic resonance imaging (fMRI) to elucidate the brain activity that is related to task set inhibition. Given the difficulties of isolating such activity, we took a novel approach: We examined how individual differences in inhibitory ability (while controlling for individual differences in noninhibitory switching ability) are related to brain activity during task switching, a period in which the new task set is activated and the no-longer-relevant set is inhibited. Investigators have found that individual differences in BI are related to genetic differences and to stable cognitive characteristics such as the tendency to engage in depressive rumination (e.g., De Lissnyder, Koster, Derakshan, & De Raedt, 2010; Markett et al., 2011; Whitmer & Gotlib, 2012), suggesting that individual differences in BI are stable and likely to be accompanied by differences at the neural level. For example, individuals who exhibit greater BI scores may be more likely, given the findings of Markett et al., to activate their BG during switching and/or, given the postulations of Mayr (2007) and Koch et al. (2010), to activate their rIFG during switching. In sum, with the present study we attempted to identify neural activity that is related to a better ability to inhibit no-longer-relevant task sets.
To examine this question in the present study, we used fMRI to identify the brain activity involved in task switching (i.e., both inhibitory and noninhibitory switching processes) by comparing activity on switch versus repeat trials (AB vs. AA). We also measured participants’ inhibitory ability with the BI paradigm in a separate behavioral session. Increased RTs for ABA as compared to CBA sequences in the BI paradigm reflect better inhibition of task set A (Mayr & Keele, 2000), because the more strongly that a task set is inhibited, the longer it will take to overcome that inhibition. The BI measure controls for switching abilities unrelated to inhibition (e.g., task set activation) and for general cognitive performance, because task A is preceded by two switches in both ABA and CBA sequences. We regressed this behavioral measure of task set inhibition upon the brain activity involved in task switching, which should isolate only the components of that brain activity that are specific to task set inhibition.
Method
Participants
A group of 27 adults (16 male, 11 female) participated in the study. The mean age of participants was 20.04 years (SD = 2.73). Participants were recruited via e-mails to an undergraduate Introduction to Psychology class and via flyers posted at the University of Colorado at Boulder.
Task design and materials
Behavioral session
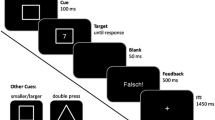
We first had participants complete a task-switching task so that we could obtain a measure of their inhibitory abilities. Our paradigm was based on the task-switching paradigm used by Yeung, Nystrom, Aronson, and Cohen (2006), with the exception that it also included a third task, so that BI could be measured. In our paradigm, participants were presented with a face (male or female), a word (one or two syllables) centered on that face, and a colored outline of a square (composed of one or two colors) around the face and word. Participants received a cue to do one of three tasks. In the face task, participants had to press “1” if the face was male and “2” if the face was female. A total of 60 male and 60 female faces were taken from a data set provided by the Max Planck Institute for Biological Cybernetics (Tübingen, Germany). In the word task, participants pressed “1” or “2,” depending on whether the word contained one or two syllables. The words were taken from a database created for a previous study of phonological processing (Poldrack et al., 1999). Each word appeared only once. In the color task, participants pressed “1” or “2,” depending on whether the square was composed of one or two colors (the colors used were red, green, yellow, and blue). In trials with two colors, the colors were randomly assigned to each of the four sides of the square. To indicate which task was to be performed, a cue appeared 200 ms before stimulus presentation and remained on for the duration. The cue surrounded the stimuli and was in the shape of a scroll for the word task, of an oval for the face task, and of a dashed square for the color task. When a participant responded, a blank screen would appear until the start of the next trial. The type of task (color, word, or face) was presented in a pseudorandom order; the tasks appeared randomly, with the exception that all task types followed each task type equally often.
In this task, four distinct trial types occurred, determined by task sequence: repeat trials, control trials, inhibitory trials, and unclassified-switch trials. Repeat trials were trials in which the same task was repeated (AA). The other trials were forms of switch trials, in that the task set of the current trial was different from that of the previous trial (e.g., BA). The trial was a control trial if the current task was different from the task completed two trials back (n – 2) and if the previous trial was also a switch trial (CBA). It was an inhibitory trial if the current task was the same as the n – 2 task and if the previous trial was also a switch trial (ABA). Lastly, the current trial was an unclassified-switch trial if it is a switch trial preceded by a repeat trial (BBA). RTs for these trial types were calculated after collapsing across task types, so that each trial type was equally composed of the face task, the word task, and the color task. Each trial type was presented in a pseudorandom order with the one additional constraint, that trial type could not be repeated more than three trials in a row. In the behavioral session, 33.3 % of the trials were AA trials, 22.2 % were BBA trials, 22.2 % were CBA trials, and 22.2 % were ABA trials.
To obtain a behavioral measure of inhibition, we compared RTs on inhibitory trials to those on control trials (ABA > CBA). Increased RTs for ABA as compared to CBA sequences in the BI paradigm reflect greater inhibition of task set A (Mayr & Keele, 2000), because the more strongly a task set is inhibited, the longer it will take to overcome that inhibition. We note that the BI measure controls for switching abilities unrelated to inhibition (e.g., task set activation) and for general cognitive performance, because task A is preceded by two switches in both ABA and CBA sequences. Nonetheless, we also measured switch costs, or the extra time that it took to switch task sets, during the behavioral session, so that we could provide converging evidence that any relation between individual differences in BI and brain activity during switching was not driven by general or noninhibitory differences in switching ability. To behaviorally measure switch costs, we compared the mean RTs on unclassified-switch and control trials to those on repeat trials [0.5 * (BBA + CBA) > AA]. Increased RTs reflected more difficulty with task switching. RTs from inhibitory trials were not included in this comparison because latencies in ABA trials also reflect the time needed to override inhibition. We used percent or proportion change (ABA/CBA) as the measure of task set inhibition because it controls for general differences in mean RTs across individuals and is the same type of contrast used to analyze imaging data (i.e., percent change in brain signal).
fMRI session
The task-switching paradigm that we presented to participants while we obtained functional scans was identical to the one used in the behavioral session, with a few notable exceptions. For example, in order to obtain an estimate of baseline brain activity and to jitter presentation of the different trial types, participants were presented with a fixation cross, instead of a cue and task stimuli, for 2,000 ms on 33 % of trials. Participants were required to look at the fixation crosses without making a response. Fixation trials were presented in a pseudorandom order with the other trial types.
The fMRI task also did not include ABA trials. As we said above, the comparison of ABA trials with CBA trials elicits brain activity involved in overcoming the inhibition of a task set (a contrast that was previously examined by Dreher & Berman, 2002). We, however, were interested in brain activity involved in exerting inhibition, and given time constraints, we opted against including ABA trials because this would have reduced the amount of time that we had to measure the brain activity of interest (i.e., brain activity elicited by the comparison of CBA and BBA trials with AA trials). The inclusion of ABA trials also would have led to technological problems (e.g., issues with counterbalancing and the ability to deconvolve brain signals specific to each trial type). It may have been possible to include ABA trials if we had substantially altered our design (e.g., to use a design similar to that of Dreher & Berman, 2002), but such designs would have had significantly less power to detect brain activity related to task switching and, if a similar design had also been used during the behavioral session in order to keep the tasks similar, to identify individual differences in BI.
In order to maximize the abilities of the fMRI task to detect brain activity related to task switching and of the behavioral task to precisely estimate individual differences in inhibitory ability, we used two slightly different sets of task parameters during the fMRI and behavioral sessions. For example, because we were only interested in the contrast between switch and repeat trials when measuring brain activity, we presented equal numbers of repeat and switch trials (i.e., 50 % of nonfixation trials were repeat trials, 25 % were unclassified-switch trials, and 25 % were control trials) during the fMRI session. During the behavioral session, however, we were primarily interested in the two types of switch trials used to calculate BI scores (i.e., ABA and CBA trials), and thus we increased the number of switch trials to 66.6 % and reduced the number of repeat trials to 33.3 % (as said above, we still included some repeat trials so that we could examine switching costs during the behavioral session).
To ensure that the same amounts of brain data were collected in the fMRI session for each participant and in each condition, regardless of RT differences, we presented a new stimulus or fixation cross every 2,000 ms. The next stimulus or fixation cross appeared, on average, 1,272 ms after a response was made. It is of note that the presentation of a fixation cross did not affect what sort of trial type would come next (e.g., task B was a switch trial in the “A–fixation–B” sequence and a repeat trial in the “B–fixation–B” sequence). Thus, the average time from a response until the presentation of the cue for the next trial varied substantially, depending on whether fixation crosses were presented between the response and the next cue (fixation crosses could repeat up to a maximum of three trials in a row). When taking into account the delay caused by fixation trials, the presentation of the cue for the next trial occurred, on average, 2,269 ms after a response was made. In the behavioral session, there was no need to present fixation crosses or to make all trials last at least 2,000 ms; therefore, on every trial, we presented the cue for the next trial 500 ms after a response. A short, constant response-to-cue interval (RCI) allowed us to run more trials in the same amount of time, and thereby to gain a more precise estimate of individuals’ inhibitory abilities.
It is of note that although parameter differences (e.g., RCI) can affect the mean amount of BI exhibited by a group of participants (Koch et al., 2010), it is not clear whether parameter differences also affect the rank order of participants’ BI scores. In other words, it is not clear whether a good inhibitor during the behavioral session would be an equally good inhibitor under a different set of parameters during the fMRI session. It is important to emphasize, however, that if parameter changes did affect the rank order of participants’ BI scores, they should reduce the reliability of our measure of inhibitory ability, which, in turn, should make it more difficult for us to find a significant correlation between individual differences in inhibitory ability and the brain activity involved in task switching. Nonetheless, we decided that the benefits of letting the parameters vary between sessions (for the reasons stated above) outweighed the costs of doing so.
Procedure
Participants completed a practice and the first part of the behavioral version of the BI task on a computer before entering the magnet (486 trials with no breaks). The second portion of the behavioral version was completed in the magnet while structural images were obtained (270 trials with no breaks). The behavioral version had a total of 756 trials. Immediately afterward, the participants performed the version in which functional images were obtained. The fMRI version had a total of 336 trials (plus 167 fixation trials), presented with no breaks.
Image acquisition and analysis
Image acquisition
Functional MRI was performed on a 3-T full-body GE scanner so as to acquire blood-oxygenation-level-dependent (BOLD) contrast using gradient echo T2*-weighted echoplanar imaging (EPI; repetition time [TR] = 2,000 ms; 256-mm field of view [FOV], 64 × 64 matrix, 29 slices, 4-mm slice thickness, 0-mm slice gap; flip angle = 90°). Slices were oriented obliquely along the AC–PC line. The first seven volumes from the run were discarded in order to allow time for the magnet to reach a steady state. In addition, T1-weighted 3-D IR-SPGR high-resolution structural images were acquired for each participant along the coronal plane (TR = 9 ms, error time [TE] = 2.0 ms, flip angle = 10°, inversion time = 500 ms; 220-mm FOV, 256 × 256 matrix, 0.87 × 0.87-mm in-plane resolution, 124 slices, 1.7-mm slice thickness) for subsequent anatomic localization. Head movement was minimized using a custom-fitted head holder consisting of polyurethane foam beads inflated to tightly mold around the head and neck.
Image analysis
Image processing and data analysis were performed using the FMRIB software library package FSL (Analysis group, FMRIB, Oxford, U.K.; www.fmrib.ox.ac.uk/fsl/). Standard preprocessing was applied: motion correction (MCFLIRT), brain extraction (BET), time-series prewhitening, registration, and spatial normalization to the Montreal Neurological Institute (MNI) high-resolution 152-T1 template. Images were resampled into this space with 3-mm isotropic voxels and smoothed with a Gaussian kernel of 8-mm full width at half-maximum to minimize noise and residual differences in gyral anatomy, resulting in an effective spatial resolution of 10.2 × 10.7 × 11.5 mm. Each normalized image was bandpass filtered (high-pass filter = 120 s) to remove high-frequency noise. FMRIB’s improved linear model (FILM) was then applied, from which statistical inferences were based on the theory of random Gaussian fields, and changes relative to the experimental conditions were modeled by convolution of single-trial epochs with the canonical hemodynamic response function to approximate the activation patterns. Using multiple regression analysis, we constructed statistical maps for each participant that represented the association between the observed time series (e.g., BOLD signal) and regressors that were created separately for switch and repeat trials. Our contrast of interest compared statistical maps of brain activity for switch trials over repeat trials.
Group analysis was performed using FSL’s higher-level FEAT analysis tool to yield statistical parameter maps (SPMs) in which all subsequent analyses were performed. SPMs were thresholded on a voxel-wise basis at z = 2.58, p = .01. To determine which brain regions were related to inhibition, an additional regression analysis was performed at the group level. We regressed participants’ BI scores (ABA > CBA) on the activity in each voxel of the brain for the switch-versus-repeat contrast (e.g., did participants who exhibited larger BI scores also exhibit more activity in voxel 1?).
We then examined whether this regression yielded significant clusters of contiguous active voxels (i.e., active voxels were those that passed the voxel-wise threshold). AFNI Alphasim, a common method for dealing with false positives in brain-imaging data, was used to determine the threshold of a significant cluster. Because of our a priori hypotheses, we first searched for significant clusters in a limited brain space that included the BG (including the putamen, globus pallidus, caudate nucleus, and nucleus accumbens) and the rIFG (all of right ventrolateral PFC, including pars triangularis/opercularis and Brodmann area [BA] 44/45). As calculated by Alphasim, a cluster that fell within this space was significant if it was larger than 82 voxels (cluster threshold set for p < .05). To determine whether any clusters were significant outside this a priori area, we used Alphasim to calculate the cluster-size threshold when examining the entire space of the brain (i.e., full-brain analysis). The cluster size threshold for the full-brain analysis was 154 voxels (p < .05). Thus, to be clear, clusters had to pass a lower threshold for significance (82 contiguous voxels) if they fell inside the a priori area of interest (i.e., the space covering the rIFG and the BG) and a higher threshold (154 voxels) if they were outside the a priori area of interest (whole-brain comparison). In sum, we regressed BI scores on each voxel and then calculated whether there were significant clusters of activated voxels. After identifying significant clusters, we then extracted the average percent signal change of all of the voxels that were part of that significant cluster for further analysis (e.g., to assess whether an outlier was driving the significance of that cluster; see Fig. 3 below).
Results
Behavioral data
From behavioral session
The average accuracy was 93.44 % (SD = 0.03). Consistent with previous research using the BI paradigm (e.g., Mayr & Keele, 2000), analyses were performed on correct RTs if the two preceding responses were also correct and if the RT was less than 2.5 standard deviations away from the participant’s mean, leaving 82.2 % of the trials for analysis. The mean RTs for the different trial types were 1,179 ms for inhibitory trials, 1,124 ms for control trials, 1,041 ms for unclassified trials, and 918 ms for repeat trials.
Two one-sample t tests demonstrated that the paradigm successfully elicited BI and switch costs. We found a significant inhibition effect (ABA/CBA), t(26) = 3.554, p = .001, with the mean RT on ABA trials being 1.049 times that on CBA trials, and significant switch costs [0.5 * (BBA + CBA) / AA], t(26) = 10.2, p < .0001, with the mean RT on switch trials being 1.18 times that on repeat trials.
From fMRI session
The average accuracy was 86.57 % (SD = 0.07). Participants responded, on average, in 755 ms on switch trials and in 700 ms on repeat trials. Analyses were performed on correct trials in which the RT was less than 2.5 standard deviations away from the participants’ means (0.2 % of trials excluded). In a one-sample t test, we found significant switch costs, t(26) = 8.564, p < .0001, with the RT on switch trials being 1.08 times that on repeat trials. Thus, behaviorally, the scanned version also elicited the expected behavioral effect.
Imaging data
General effects associated with task switching
To determine whether our paradigm elicited brain activity similar to that found in other studies of task switching, we contrasted brain activity during switch trials with that on repeat trials. Table 1 presents significant clusters (whole-brain corrected for significance when using a voxel-wise threshold at z = 3.8, p < .0001). This contrast revealed a very large cluster of activity, extending through the parietal and occipital lobes, with a peak in the precuneus. It also revealed a large cluster with a peak in the supplementary motor cortex extending medially to the dorsal anterior cingulate and laterally to dorsolateral prefrontal cortex. Activity was also observed in the putamen (a subsection of the BG), premotor cortex, insula, and thalamus (see Fig. 1). Hence, the task activated the network of brain regions typically involved in task switching (e.g., Crone et al., 2006; Rushworth et al., 2002; Wager et al., 2004, 2005).
Brain activity correlated with the behavioral measure of inhibition
To isolate brain activity during task switching that was related to individual differences in the ability to inhibit the previous task set, we correlated the behavioral measure of inhibition (ABA > CBA) from each participant with his or her brain activity for the contrast of switch versus repeat trials (AB > AA). As is shown in Fig. 2 and Table 2, increased behavioral inhibition was associated with increased activity in the left putamen, the right putamen, and bilaterally in the supplementary motor area (SMA)/premotor cortex (BA 6). The activity in the SMA/premotor cortex appears to include both caudal and rostral divisions (MNI coordinates on the y-axis extended from −18 to 2). The scatterplots shown in Fig. 3 reveal that outliers did not drive these correlations.
Scatterplots. On the y-axis is the percent signal change of brain activity for switch trials > repeat trials. On the x-axis is reaction time proportion (ABA/CBA). Increased backward inhibition is associated with increased brain activity in (A) left putamen, (B) right putamen, (C) left BA 6, and (D) right BA 6
It is important to note that the behavioral measure of BI controls for noninhibitory switching abilities (e.g., the activation of the new task set) because both CBA and ABA sequences require two switches in a row. In other words, an individual who is bad at switching should be equally bad at the end of both CBA and ABA sequences, because both sequences involve two switches in a row. Thus, the only individual differences measured by the CBA-versus-ABA comparison is how strongly participants inhibited task set A when they switched away from it. However, to provide converging evidence that brain activity in the BG and SMA/premotor cortex was specific to individual differences in the ability to inhibit a task set and not to the ability to activate the task set, we also conducted separate multiple regression analyses upon the average percent signal change in each significant cluster, with both BI scores and switch costs from the behavioral version entered as predictors. When the regression model included each individual’s switch costs and BI scores, the BI scores remained significantly related to the average percent signal change in each cluster (all ps < .05). When controlling for individual differences in BI, however, switch costs were not significantly related to brain activity in any of these four clusters (all ps > .2) except the right putamen (p = .026), suggesting that the activity in these clusters was primarily related to inhibitory abilities. Thus, brain activity in these four clusters (left and right putamen, left and right SMA/premotor cortex) appears to be primarily related to the ability to inhibit the previous task set and not to switching abilities unrelated to inhibition.
Discussion
To date, because of methodological difficulties, investigators have not been able to isolate brain activity during task switching that was specific to the inhibition of the previous task set. In the present study, we used a novel individual-differences approach to identify brain activity that was related to task set inhibition, in that we regressed individual differences in the ability to inhibit a previous task set—that is, participants’ BI scores—onto brain activity during switching—that is, brain activity involved in inhibiting a no-longer-relevant task set and activating the new task set. We found that individuals who are better at inhibiting the previous task set, when controlling for their noninhibitory switching abilities, exhibited increased bilateral activation in the putamen, a section of the BG, and the SMA/premotor cortex (BA 6) during task switching.
The finding that individual differences in BI predict brain activity in the BG during task switching is consistent with the findings of Markett et al. (2011) that a polymorphism in the DRD2 gene is related to individual differences in BI. Because the DRD2 gene affects D2 receptor density primarily in the BG, this past work suggested that neural activity in the BG should be related to individual differences in BI. The present results directly support this formulation. It is not currently clear, however, what the exact role of the BG is in inhibition. In this context, though, it is noteworthy that the BG, especially the putamen and globus pallidus, functions as a gatekeeper to working memory. Activation of D2 receptors by dopamine leads to activation of the indirect pathway of the BG, which inhibits the thalamus and thereby leads to suppression of task-irrelevant information and decreased updating of irrelevant information into working memory (e.g., Frank, Loughry, & O’Reilly, 2001; McNab & Klingberg, 2008; O’Reilly & Frank, 2006). We speculate that the indirect pathway of BG is involved in removing no-longer-relevant task sets from working memory via the region’s inhibitory influence over the thalamus. Clearly, future work is needed to further examine the role of BG in task set inhibition.
We also found that better task set inhibition across participants was related to increased activity in the SMA/premotor area (BA 6). The SMA and adjacent premotor cortex are areas known for their role in representing action rules, stimulus–response mappings, and response selection based on arbitrary cues (Chouinard & Paus, 2006; Donohue, Wendelken, & Bunge, 2008). As such, our findings are consistent with the argument that BI is primarily triggered by conflict during response selection, as compared to other processing stages (e.g., stimulus attribute selection; see, e.g., Philipp, Jolicœur, Falkenstein, & Koch, 2007; Schneider & Verbruggen, 2008; Schuch & Koch, 2003).
The exact neural mechanism by which activity in the SMA/premotor area is related to the ability to inhibit a previous task set, however, is unclear. In this context, it is noteworthy that previous research has suggested that increased inhibitory activity in a brain region can lead to a greater, instead of a smaller, fMRI signal, because of increased activation of gamma-aminobutyric acid interneurons in that region (see Logothetis, 2008). Therefore, it is possible that increased activity in the SMA/premotor area could reflect greater inhibition of the stimulus–response associations in individuals who are better at task set inhibition. Clearly, however, this speculation needs to be directly examined in future research. Lastly, note that premotor cortex receives direct projections from the BG (Strick, 2004), which may suggest that the BG influences activity in the premotor cortex during BI.
Also note that it is possible that our measure of inhibitory ability (the RT difference between ABA and CBA trials) might, in part, reflect the ability to overcome the inhibition of the initial task set (e.g., the initial task A) and that the regression of BI scores onto brain activity during a task switch may have therefore partially elicited brain activity related to the ability to overcome task set inhibition. This alternative account, however, is unlikely to be valid for a few reasons. First, increased BI scores were significantly related to decreased switch costs (r = −.444, p = .02). If increased BI scores were primarily related to difficulties in reactivating an inhibited task set, individuals with increased BI scores should be worse at task switching, not better, as we observed. Second, we measured brain activity when participants were switching away from task A (AB) but not when switching back to a recently inhibited task set (ABA), which is when brain activity should reflect the cognitive processes involved in overcoming inhibition. Indeed, the fMRI version of the task did not even include any ABA trials, but only trials in which there would be less inhibition to overcome—control trials (CBA) and unclassified-switch trials (BBA). Hence, if our fMRI contrast did not elicit brain activity involved in overcoming inhibition, the regression of BI scores onto this brain activity also would not elicit brain activity involved in overcoming inhibition. Third, Dreher and Berman (2002) previously examined the brain activity involved in overcoming the inhibition of task sets (Dreher & Berman, 2002) by comparing ABA trials to CBA trials. They found activity in a different region, right lateral PFC, from the ones found in the present study, suggesting that our contrast of interest did not elicit activity related to overcoming inhibition (note that even the use of yet more liberal thresholds did not elicit activity in this region in our study). In sum, brain activity in the BG and SMA/premotor cortex is likely to be related to the ability to exert task set inhibition, not to the ability to overcome such inhibition.
In conclusion, in this study we found that individuals who showed more inhibition of previous task sets, as measured behaviorally, showed increased activity in the putamen (a portion of the BG) and in SMA/premotor cortex (BA 6) during task switching. These findings suggest that activity in these regions plays a role in task set inhibition. Future research, however, will be needed to identify the role that these regions play in task set inhibition.
References
Aron, A. R., Monsell, S., Sahakian, B. J., & Robbins, T. W. (2004a). A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain, 127, 1561–1573. doi:10.1093/brain/awh169
Aron, A. R., Robbins, T. W., & Poldrack, R. A. (2004b). Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences, 8, 170–177. doi:10.1016/j.tics.2004.02.010
Chouinard, P., & Paus, T. (2006). The primary motor and premotor areas of the human cerebral cortex. Neuroscientist, 12, 143–152.
Crone, E. A., Wendelken, C., Donohue, S. E., & Bunge, S. A. (2006). Neural evidence for dissociable components of task-switching. Cerebral Cortex, 16, 475–486. doi:10.1093/cercor/bhi127
De Lissnyder, E., Koster, E. H. W., Derakshan, N., & De Raedt, R. (2010). The association between depressive symptoms and executive control impairments in response to emotional and non-emotional information. Cognition & Emotion, 24, 264–280.
Depue, B. E., Curran, T., & Banich, M. T. (2007). Prefrontal regions orchestrate suppression of emotional memories via a two-phase process. Science, 317, 215–219.
Donohue, S. E., Wendelken, C., & Bunge, S. A. (2008). Neural correlates of preparation for action selection as a function of specific task demands. Journal of Cognitive Neuroscience, 20, 694–706.
Dreher, J. C., & Berman, K. F. (2002). Fractionating the neural substrate of cognitive control processes. Proceedings of the National Academy of Sciences, 29, 14595–14600.
Fales, C. L., Vanek, Z. F., & Knowlton, B. J. (2006). Backward inhibition in Parkinson’s disease. Neuropsychologia, 44, 1041–1049. doi:10.1016/j.neuropsychologia.2005.11.002
Frank, M. J., Loughry, B., & O’Reilly, R. C. (2001). Interactions between frontal cortex and basal ganglia in working memory: A computational model. Cognitive, Affective, & Behavioral Neuroscience, 1, 137–160. doi:10.3758/CABN.1.2.137
Karayanidis, F., Mansfield, E. L., Galloway, K. L., Smith, J. L., Provost, A., & Heathcote, A. (2009). Anticipatory reconfiguration elicited by fully and partially informative cues that validly predict a switch in task. Cognitive, Affective, & Behavioral Neuroscience, 9, 202–215. doi:10.3758/CABN.9.2.202
Koch, I., Gade, M., Schuch, S., & Philipp, A. M. (2010). The role of inhibition in task switching: A review. Psychonomic Bulletin & Review, 17, 1–14. doi:10.3758/PBR.17.1.1
Logothetis, N. K. (2008). What we can do and what we cannot do with fMRI. Nature, 12, 869–878.
Markett, S., Montag, C., Walter, N. T., Plieger, T., & Reuter, M. (2011). On the molecular genetics of flexibility: The case of task-switching, inhibitory control and genetic variants. Cognitive, Affective, & Behavioral Neuroscience, 11, 644–651. doi:10.3758/s13415-011-0058-6
Mayr, U. (2007). Inhibition of task sets. In D. S. Gorfein & C. M. MacLeod (Eds.), Inhibition in cognition (pp. 27–44). Washington, DC: American Psychological Association.
Mayr, U., Diedrichsen, J., Ivry, R., & Keele, S. W. (2006). Dissociating task-set selection from task-set inhibition in the prefrontal cortex. Journal of Cognitive Neuroscience, 18, 14–21.
Mayr, U., & Keele, S. W. (2000). Changing internal constraints on action: The role of backward inhibition. Journal of Experimental Psychology: General, 129, 4–26. doi:10.1037/0096-3445.129.1.4
McNab, F., & Klingberg, T. (2008). Prefrontal cortex and basal ganglia control access to working memory. Nature Neuroscience, 11, 103–107. doi:10.1038/nn2024
Monsell, S. (2003). Task switching. Trends in Cognitive Sciences, 7, 134–140. doi:10.1016/S1364-6613(03)00028-7
Nicholson, R., Karayanidis, F., Davies, A., & Michie, P. T. (2006). Components of task-set reconfiguration: Differential effects of switch-to and switch-away cues. Brain Research, 1121, 160–176.
O’Reilly, R. C., & Frank, M. J. (2006). Making working memory work: A computational model of learning in the frontal cortex and basal ganglia. Neural Computation, 18, 283–328.
Philipp, A. M., Jolicœur, P., Falkenstein, M., & Koch, I. (2007). Response selection and response execution in task switching: Evidence from a go-signal paradigm. Journal of Experimental Psychology: Learning, Memory, and Cognition, 33, 1062–1075. doi:10.1037/0278-7393.33.6.1062
Poldrack, R. A., Wagner, A. D., Prull, M., Desmond, J. E., Glover, G. H., & Gabrieli, J. D. E. (1999). Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage, 10, 15–35.
Risch, N., Herrell, R., Lehner, T., Liang, K. Y., Eaves, L., Hoh, J., . . . Merikangas, K. R. (2009). Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta-analysis. Journal of the American Medical Association, 301, 2462–2471. doi:10.1001/jama.2009.878
Rushworth, M. F. S., Hadland, K. A., Paus, T., & Sipila, P. K. (2002). Role of the human medial frontal cortex in task switching: A combined fMRI and TMS study. Journal of Neurophysiology, 87, 2577–2592.
Schneider, D. W., & Verbruggen, F. (2008). Inhibition of irrelevant category–response mappings. Quarterly Journal of Experimental Psychology, 61, 1629–1640.
Schuch, S., & Koch, I. (2003). The role of response selection for inhibition of task sets in task shifting. Journal of Experimental Psychology: Human Perception and Performance, 29, 92–105. doi:10.1037/0096-1523.29.1.92
Sinai, M., Goffaux, P., & Phillips, N. A. (2007). Cue- versus response-locked processes in backward inhibition: Evidence from ERPs. Psychophysiology, 44, 596–609. doi:10.1111/j.1469-8986.2007.00527.x
Stelzel, C., Basten, U., Montag, C., Reuter, M., & Fiebach, C. J. (2010). Frontostriatal involvement in task switching depends on genetic differences in d2 receptor density. Journal of Neuroscience, 30, 14205–14212. doi:10.1523/JNEUROSCI.1062-10.2010
Strick, P. K. (2004). Basal ganglia and cerebellar circuits with the cerebral cortex. In M. S. Gazzaniga (Ed.), The cognitive neurosciences III (pp. 453–461). Cambridge, MA: MIT Press.
Wager, T. D., Jonides, J., Smith, E. E., & Nichols, T. E. (2005). Toward a taxonomy of attention shifting: Individual differences in fMRI during multiple shift types. Cognitive, Affective, & Behavioral Neuroscience, 5, 127–143. doi:10.3758/CABN.5.2.127
Wager, T. D., Reading, S., & Jonides, J. (2004). Neuroimaging studies of shifting attention: A meta-analysis. NeuroImage, 22, 1679–1693.
Whitmer, A. J., & Gotlib, I. H. (2012). Switching and backward inhibition in major depressive disorder: The role of rumination. Journal of Abnormal Psychology, 121(3), 570–578. doi:10.1037/a0027474
Yeung, N., Nystrom, L. E., Aronson, J. A., & Cohen, J. D. (2006). Between-task competition and cognitive control in task switching. Journal of Neuroscience, 26, 1429–1438. doi:10.1523/JNEUROSCI.3109-05.2006
Author note
This research was supported by National Institute of Mental Health Grant No. P50 079485 (to the Interdisciplinary Behavioral Science Center on the Determinants of Executive Function & Dysfunction at the University of Colorado at Boulder, M. Banich, PI). The authors thank Kirsten Orcutt and Paula Villar for their help in running the participants. We also thank Luka Ruzic and Greg Burgess, for their technical assistance with the fMRI analyses, and Nick Yeung, for sharing with us the stimuli used in the Yeung et al. (2006) study. Lastly, the authors thank the members of the Center for their feedback and comments on the research presented here.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Whitmer, A.J., Banich, M.T. Brain activity related to the ability to inhibit previous task sets: an fMRI study. Cogn Affect Behav Neurosci 12, 661–670 (2012). https://doi.org/10.3758/s13415-012-0118-6
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-012-0118-6