Abstract
How does switching tasks affect our ability to monitor and adapt our behavior? Largely independent lines of research have examined how individuals monitor their actions and adjust to errors, on the one hand, and how they are able to switch between two or more tasks, on the other. Few studies, however, have explored how these two aspects of cognitive–behavioral flexibility interact. That is, how individuals monitor their actions when task rules are switched remains unknown. The present study sought to address this question by examining the action-monitoring consequences of response switching—a form of task switching that involves switching the response that is associated with a particular stimulus. We recorded event-related brain potentials (ERPs) while participants performed a modified letter flanker task in which the stimulus–response (S–R) mappings were reversed between blocks. Specifically, we examined three ERPs—the N2, the error-related negativity (ERN), and the error positivity (Pe)—that have been closely associated with action monitoring. The findings revealed that S–R reversal blocks were associated with dynamic alterations of action-monitoring brain activity: the N2 and ERN were enhanced, whereas the Pe was reduced. Moreover, participants were less likely to adapt their posterror behavior in S–R reversal blocks. Taken together, these data suggest that response switching results in early enhancements of effortful control mechanisms (N2 and ERN) at the expense of reductions in later response evaluation processes (Pe). Thus, when rules change, our attempts at control are accompanied by less attention to our actions.
Similar content being viewed by others
In order to adapt to changing conditions, humans must be able to successfully modify their behavior. Overriding the rules that we adhere to on a daily basis requires substantial attention and effort (Gopher, Armony, & Greenshpan, 2000). For example, to someone who is accustomed to driving on the right side of the road, driving in the United Kingdom for the first time can be particularly challenging, as the “rules of the road” are reversed. To adjust to the unfamiliar flow of traffic, the driver must focus on the current rules and/or successfully inhibit the urge to drive on the side that he or she is most accustomed to. As more attentional resources become devoted to monitoring for new conflicts and inhibiting old habits, fewer resources are available to consciously evaluate the actual driving performance. Thus, the driver may be less aware of slip-ups in this heightened conflict-monitoring state. Previous studies from different research perspectives have investigated how individuals monitor their behaviors and how they adapt to rule reversals. However, no previous study has investigated how individuals are able to monitor their responses in the context of changing response rule conditions. This is the focus of the present study. Here, we briefly review relevant findings from action-monitoring and task-switching research before describing an integrative experiment that taps into both areas.
Action monitoring
Action monitoring involves recognizing when conflicts and errors occur and implementing appropriate behavioral adjustments to overcome them (e.g., Ridderinkhof, Ullsperger, Crone, & Nieuwenhuis, 2004). That is, it is focused on how individuals keep track of their actions. The conflict-monitoring theory suggests that the presence of “conflict” in the information-processing stream triggers strategic adjustments in cognitive control that serve to reduce conflict in subsequent performance (Botvinick, Braver, Barch, Carter, & Cohen, 2001). In this context, conflict is operationalized as the simultaneous activation of multiple mutually exclusive response tendencies (Botvinick, Cohen, & Carter, 2004). For example, incongruent trials (e.g., >><>>) are characterized by increased reaction times (RTs) and error rates, as compared to congruent trials (e.g., <<<<), which activate only one response tendency (e.g., Botvinick et al., 2001).
Action-monitoring processes are most often localized to areas of the medial frontal cortex, especially the anterior cingulate cortex (ACC; Bush, Luu, & Posner, 2000; Ridderinkhof et al., 2004). The ACC receives input from a variety of cognitive and affective systems (Devinsky, Morrell, & Vogt, 1995), uses this input to detect conflict or suboptimal performance (Carter & van Veen, 2007; Holroyd & Coles, 2002), and signals the need for necessary resources to improve task performance (Kerns et al., 2004; MacDonald, Cohen, Stenger, & Carter, 2000; van Veen & Carter, 2006). Although the precise role of the ACC in action monitoring is still under debate (Brown, 2011; Grinband et al., 2011a, 2011b; Yeung, Cohen, & Botvinick, 2011), considerable evidence has suggested that its primary function in this regard is to signal whenever conflicts occur (e.g., Carter et al., 1998; Weissman, Giesbrecht, Song, Mangun, & Woldorff, 2003).
Electrophysiological studies have indexed conflict using the N2, a negative-going event-related brain potential (ERP) reaching maximal amplitude at fronto-central electrode sites between 200 and 300 ms after stimulus onset, that has been localized to the ACC (van Veen & Carter, 2002). Although it is a stimulus-locked ERP component, the N2 has been consistently thought to reflect processes associated with responses, including response inhibition (Falkenstein, Hoormann, & Hohnsbein, 1999; Tillman & Wiens, 2011) and response conflict (Yeung, Botvinick, & Cohen, 2004; Yeung & Cohen, 2006). The hypothetical mechanism that the N2 reflects is a process of controlling incorrect response preparation (see Folstein & Van Petten, 2008, for a review). Much of the evidence for this control account comes from the flanker task: Incongruent stimuli prime the incorrect response tendency and elicit larger N2 amplitudes, as compared to congruent stimuli (Gehring, Gratton, Coles, & Donchin, 1992; Kopp, Mattler, Goertz, & Rist, 1996).
Performance errors are also associated with increased ACC activity (Carter et al., 1998; Holroyd et al., 2004) and have been at the heart of action-monitoring research and theory (Gehring, Liu, Orr, & Carp, 2012; Holroyd & Coles, 2002; Yeung et al., 2004). Electrophysiological studies have identified putative markers of ACC activity associated with errors, such as the error-related negativity (ERN; Gehring, Goss, Coles, Meyer, & Donchin, 1993). The response-locked ERN is a sharp negative deflection in the ERP that peaks at fronto-central sites between 0 and 150 ms following response errors, and is consistently localized to the ACC (Dehaene, Posner, & Tucker, 1994; Herrmann, Römmler, Ehlis, Heidrich, & Fallgatter, 2004; van Veen & Carter, 2002). Whether it reflects the explicit detection of an error (Scheffers & Coles, 2000), a reinforcement-learning signal (Holroyd & Coles, 2002) or the detection of response conflict on error trials (Yeung et al., 2004), the general consensus is that the ERN is the brain’s first signal that something has gone awry.
The conflict-monitoring theory claims that the ERN is the result of the response conflict between the enacted erroneous response and subsequent corrective response (Yeung et al., 2004). The theory predicts that the degree to which the correct response tendency is “activated” or “primed” determines the amplitude of the ERN. Several experiments have supported this prediction. For example, studies have found increased ERN amplitudes when accuracy is emphasized over speed (Arbel & Donchin, 2009; Falkenstein, Hoormann, Christ, & Hohnsbein, 2000; Gehring et al., 1993) and when the stimuli are bright (Yeung, Ralph, & Nieuwenhuis, 2007). These conditions are thought to increase the tendency to respond correctly, and when errors are committed, the two response tendencies (error and correct) conflict with one another, producing larger ERN amplitudes.
The error positivity (Pe) follows the ERN, reaching maximal amplitude around centro-parietal sites between 200 and 500 ms after an erroneous response (Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991). Although early investigations also localized the Pe to the ACC (Dehaene et al., 1994; Herrmann et al., 2004; van Veen & Carter, 2002), more recent studies have suggested possible alternative neural generators as well, such as the anterior insular cortex (Overbeek, Nieuwenhuis, & Ridderinkhof, 2005; Ullsperger, Harsay, Wessel, & Ridderinkhof, 2010). The latter suggestion is consistent with the broader scalp distribution that is characteristic of the Pe. Unlike the ERN and N2, the Pe has not yet been incorporated into a formal action-monitoring model (Overbeek et al., 2005; Orr & Carrasco, 2011). Nonetheless, increasing evidence suggests that the Pe reflects the visceral awareness and conscious recognition of having made a mistake (Endrass, Franke, & Kathmann, 2005; Murphy, Robertson, Allen, Hester, & O’Connell, 2012; Nieuwenhuis, Ridderinkhof, Blom, Band, & Kok, 2001; O’Connell et al., 2009; Shalgi, Barkan, & Deouell, 2009). The growing consensus is that the Pe reflects processes related to errors (i.e., awareness or evaluation of errors) per se, whereas the ERN might index a more general conflict signal (Hughes & Yeung, 2011). This account is also consonant with studies that have found that the Pe, but not the ERN, predicted remedial actions following errors (i.e., posterror slowing or posterror accuracy; Frank, D’Lauro, & Curran, 2007; Hajcak, McDonald, & Simons, 2003; Moser, Schroder, Heeter, Moran, & Lee, 2011; Nieuwenhuis et al., 2001).
In sum, three ERPs—the N2, ERN, and Pe—are thought to reflect similar yet dissociable processes related to how we monitor our actions. Critically, all three ERPs are tightly linked with response processes: Whereas the N2 may reflect control efforts to avoid responding incorrectly, the ERN may reflect an initial response conflict signal following errors, and the Pe reflects conscious error awareness.
Task switching
When people switch between two or more tasks, they are slower and more error prone (Monsell, 2003). That is to say, switching tasks is costly. The origins of switch costs are a matter of some debate (Kiesel et al., 2010), but one process for which there is striking empirical support is proactive interference triggered by stimuli that have been associated with more than one task. For example, when subjects switch between naming the word and naming the picture of word–picture compounds, the word-reading RT is increased if that particular stimulus has previously been picture-named—even 100 trials after the initial stimulus (Waszak, Hommel, & Allport, 2003). More generally, once a stimulus has been associated with a particular response, any later presentations of that stimulus will evoke that same response (Pavlov, 1927). When the original stimulus–response (S–R) mapping is repeated, this “binding” facilitates performance, but when the rules change (i.e., when the same stimulus is now associated with a different response), these task-switching results show that the binding is detrimental to performance.
Rushworth and colleagues have distinguished between two different types of task switching: response switching, which involves switching the response (e.g., left or right hand) associated with a particular stimulus (e.g., red triangle), and visual switching, which involves switching attention between different sensory dimensions of a stimulus (e.g., a red triangle and a green square; Rushworth, Hadland, Paus, & Sipila, 2002; Rushworth, Passingham, & Nobre, 2002). This is a crucial distinction, because these two types of switching are likely subserved by different neural mechanisms. Selective attention is likely more involved in visual switching, whereas selecting among competing response options is likely more involved in response switching. Anatomically, selective attention involves parietal regions, such as superior parietal cortex, whereas response selection engages frontal networks, particularly the dorsolateral frontal cortex and ACC (Blasi et al., 2007; Casey et al., 2000; Liston, Matalon, Hare, Davidson, & Casey, 2006; Miller & Cohen, 2001). These data are consistent with the extensive anatomical connections between the ACC and regions involved in motor control (Devinsky et al., 1995; Paus, 2001; Picard & Strick, 2001). Therefore, one critical difference between response switching and visual switching is that response switching reliably elicits response conflict and concomitant enhanced ACC activity (Hsieh & Wu, 2011; Rushworth, Hadland, et al., 2002). Thus, response switching is of particular relevance to our interest here in studying how people monitor their responses.
In sum, switching tasks is costly, in terms of RTs and error rates. Although the mechanisms underlying switch costs are not yet fully understood, it is clear that once a response has been associated with a stimulus, a later presentation of that stimulus will evoke that response tendency. If that tendency is now incorrect, as when the response rules change, this should generate interference.
Relationships between action monitoring and task switching
Although the topics of task switching and action monitoring have each developed rich literatures in their own right, very few studies have investigated the influence of task switching on action monitoring per se (Kiesel et al., 2010). That is, very few empirical data have addressed the question, “How does switching tasks influence how we monitor and adapt our actions?” In one recent exception, the ERN and Pe were examined in a paradigm that alternated between a single-task condition and a task-switching condition (Tanaka, 2009). In that study, the Pe was reduced in the task-switching condition, but the ERN was unchanged. Thus, conscious error awareness—indexed by Pe amplitude—was attenuated during task switching and may have been responsible for the poorer performance in the task-switching condition (Tanaka, 2009). In a more recent study, action-monitoring ERPs were examined using a randomized-runs procedure (Ikeda & Hasegawa, 2012). In this task, participants were presented with stimuli consisting of a digit and a letter. Each run of trials began with a task cue that indicated which aspect of the stimulus was to be attended to. If the task cue for a particular run was the same as in the previous run, it was considered a “repeat” run; otherwise, it was considered a “switch” run. Ikeda and Hasegawa found that task-confusion errors—errors within switch runs caused by the execution of the previous (and irrelevant) task set—were characterized by reduced ERN and Pe amplitudes. These results suggested that conflict monitoring and error awareness were attenuated during task switching.
Although Tanaka’s (2009) and Ikeda and Hasegawa’s (2012) experiments provide preliminary insights into how task switching influences action-monitoring processes, the task designs in these studies may have precluded a complete understanding of how task switching influences how we monitor our actions. Most critically, Tanaka (2009) and Ikeda and Hasegawa (2012) both employed visual-switching tasks—as opposed to response-switching tasks—in their studies. That is, they had participants direct attention to different aspects of the stimulus, rather than switch between response rules. A recent ERP study that compared visual- and response-switching tasks found that the stimulus-locked frontal N2 was increased on switch trials, but only in the response-switching task. The authors concluded that an enhanced N2 reflected response suppression, consistent with the notion that ACC-generated action-monitoring components are selectively affected by response-switching tasks (Hsieh & Wu, 2011). As noted above, visual- and response-switching processes engage dissociable brain networks, such that the latter more consistently elicits ACC activity (e.g., Rushworth, Hadland, et al., 2002). Action-monitoring processes subserved by the ACC are primarily engaged at the response level, be it selecting among response options or monitoring for response conflict (Botvinick et al., 2004; Holroyd & Coles, 2002; Ridderinkhof et al., 2004; Yeung et al., 2004). Therefore, although Tanaka (2009) and Ikeda and Hasegawa (2012) examined visual-switching effects on action monitoring, and Hsieh and Wu examined the neural correlates of response switching, no study to date has examined how the full gamut of neural and behavioral correlates of error and conflict monitoring are modulated by response switching. This was the aim of the present study, and in this way, we were able to offer novel insights into the task switching–action monitoring relationship.
Described in more detail below, we employed a letter flanker task in which we reversed the S–R mappings between blocks and introduced new stimuli every second block, so that the S–R mapping afterward would be “fresh.” In this way, we were able to induce response switching in postreversal (switch) blocks, in which the previous (nonswitch) block’s mapping interfered with the current mapping. We predicted that switch blocks would be characterized by switch costs of increased RTs and error rates. We further predicted increased overall N2 amplitudes during switch blocks, consistent with previous task-switching (Mueller, Swainson, & Jackson, 2009) and response-switching (Hsieh & Wu, 2011; Rushworth, Passingham, & Nobre, 2002) ERP studies. Given the mixed findings of ERN modulation with task switching (i.e., unchanged [Tanaka, 2009] or attenuated [Ikeda & Hasegawa, 2012]), we were agnostic as to whether or how the S–R reversal would influence this component. Reduced Pe amplitude has been demonstrated in visual task switching (Ikeda & Hasegawa, 2012; Tanaka, 2009), so there was some reason to expect reduced Pe amplitude in switch blocks in the present study, especially if this co-occurred with behavioral evidence of reduced attention to errors. Given that adjusting to errors and conflicts is an essential consequence of action monitoring, we also examined how posterror behavioral responses (see Danielmeier & Ullsperger, 2011, for a review) were modulated by the S–R reversal.
Method
Participants
A group of 67 undergraduates (36 female, 31 male) were recruited from Michigan State University’s research pool and received course credit for their participation.Footnote 1 All participants provided written informed consent prior to beginning the experiment.
As will be noted in the Results section, error rates and RTs were significantly higher in switch blocks. Because the ERN amplitude is negatively correlated with error likelihood (e.g., Holroyd & Coles, 2002; Maier, Steinhauser, & Hübner, 2010), this potentially confounded meaningful ERP comparisons between the two block types. To check that our overall results were not affected by the confound, we duplicated our ERP analysis on a subsample of 29 participants (17 female, 12 male) who performed similarly (less than or equal to three errors difference) in nonswitch and switch blocks and compared the results to those from the overall sample.
Task
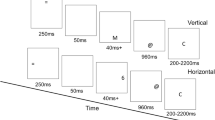
Participants completed a letters version of the Eriksen flanker task (Eriksen & Eriksen, 1974). The participants were instructed to respond to the center letter (target) of a five-letter string. Each five-letter string was either congruent (e.g., MMMMM) or incongruent (e.g., MMNMM). The characters were displayed in a standard white font on a black background, and they subtended 1.3° of visual angle vertically and 9.2° horizontally. All of the stimuli were presented on a Pentium R Dual Core computer, with Presentation software (Neurobehavioral Systems, Inc.) being used to control the presentation and timing of all stimuli, the determination of response accuracy, and the measurement of reaction times.
During each trial, flanking letters were presented 35 ms prior to target letter onset, and all five letters remained on the screen for a subsequent 100 ms (the total trial time was 135 ms). A fixation cross (+) was presented during the intertrial interval (from flanker stimuli on trial n to flanker stimuli on trial n + 1), which varied randomly between 1,200 and 1,700 ms at 100-ms intervals. Performance feedback was not given. The experimental session included 480 trials, grouped into 12 blocks of 40 trials, during which accuracy and speed were equally emphasized. The primary response-switching manipulation involved reversing S–R mappings within a block pair (e.g., left button for a target “M” in Block 1, right button for a target “M” in Block 2). Letters making up the trial stimuli differed across block pairs: Blocks 1 and 2, “M” and “N”; Blocks 3 and 4, “F” and “E”; Blocks 5 and 6, “O” and “Q”; Blocks 7 and 8, “T” and “I”; Blocks 9 and 10, “V” and “U”; Blocks 11 and 12, “P” and “R.” Prior to each block, instructions regarding the letter–mouse button assignments (the S–R mappings) were presented on the computer screen. In the following discussion, we will refer to the blocks in which we initially presented specific letter pairs as “nonswitch blocks,” and the blocks that were performed after S–R mappings were reversed as “switch blocks.” Nonswitch and switch blocks each comprised 240 total trials. This design elicited a sufficient number of errors per cell for reliable ERP analyses (i.e., six or more; Olvet & Hajcak, 2009).
Following the flanker task, the cap and sensors were removed, and participants completed a series of self-report questionnaires that are not considered here, but that have been reported elsewhere (see note 1).
Psychophysiological recording and data reduction
After giving written consent, the participants were seated approximately 60 cm in front of a computer monitor and were fitted with a stretch-lycra cap. Continuous electroencephalographic activity was recorded using the ActiveTwo BioSemi system (BioSemi, Amsterdam, The Netherlands). Recordings were taken from 64 Ag–AgCl electrodes placed in accordance with the 10/20 system. In addition, two electrodes were placed on the left and right mastoids. Electro-oculogram activity generated by eye movements and blinks was recorded at FP1 and at three additional electrodes placed inferior to the left pupil and on the left and right outer canthi (all approximately 1 cm from the pupil). During data acquisition, the common-mode-sense active electrode and driven-right-leg passive electrode formed the ground, as per BioSemi’s design specifications. All signals were digitized at 512 Hz using ActiView software (BioSemi).
Offline analyses were performed using BrainVision Analyzer 2 (BrainProducts, Gilching, Germany). Scalp electrode recordings were re-referenced to the numeric mean of the mastoids and band-pass filtered with cutoffs of 0.1 and 30 Hz (12 dB/oct rolloff). Ocular artifacts were corrected using the method developed by Gratton, Coles, and Donchin (1983). The stimulus- and response-locked data were segmented into individual epochs beginning 100 ms before response/stimulus onset and continuing for 800 ms following the stimulus/response. Physiological artifacts were detected using a computer-based algorithm, such that trials in which the following criteria were met were rejected: a voltage step exceeding 50 μV between contiguous sampling points, a voltage difference of more than 200 μV within a trial, or a maximum voltage difference less than 0.5 μV within a trial. Trials were also removed from subsequent analyses if the RT fell outside of a 200–800 ms time window. Average activity in the 100-ms window preceding stimulus (N2) or response (ERN, Pe) onset was used as the baseline. For each participant, trials that occurred during blocks in which a particular letter pair was presented for the first time (e.g., Blocks 1, 3, and 5) were averaged in order to quantify nonswitch ERPs, and trials from blocks in which the S–R mappings for the letter pairs were reversed (e.g., Blocks 2, 4, and 6) were averaged in order to quantify switch ERPs. The N2 was defined as the average voltage in the 200- to 300-ms poststimulus window following target onset. The ERN and its correct-trial counterpart, the correct-response negativity (CRN), were defined as the average activity occurring in the 0- to 100-ms postresponse window. Finally, the Pe and its correct-response counterpart were defined as the average activity in the 150- to 350-ms window following a response.
Overview of the analyses Footnote 2
Behavioral data were submitted to repeated measures analyses of variance (ANOVAs). Overall error rates were submitted to a one-way block type (nonswitch vs. switch) ANOVA. Overall RTs were submitted to a 2 (accuracy: error vs. correct) × 2 (block type: nonswitch vs. switch) ANOVA. To examine posterror adjustments, RT and error rates following error and correct trials were analyzed using separate 2 (response type: posterror vs. postcorrect) × 2 (block type: nonswitch vs. switch) ANOVAs. We also conducted a trial-by-trial analysis to examine the time course of the response-switching effect using a 2 (block type: nonswitch vs. switch) × 40 (trial) ANOVA.
For ERP analyses, the N2 was analyzed on correct trials using a 5 (site: Fz, FCz, Cz, CPz, Pz) × 2 (congruency: congruent vs. incongruent) × 2 (block type: nonswitch vs. switch) ANOVA. The ERN and Pe were analyzed using separate 5 (site: Fz, FCz, Cz, CPz, Pz) × 2 (accuracy: error vs. correct) × 2 (block type: nonswitch vs. switch) repeated measures ANOVA. Identical behavioral and ERP ANOVAs were run for the subsample participants to ensure that any ERP effects were not simply due to error rate differences. For the sake of brevity, we will only report the ERP effects and interactions involving block type (but see Table S1 in the supplemental materials for all of the effects and interactions). Partial eta squared (η 2p ) is reported as an estimate of effect size, such that .05 is considered a small effect, .1 a medium effect, and .2 a large effect (Cohen, 1969).
Results
Overall behavioral results
As expected, RTs were shorter on error trials (M = 364.87, SD = 51.86) than on correct trials (M = 436.57, SD = 39.60) [F(1, 66) = 467.39, p < . 001, η 2p = .88], confirming the typical speed–accuracy trade-off. As can be seen in Fig. 1, the effects of block type were in line with our expectations: Overall RTs were longer in switch (M = 411.92, SD = 48.12) than in nonswitch (M = 383.42, SD = 38.20) blocks [F(1, 66) = 56.33, p < .001, η 2p = .46], and error rates were also higher in switch (M = 9.01 %, SD = 5.16 %) than in nonswitch (M = 8.18 %, SD = 5.57 %) blocks [F(1, 66) = 3.93, p = .05, η 2p = .06], confirming the main effect of block type for both RTs and error rates.
Behavioral results for the full sample (n = 67). Block type effects for reaction times (left) and error rates (right) for overall performance (top) and for posterror performance (middle). For posterror performance, reaction times (RTs) and error rates were calculated as the difference between posterror trials and postcorrect trials. Note that the negative posterror error rate in the nonswitch blocks indicates that more accurate responding followed error trials, and the opposite is true for switch blocks. The bottom graph depicts a trial-by-trial analysis of overall RTs. Error bars represent ±1 SEM. * p < .05. ** p < .01
Finally, the interaction between accuracy and block type was significant for RTs [F(1, 66) = 28.17, p < .001, η 2p = .30]: Although both error-trial and correct-trial RTs were longer in switch blocks, the switching effect was greater on error trials (difference: M = 42.69, SD = 49.63) than on correct trials (difference: M = 14.31, SD = 20.68) [t(66) = 5.31, p < .001]. Follow-up t tests likewise showed that the difference between correct- and error-trial RTs was smaller in switch blocks (M = 64.40, SD = 36.43) than in nonswitch blocks (M = 92.78, SD = 37.44) [t(66) = 5.31, p < .001]. Thus, in relative terms, error trials were slower in switch blocks than in nonswitch blocks. Because the intertrial interval was the same (on average) for switch and nonswitch blocks, longer error-trial RTs in switch blocks would have left less time available for posterror processing.
Posterror adaptation
Posterror slowing (PES)
Regardless of block type, correct responses were slower on trials after error trials (M = 473.07, SD = 55.55) than on trials after correct trials (M = 431.86, SD = 40.59) [F(1, 66) = 112.67, p < .001, η 2p = .63], confirming the typical PES effect. In addition, a significant interaction between response type and block type emerged [F(1, 66) = 5.86, p < .05, η 2p = .08], indicating that participants slowed down more after errors in switch blocks. Follow-up t tests showed that PES, measured as the difference between posterror correct RTs minus postcorrect correct RTs, was larger in switch blocks (M = 48.05, SD = 40.26) than in nonswitch blocks (M = 32.73, SD = 41.27) [t(66) = 2.42, p < .05].
Posterror error rate
Regardless of block type, error rates tended to be numerically, but not statistically, higher on posterror trials (M = 9.73 %, SD = 7.02 %) than on postcorrect trials (M = 8.58 %, SD = 5.21 %) [F(1, 66) = 2.23, p = .14, η 2p = .03]. A main effect of block type emerged, such that postresponse error rates were higher in switch blocks (M = 10.35 %, SD = 6.43 %) than in nonswitch blocks (M = 7.83 %, SD = 6.05 %) [F(1, 66) = 9.90, p < .01, η 2p = .13]. The Response Type × Block Type interaction also emerged for postresponse error rates [F(1, 66) = 9.09, p < .005, η 2p = .12]. In nonswitch blocks, posterror error rates (PE ER; M = 7.28 %, SD = 7.90 %) were lower than postcorrect error rates (PC ER; M = 8.38 %, SD = 5.65 %); the opposite pattern was true of switch blocks (PE ER, M = 11.91 %, SD = 10.21 %; PC ER, M = 8.78 %, SD = 5.28 %). Consequently, the postresponse error rate difference (PE ER minus PC ER) was significantly enhanced in switch blocks (M = 3.13 %, SD = 9.94 %) as compared to nonswitch blocks (M = –1.11 %, SD = 6.51 %) [t(66) = 3.02, p < .005]. That is, relative to postcorrect responses, participants responded more accurately after errors in nonswitch blocks, but less accurately after errors in switch blocks.
These results suggest that in nonswitch blocks, participants responded more slowly and more accurately following their errors. In contrast, in switch blocks, participants responded more slowly, yet less accurately, following their errors. These findings suggest that response switching results in inefficient posterror behavioral adjustments, which should then be reflected in relevant ERPs.
Trial-by-trial analysis
The bottom panel of Fig. 1 shows RTs separated by block type and trial. The figure suggests that, except for the first trial, the effect of response switching was constant across the block. In our analyses, we omitted the first trial, on the grounds that this reflected additional processes specific to that trial (Altmann & Gray, 2008). For RTs, the main effect of block type was again significant [F(1, 66) = 35.21, p < .001, η 2p = .35], such that switch block RTs were longer than nonswitch block RTs. The main effect of trial was also significant [F(1, 66) = 3.99, p < .001, η 2p = . 06], and follow-up polynomial contrasts indicated that the trend was linear [F(1, 66) = 25.11, p < .001, η 2p = .28], indicative of within-run slowing (Altmann, 2002). However, the interaction between block type and trial was not significant [F(1, 66) = 1.50, p > .05, η 2p = .02], suggesting that, relative to nonswitch blocks, switch blocks were associated with longer RTs on any given trial.
For error rates, the main effect of block type was again significant [F(1, 66) = 4.87, p < .05, η 2p = .07]; switch blocks were associated with higher error rates than were nonswitch blocks. However, the main effect of trial was not significant [F(1, 66) = 1.09, p > .34, η 2p = .02], indicating that error rates were more or less consistent throughout a given block. The interaction between block type and trial was also nonsignificant [F(1, 66) < 1, p > .90, η 2p = .01], suggesting that switch blocks were associated with higher error rates throughout the block.
The absence of any hint of an interaction between block type and trial links the main effect of block type to priming of the incorrect response mapping by individual stimuli. Had the incorrect response mapping not been associatively linked to task stimuli, we would have expected it to show some evidence of decay across a block (Altmann & Gray, 2008), and thus some decrease in the effect of block type across trials.
ERPs
N2
Figure 2 presents stimulus-locked ERP waveforms. In the 200- to 300-ms post-stimulus-onset window, the main effect of block type was significant [F(1, 66) = 29.48, p < .001, η 2p = .31], indicating that overall N2 amplitudes were increased (i.e., more negative) in switch blocks (M = 0.84, SD = 3.83) as compared to nonswitch blocks (M = 1.75, SD = 4.02). The interaction between site and block type was also significant [F(4, 264) = 29.16, p < .001, η 2p = .31], indicating that the effect of block type was largest at fronto-central recording sites (see the supplemental materials, Fig. S3). Critically, none of the interactions between congruency and block type were significant (Fs < 1.25, ps > .28, η 2p s < .03). This null interaction suggests that response-switching conflict was independent of flanker interference conflict (see also the supplemental materials).
Stimulus-locked grand-average ERP waveforms depicting the N2. For illustrative purposes only, waveforms are averaged across congruencies, as the congruency effects on N2 did not differ as a function of block type (see the Results section). Time 0 represents target letter onset
ERN
Figure 3 presents the ERNs in switch and nonswitch blocks. Importantly, in the context of the present study, a main effect of block type emerged [F(1, 66) = 11.91, p = .001, η 2p = .15], indicating greater overall postresponse negativity in switch blocks than in nonswitch blocks. Moreover, the interaction between accuracy and block type was significant [F(1, 66) = 6.80, p < .05, η 2p = .09]. Follow-up t tests revealed that the ERN was larger on switch-block trials (M = –3.55, SD = 3.77) than on nonswitch-block trials (M = –2.31, SD = 3.37) [t(66) = 3.26, p < . 01]. In contrast, the CRN did not differ between switch (M = –0.75, SD = 2.63) and nonswitch blocks (M = –0.58, SD = 2.74) [t(66) = 1.17, p > .24]. The ERN difference score (ERN minus CRN) was significantly enhanced in switch blocks (M = –2.80, SD = 3.53) as compared to nonswitch blocks (M = –1.73, SD = 3.44) [t(66) = 2.61, p < .05]. Although the Site × Block Type interaction was significant [F(4, 264) = 9.67, p < .001, η 2p = .13], critically, the Site × Block Type × Accuracy interaction was not [F(4, 264) = 1.69, p > .18, η 2p = . 03].
Pe
Figure 3 also depicts the Pe. The main effect of block type was significant [F(1, 66) = 9.25, p < .01, η 2p = .12], as was the interaction between accuracy and block type [F(1, 66) = 27.04, p < .001, η 2p = .29]. Follow-up t tests revealed that the Pe was significantly reduced in switch blocks (M = 4.40, SD = 6.25) relative to nonswitch blocks (M = 7.52, SD = 6.47) [t(66) = 5.00, p < .001]. Conversely, the correct-trial Pe was not different between switch (M = –7.59, SD = 3.84) and nonswitch (M = –7.82, SD = 3.88) blocks [t(66) = 1.17 p = .25]. Finally, the Pe difference amplitude (Pe minus correct-trial Pe) was reduced in switch blocks (M = 12.00, SD = 6.51) as compared to nonswitch blocks (M = 15.35, SD = 7.04) [t(66) = 5.62, p < .001]. The interaction between site and block type [F(4, 264) = 22.18, p < .001, η 2p = .25] was significant. More importantly, the site, block type, and accuracy interaction was significant [F(4, 264) = 10.83, p < .001, η 2p = .14], indicating that the reduced Pe (and Pe difference) in switch blocks was most pronounced at centro-parietal sites (see Supplemental Fig. S3).
Subsample results
The subsample analyses revealed that, although RTs were still longer in switch blocks [F(1, 28) = 31.76, p < .001, η 2p = .53], the overall error rate—by design—was not significantly different between block types [F(1, 28) = 1.65, p = .21, η 2p = .06]. Thus, the subsample of participants provided for an additional check of the ERP analyses.
The ERP results in the subsample were identical to those reported above. Overall N2 amplitudes were larger in switch than in nonswitch blocks [F(1, 28) = 11.61, p < .01, η 2p = .29], but there was no interaction between block type and congruency (Fs < 1, ps > .44, η 2p s < .04). For the ERN, the interaction between block type and accuracy was significant [F(1, 28) = 4.53, p < .05, η 2p = .14]; the ERN was enhanced within switch blocks as compared to nonswitch blocks, but the CRN was not different between block types. This interaction also emerged for the Pe [F(1, 28) = 15.76, p < .001, η 2p = .36]; the Pe was significantly reduced in switch blocks as compared to nonswitch blocks, but the correct-trial Pe was not different between block types. For all three components, all interactions with site were qualitatively similar to those reported above. In sum, the subsample results provide evidence that the switch-block ERP effects reported above are not simply due to error rate confounds.
Relationship between ERPs and behavior (overall sample)
Given that response switching had various influences on both overall ERPs and the behavioral measures, we next examined whether it also influenced brain–behavior relationships. The ERN and Pe have both been associated with error-related behavioral measures, such as PES and posterror accuracy (e.g., Gehring et al., 1993; Hajcak et al., 2003). Correlations between the ERN, Pe, error-trial RT, PES, and posterror error rate in the nonswitch and switch blocks are depicted in Table 1. None of the behavioral measures were related to the ERN in either block type. In switch blocks only, the posterror error rate was negatively related to Pe amplitude, consistent with previous studies (e.g., Frank et al., 2007; Moser et al., 2011). Finally, within switch blocks, longer error-trial RTs were related to both smaller Pe amplitudes and decreased posterror accuracy. As we have already noted, longer error-trial RTs led to shorter posterror time windows during switch blocks. These relationships, therefore, suggest that shorter posterror time windows resulted in attenuated Pe and posterror accuracy.
Discussion
The present study was designed to examine response-switching influences on action monitoring. Although previous studies have examined the underlying mechanisms of response switching (e.g., Hsieh & Wu, 2011) and visual-switching influences on action monitoring (Ikeda & Hasegawa, 2012; Tanaka, 2009), no previous study has examined the action-monitoring consequences of response switching. We employed a flanker task with S–R reversals between blocks, creating response-switching conflicts in the switch blocks. Response switching had several behavioral effects and differentially modulated action-monitoring ERPs: The overall N2 and ERN were enhanced, but Pe was reduced. Because action-monitoring research and task-switching research have evolved more or less in parallel to one another (Kiesel et al., 2010), this integrative approach can shed new light on how individuals process and adjust to conflicts and errors in the context of changing response rule requirements. Here, we discuss our findings in the context of current theories.
Response switching and S–R reversals: Stimulus-primed response conflict (SPRC)
RTs and error rates were substantially increased during switch blocks (see Fig. 1), akin to switch costs in other task-switching studies (Monsell, 2003). It is again critical to note that our paradigm was a response-switching task in vwhich the target stimulus was always the central letter but the response rules were reversed. Thus, our task elicited response conflict (Rushworth, Hadland, et al., 2002). Previous work has demonstrated that a stimulus can elicit long-lasting proactive interference whenever the response it is initially paired with changes (Shiu & Chan, 2006). This finding extends to situations in which the stimulus was initially paired with a different higher-level task context (Waszak et al., 2003). Because our procedure used identical stimuli and responses in nonswitch and switch blocks, but with reversed response mappings, we suggest that each stimulus within a given switch block evoked both the current (switch) and the previous (nonswitch) blocks’ S–R mappings. For example, a target “M” in Block 2 (a switch block) would elicit both the currently correct response (e.g., left button) and the previously correct but currently incorrect response (e.g., right button) that was learned during Block 1 (the nonswitch block).Footnote 3 We refer to the ensuing interference between the simultaneously activated relevant and irrelevant S–R mappings and response tendencies as “stimulus-primed response conflict,” or SPRC. The role of the stimulus in driving this conflict is evident from the lack of interaction of this effect with trial (Fig. 1, bottom panel). If the old response mapping were not continually primed by the current stimulus, we would expect some decay of this representation across the block (Altmann & Gray, 2008), and thus a decrease in switch costs across the block. Because conflict may be brought about by any source that introduces processing variability or noise (Yeung et al., 2011), SPRC can be considered a legitimate source of conflict.
SPRC influenced several aspects of action monitoring on a broad, block-to-block level, in which overall RTs and error rates were heightened across switch blocks. Independent of this more “background” conflict restricted to switch blocks, flanker interference conflict operated on a trial-to-trial basis, with increased RTs and error rates on incongruent trials. SPRC was dissociated from other flanker interference effects: The Gratton and N2 difference effects were not different between nonswitch and switch blocks (see the supplemental materials). This finding permits analysis of how response switching uniquely and independently influenced action monitoring.
Conflict control processing: N2 and ERN
As we noted earlier, even though the flanker N2 is stimulus locked, it is generally viewed as a marker of control over incorrect response tendencies (Folstein & Van Petten, 2008). Here, in line with our predictions, we found that the N2 was higher in switch blocks, which supports this interpretation: We attribute the enhanced N2 on switch blocks to suppression of incorrect response tendencies when response rules were changed. In other words, N2 reflected attempts to inhibit SPRC. In previous work, the flanker N2 has been interpreted in terms of inhibiting the response primed by incongruent distractors (e.g., Tillman & Wiens, 2011). Our own data suggest the two effects on N2 were additive (not interactive), such that amplitudes were smallest on congruent nonswitch flankers and largest on incongruent switch flankers. Thus, taken together with previous response-switching work (e.g., Hsieh & Wu, 2011; Rushworth, Passingham, & Nobre, 2002), our results suggest that N2 may reflect a mechanism specific to inhibition of incorrect response tendencies primed directly by the stimulus.
The ERN was also enhanced during switch blocks. This finding, interpreted according to the conflict theory (Yeung et al., 2004), suggests that posterror target processing was enhanced during switch blocks. Enhanced target processing is a compensatory strategy for controlling response selection that has been observed when tasks become more challenging (Sarter, Gehring, & Kozak, 2006). Here it is possible that a “ramping up” of target processing, indexed by increased ERNs, occurred when initial control attempts (i.e., response inhibition) failed on error trials. Thus, whereas an enhanced N2 reflected the successful inhibition of the previous block’s mapping—leading to correct responses—enhanced ERN reflected continued processing of the target stimulus after control was unsuccessful (on error trials). This interpretation is consistent with the absence of a correlation between the switching effect on the N2 and the switching effect on the ERN (r = –.13, p = .29), which suggests that the switching effect was registering on different processes.
The enhanced ERN in switch blocks is inconsistent with previous task-switching studies that have found no modulation of the ERN (Tanaka, 2009) or a decreased ERN (Ikeda & Hasegawa, 2012) during switching. This inconsistency highlights the difference between the visual-switching procedures used in those previous studies and the response-switching procedure used here. Indeed, the two types of switching are each associated with distinct regional brain activity and ERP modulation (e.g., Hsieh & Wu, 2011; Rushworth, Passingham, & Nobre, 2002). Specifically, response switching seems to consistently modulate the putative markers of ACC activity (e.g., Brass, Wenke, Spengler, & Waszak, 2009; Parris, Thai, Benattayallah, Summers, & Hodgson, 2007; Rushworth, Hadland, et al., 2002). In visual switching, task confusions are possible in which either response seems like it might be correct, especially when the incorrect task set is executed. Ikeda and Hasegawa argued that such confusions blur the distinction between correct and error trials and thus lead to a reduced ERN. In these terms, we would say that priming of the incorrect response mapping in our procedure accentuated the distinction between correct and error trials, leading to an enhanced ERN.
Behavioral adjustments and error processing
Posterror adjustments have been incorporated into several different action-monitoring theories, although their underlying mechanisms are not yet fully understood (Danielmeier & Ullsperger, 2011). The conflict-monitoring theory suggests that PES reflects an increased response threshold in posterror trials in order to adjust speed–accuracy trade-offs to improve performance (Botvinick et al., 2001). However, PES is not always correlated with posterror accuracy (e.g., Carp & Compton, 2009; Danielmeier & Ullsperger, 2011), and some studies have shown that PES coincides with decreased posterror accuracy (e.g., Fiehler, Ullsperger, & von Cramon, 2005). Indeed, increasing evidence suggests that posterror evaluation is associated with considerable interference with subsequent trial processing, especially when there is little time between the error and the subsequent trial; posterror responding is slower and less accurate with decreasing response–stimulus intervals (Jentzsch & Dudschig, 2009).
Nonswitch blocks were associated with adaptive posterror adjustments: Error trials were followed by slower and more accurate responses. This fits with the conflict theory, in that posterror conflict detection triggered subsequent control processes that improved performance (Botvinick et al., 2001). In contrast, switch blocks were associated with increased PES and decreased posterror accuracy, indicative of maladaptive adjustments. Therefore, conflict detection failed to implement sufficient control processes to improve performance. We attribute this lack of posterror control to the changing response dynamics that characterized the switch blocks. Specifically, because intertrial intervals were the same (on average) across switch and nonswitch blocks, longer error-trial RTs during switch blocks reduced the time window between the error response on trial n and the subsequent stimulus on trial n + 1. That is, participants “ran out of time” before they could appropriately evaluate their incorrect responses and improve their performance on the next trial (e.g., Danielmeier, Eichele, Forstmann, Tittgemeyer, & Ullsperger, 2011; Jentzsch & Dudschig, 2009; King, Korb, von Cramon, & Ullsperger, 2010). Indeed, the difference in error-trial RTs between switch and nonswitch blocks was highly correlated with the difference in posterror error rates between block types (r = .50, p < .001).
The Pe findings support the idea that “running out of time” was associated with insufficient error evaluation. Pe was reduced in switch blocks, consistent with previous studies (Ikeda & Hasegawa, 2012; Tanaka, 2009), suggesting that overall conscious awareness of and attention to errors was attenuated or delayed (Hughes & Yeung, 2011; Murphy et al., 2012; Ridderinkhof, Ramautar, & Wijnen, 2009). If SPRC extended the time needed for processing through response selection, it would have compressed the time available for later processes to generate conscious error awareness (Ullsperger et al., 2010). Increasing evidence has linked the Pe to conscious awareness and posterror behavioral adjustments (Frank et al., 2007; Murphy et al., 2012; Steinhauser & Yeung, 2010). It stands to reason, then, that slower error RTs would lead to a reduced opportunity for error awareness, and thus to both lower Pe amplitudes and less successful posterror adjustment.
Alternative accounts
The reinforcement-learning theory of the ERN is another influential action-monitoring model, which posits that the ERN is a signal to the ACC indicating that performance is worse than expected (Holroyd & Coles, 2002). In this way, the ACC is trained to select the appropriate responses and improve performance at the task at hand, and it becomes more activated as the “nature of the stimuli or the response options make the mappings more difficult to implement” (Holroyd & Coles, 2002, p. 695). The increased ERN during switch blocks is consistent with this view, to the extent that the implementation of S–R mappings was more difficult in these blocks. However, the reinforcement-learning theory does not explicitly incorporate the N2 (Baker & Holroyd, 2011), and therefore cannot account for the present findings to the extent that the conflict theory can.
Grinband et al. (2011b) have recently proposed that activation in medial frontal cortex can be accounted for by time on task, such that larger RTs correlate with more brain activity in this region. The time-on-task theory claims that this correlation challenges effects related to conflict (Botvinick et al., 2001; Yeung et al., 2004) and error likelihood (Brown & Braver, 2005). In the present study, switch blocks were associated with increased RTs and increased ERN and N2, a finding in line with the time-on-task account, given that these two ERPs are consistently localized to the ACC (Dehaene et al., 1994; van Veen & Carter, 2002). Nonetheless, the time-on-task theory lacks an intervening psychological construct—an underlying mechanism—that the conflict-monitoring theory provides (Yeung et al., 2011), and therefore offers a conceptually weaker, yet plausible, explanation of the present data at this time. That is to say, time on task cannot provide a meaningful interpretation of our results, other than suggesting that increased RTs accounted for the ERN and N2 findings during switch blocks.
Conclusion
We have described a fairly comprehensive picture of how action-monitoring processes are influenced by a subtle yet powerful manipulation of reversing stimulus–response rules. Reversing the rules resulted in a type of proactive interference that we refer to as stimulus-primed response conflict, in which each stimulus in switch blocks simultaneously primed the previous (irrelevant) S–R mapping and the current (relevant) mapping, creating interference between the two mappings. This conflict was evident in increased RTs and error rates. Successful resolution of this conflict on correct trials required additional processes such as response inhibition, as reflected in an enhanced N2. When response-selection control was unsuccessful—on error trials—compensatory mechanisms of enhanced postresponse target processing were engaged, as reflected in an increased ERN. However, the costs of this additional processing produced shorter response–stimulus intervals following errors, resulting in reduced error awareness, as reflected in an attenuated Pe and impaired posterror adjustments. Thus, reversing the rules via response switching is associated with enhanced effortful control on correct trials, and inefficient action monitoring and compromised awareness after errors.
The results from this study may have implications for real-world situations. For example, the hypothetical driver that we described earlier may be under constraints similar to SPRC. Monitoring for conflicts in the new context requires engaging in processing the driving environment and/or inhibiting the rules previously learned while focusing on the current driving rules. As more resources are taken by these compensatory mechanisms, fewer resources are available to consciously evaluate the driving performance, resulting in slower and more error-prone driving. While these considerations are speculative, the present study provides novel and important contributions to the literature, and represents an integrative approach to studying the time course of action-monitoring consequences associated with response switching. Future studies directly comparing the action-monitoring consequences of visual- and response-switching tasks are warranted.
Notes
See the supplemental materials for additional analyses of the congruency effect and the Gratton effect.
Note that the same interference would not occur during nonswitch blocks, because it was during these blocks that the S–R mappings for a particular letter pair were first learned. Previous work had suggested that proactive-interference switch costs linked to a stimulus only occur when the stimulus had previously appeared in an alternative, currently irrelevant task context (Waszak et al., 2003). In the present procedure, the letters making up the trial stimuli changed every two blocks, such that each letter pair had one nonswitch block and one switch block (see the Method section). Each nonswitch block introduced a new S–R mapping, and each switch block reversed this mapping. Thus, the transition from a given switch block to the subsequent nonswitch block would not have produced any proactive interference, because the letters making up the stimuli were different.
References
Altmann, E. M. (2002). Functional decay of memory for tasks. Psychological Research, 66, 287–297. doi:10.1007/s00426-002-0102-9
Altmann, E. M., & Gray, W. D. (2008). An integrated model of cognitive control in task switching. Psychological Review, 115, 602–639. doi:10.1037/0033-295X.115.3.602
Arbel, Y., & Donchin, E. (2009). Parsing the componential structure of post-error ERPs: A principal component analysis of ERPs following errors. Psychophysiology, 46, 1179–1189. doi:10.1111/j.1469-8986.2009.00857.x
Baker, T. E., & Holroyd, C. B. (2011). Dissociated roles of the anterior cingulate cortex in reward and conflict processing as revealed by the feedback error-related negativity and N200. Biological Psychology, 87, 25–34. doi:10.1016/j.biopsycho.2011.01.010
Blasi, G., Goldberg, T. E., Elvevåg, B., Rasetti, R., Bertolino, A., Cohen, J., & Mattay, V. S. (2007). Differentiating allocation of resources and conflict detection within attentional control processing. European Journal of Neuroscience, 25, 594–602. doi:10.1111/j.1460-9568.2007.05283.x
Botvinick, M. M., Braver, T. S., Barch, D. M., Carter, C. S., & Cohen, J. D. (2001). Conflict monitoring and cognitive control. Psychological Review, 108, 624–652. doi:10.1037/0033-295X.108.3.624
Botvinick, M. M., Cohen, J. D., & Carter, C. S. (2004). Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences, 8, 539–546. doi:10.1016/j.tics.2004.10.003
Brass, M., Wenke, D., Spengler, S., & Waszak, F. (2009). Neural correlates of overcoming interference from instructed and implemented stimulus–response associations. Journal of Neuroscience, 29, 1766–1772. doi:10.1523/JNEUROSCI.5259-08.2009
Brown, J. W. (2011). Medial prefrontal cortex activity correlates with time-on-task: What does this tell us about theories of cognitive control? NeuroImage, 57, 314–315. doi:10.1016/j.neuroimage.2011.04.028
Brown, J. W., & Braver, T. S. (2005). Learned predictions of error likelihood in the anterior cingulate cortex. Science, 307, 1118–1121.
Bush, G., Luu, P., & Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4, 215–222. doi:10.1016/S1364-6613(00)01483-2
Carp, J., & Compton, R. J. (2009). Alpha power is influenced by performance errors. Psychophysiology, 46, 336–343. doi:10.1111/j.1469-8986.2008.00773.x
Carter, C. S., Braver, T. S., Barch, D. M., Botvinick, M. M., Noll, D., & Cohen, J. D. (1998). Anterior cingulate cortex, error detection, and the online monitoring of performance. Science, 280, 747–749. doi:10.1126/science.280.5364.747
Carter, C. S., & van Veen, V. (2007). Anterior cingulate cortex and conflict detection: An update of theory and data. Cognitive, Affective, & Behavioral Neuroscience, 7, 367–379. doi:10.3758/CABN.7.4.367
Casey, B. J., Thomas, K. M., Welsh, T. F., Badgaiyan, R. D., Eccard, C. H., Jennings, J. R., & Crone, E. A. (2000). Dissociation of response conflict, attentional selection, and expectancy with functional magnetic resonance imaging. Proceedings of the National Academy of Sciences, 97, 8728–8733. doi:10.1073/pnas.97.15.8728
Cohen, J. (1969). Statistical power analysis for the behavioral sciences. New York, NY: Academic Press.
Danielmeier, C., Eichele, T., Forstmann, B. U., Tittgemeyer, M., & Ullsperger, M. (2011). Posterior medial frontal cortex activity predicts post-error adaptations in task-related visual and motor areas. Journal of Neuroscience, 31, 1780–1789. doi:10.1523/JNEUROSCI.4299-10.2011
Danielmeier, C., & Ullsperger, M. (2011). Post-error adjustments. Frontiers in Psychology, 2, 1–10. doi:10.3389/psyg.2011.00233
Dehaene, S., Posner, M. I., & Tucker, D. M. (1994). Localization of a neural system for error detection and compensation. Psychological Science, 5, 303–305. doi:10.1111/j.1467-9280.1994.tb00630.x
Devinsky, O., Morrell, M. J., & Vogt, B. A. (1995). Contributions of anterior cingulate cortex to behaviour. Brain, 118, 279–306. doi:10.1093/brain/118.279
Endrass, T., Franke, C., & Kathmann, N. (2005). Error awareness in a saccade countermanding task. Journal of Psychophysiology, 19, 275–280. doi:10.1027/0269-8803.19.4.275
Eriksen, B. A., & Eriksen, C. W. (1974). Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics, 16, 143–149. doi:10.3758/BF03203267
Falkenstein, M., Hohnsbein, J., Hoormann, J., & Blanke, L. (1991). Effects of crossmodal divided attention on late ERP components: II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology, 78, 447–455. doi:10.1016/0013-4694(91)90062-9
Falkenstein, M., Hoormann, J., Christ, S., & Hohnsbein, J. (2000). ERP components on reaction errors and their functional significance: A tutorial. Biological Psychology, 51, 87–107. doi:10.1016/S0301-0511(99)00031-9
Falkenstein, M., Hoormann, J., & Hohnsbein, J. (1999). ERP components in go/nogo tasks and their relation to inhibition. Acta Psychologica, 101, 267–291. doi:10.1016/S0001-6918(99)00008-6
Fiehler, K., Ullsperger, M., & von Cramon, D. Y. (2005). Electrophysiological correlates of error correction. Psychophysiology, 42, 72–82. doi:10.1111/j.1469-8986.2005.00265.x
Folstein, J. R., & Van Petten, C. (2008). Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology, 45, 152–170. doi:10.1111/j.1468-8986.2007.00602.x
Frank, M. J., D’Lauro, C. D., & Curran, T. (2007). Cross-task individual differences in error processing: Neural, electrophysiological, and genetic components. Cognitive, Affective, and Behavioral Neuroscience, 7, 297–308. doi:10.3758/CABN.7.4.297
Gehring, W. J., Goss, B., Coles, M. G. H., Meyer, D. E., & Donchin, E. (1993). A neural system for error detection and compensation. Psychological Science, 4, 385–390. doi:10.1111/j.1467-9280.1993.tb00586.x
Gehring, W. J., Gratton, G., Coles, M. G. H., & Donchin, E. (1992). Probability effects on stimulus evaluation and response processes. Journal of Experimental Psychology: Human Perception and Performance, 18, 198–216. doi:10.1037/0096-1523.18.1.198
Gehring, W. J., Liu, Y., Orr, J. M., & Carp, J. (2012). The error-related negativity (ERN/Ne). In S. J. Luck & E. Kappenman (Eds.), Oxford handbook of event-related potential components (pp. 231–291). New York, NY: Oxford University Press.
Gopher, D., Armony, L., & Greenshpan, Y. (2000). Switching tasks and attention policies. Journal of Experimental Psychology: General, 129, 308–339. doi:10.1037/0096-3445.129.3.308
Gratton, G., Coles, M. G. H., & Donchin, E. (1983). A new method for the off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology, 55, 468–484. doi:10.1016/0013-4694(83)90135-9
Grinband, J., Savitskaya, J., Wager, T. D., Teichert, T., Ferrera, V. P., & Hirsch, J. (2011a). Conflict, error likelihood, and RT: Response to Brown & Yeung et al. NeuroImage, 57, 320–322. doi:10.1016/j.neuroimage.2011.04.027
Grinband, J., Savitskaya, J., Wager, T. D., Teichert, T., Ferrera, V. P., & Hirsch, J. (2011b). The dorsal medial frontal cortex is sensitive to time on task, not response conflict or error likelihood. NeuroImage, 57, 303–311. doi:10.1016/j.neuroimage.2010.12.027
Hajcak, G., McDonald, N., & Simons, R. F. (2003). To err is autonomic: Error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology, 40, 895–903. doi:10.1111/1469-8986.00107
Herrmann, M. J., Römmler, J., Ehlis, A., Heidrich, A., & Fallgatter, A. J. (2004). Source localization (LORETA) of the error-related-negativity (ERN/Ne) and positivity (Pe). Cognitive Brain Research, 20, 294–299. doi:10.1016/j.cogbrainres.2004.02.013
Holroyd, C. B., & Coles, M. G. (2002). The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review, 109, 679–709. doi:10.1037/0033-295X.109.4.679
Holroyd, C. B., Nieuwenhuis, S., Yeung, N., Nystrom, L., Mars, R. B., Coles, M. G., & Cohen, J. D. (2004). Dorsal anterior cingulate cortex shows fMRI response to internal and external error signals. Nature Neuroscience, 7, 497–498. doi:10.1038/nn1238
Hsieh, S., & Wu, M. (2011). Electrophysiological correlates of preparation and implementation for different types of task shifts. Brain Research, 1423, 41–52. doi:10.1016/j.brainres.2011.09.018
Hughes, G., & Yeung, N. (2011). Dissociable correlates of response conflict and error awareness in error-related brain activity. Neuropsychologia, 49, 405–415. doi:10.1016/j.neuropsychologia.2010.11.036
Ikeda, K., & Hasegawa, T. (2012). Task confusion after switching revealed by reductions of error-related ERP components. Psychophysiology, 49, 427–440. doi:10.1111/j.1469-8986.2011.01295.x
Jentzsch, I., & Dudschig, C. (2009). Why do we slow down after an error? Mechanisms underlying the effects of post-error slowing. Quarterly Journal of Experimental Psychology, 62, 209–218. doi:10.1080/17470210802240655
Kerns, J. G., Cohen, J. D., MacDonald, A. W., Cho, R. Y., Stenger, V. A., & Carter, C. S. (2004). Anterior cingulate conflict monitoring and adjustments in control. Science, 303, 1023–1026. doi:10.1126/science.1089910
Kiesel, A., Steinhauser, M., Wendt, M., Falkenstein, M., Jost, K., Philipp, A. M., & Kock, I. (2010). Control and interference in task switching—A review. Psychological Bulletin, 136, 849–874. doi:10.1037/a0019842
King, J. A., Korb, F. M., von Cramon, D. Y., & Ullsperger, M. (2010). Post-error behavioral adjustments are facilitated by activation and suppression of task-relevant and task-irrelevant information processing. Journal of Neuroscience, 30, 12759–12769. doi:10.1523/JNEUROSCI.3274-10.2010
Kopp, B., Mattler, U., Goertz, R., & Rist, F. (1996). N2, P3, and the lateralized readiness potential in a nogo task involving selective response priming. Electroencephalography and Clinical Neurophysiology, 99, 19–27. doi:10.1016/0921-884X(96)95617-9
Liston, C., Matalon, S., Hare, T. A., Davidson, M. C., & Casey, B. J. (2006). Anterior cingulate and posterior parietal cortices are sensitive to dissociable forms of conflict in a task-switching paradigm. Neuron, 50, 643–653. doi:10.1016/j.neuron.2006.04.015
MacDonald, A. W., Cohen, J. D., Stenger, V. A., & Carter, C. S. (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science, 288, 1835–1838. doi:10.1126/science.288.5472.1835
Maier, M. E., Steinhauser, M., & Hübner, R. (2010). Effects of response-set size on error-related brain activity. Experimental Brain Research, 202, 571–581. doi:10.1007/s00221-010-2160-3
Miller, E. K., & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Reviews in Neuroscience, 24, 167–202. doi:10.1146/annrev.neuro.24.1.167
Monsell, S. (2003). Task switching. Trends in Cognitive Sciences, 7, 134–140. doi:10.1016/S1364-6613(03)00028-7
Moran, T. P., Taylor, D., & Moser, J. S. (in press). Sex moderates the relationship between worry and performance monitoring brain activity in undergraduates. International Journal of Psychophysiology. doi:10.1016/j.ijpsycho.2012.05.005
Moser, J. S., Moran, T. P., & Jendrusina, A. A. (2012). Parsing relationships between dimensions of anxiety and action monitoring brain potentials in female undergraduates. Psychophysiology, 49, 3–10. doi:10.1111/j.1469-8986.2011.01279.x
Moser, J. S., Schroder, H. S., Heeter, C., Moran, T. P., & Lee, Y.-H. (2011). Mind your errors: Evidence for a neural mechanism linking growth mindset to adaptive post-error adjustments. Psychological Science, 22, 1484–1489. doi:10.1177/0956797611419520
Mueller, S. C., Swainson, R., & Jackson, G. M. (2009). ERP indices of persisting and current inhibitory control: a study of saccadic task switching. NeuroImage, 45, 191–197. doi:10.1016/j.neuroimage.2008.11.019
Murphy, P. R., Robertson, I. H., Allen, D., Hester, R., & O’Connell, R. G. (2012). An electrophysiological signal that precisely tracks the emergence of error awareness. Frontiers in Human Neuroscience, 6(65), 1–16. doi:10.3389/fnhum.2012.00065
Nieuwenhuis, S., Ridderinkhof, K. R., Blom, J., Band, G. P., & Kok, A. (2001). Error-related brain potentials are differentially related to awareness of response errors: Evidence from an antisaccade task. Psychophysiology, 38, 752–760. doi:10.1017/S0048577201001111
O’Connell, R. G., Bellgrove, M. A., Dockree, P. M., Lau, A., Hester, R., Garavan, H., & Robertson, I. H. (2009). The neural correlates of deficient error awareness in attention-deficit hyperactivity disorder (ADHD). Neuropsychologia, 47, 1149–1159. doi:10.1016/j.neuropsychologia.2009.01.011
Olvet, D. M., & Hajcak, G. (2009). The stability of error-related brain activity with increasing trials. Psychophysiology, 46, 957–961. doi:10.1111/j.1467-8986.2009.00848.x
Orr, J. M., & Carrasco, M. (2011). The role of the error positivity in the conscious perception of errors. Journal of Neuroscience, 31, 5891–5892. doi:10.1523/JNEUROSCI.0279-11.2011
Overbeek, T. J. M., Nieuwenhuis, S., & Ridderinkhof, K. R. (2005). Dissociable components of error processing: On the functional significance of the Pe vis-à-vis the ERN/Ne. Journal of Psychophysiology, 19, 319–329. doi:10.1027/0269-8803.19.4.319
Parris, B. A., Thai, N. J., Benattayallah, A., Summers, I. R., & Hodgson, T. L. (2007). The role of lateral prefrontal cortex and anterior cingulate in stimulus–response association reversals. Journal of Cognitive Neuroscience, 19, 13–24. doi:10.1162/jocn.2007.19.1.13
Paus, T. (2001). Primate anterior cingulate cortex: Where motor control drive and cognitive interface. Nature Reviews Neuroscience, 2, 417–424. doi:10.1038/35077500
Pavlov, I. P. (1927). Conditioned reflexes (G. V. Anrep, Trans.). London, U.K.: Oxford University Press.
Picard, N., & Strick, P. L. (2001). Imaging the premotor areas. Current Opinions in Neurobiology, 11, 663–672. doi:10.1016/S0959-4388(01)00266-5
Ridderinkhof, K. R., Ullsperger, M., Crone, E. A., & Nieuwenhuis, S. (2004). The role of the medial frontal cortex in cognitive control. Science, 306, 443–447. doi:10.1126/science.1100301
Ridderinkhof, K. R., Ramautar, J. R., & Wijnen, J. G. (2009). To Pe or not to Pe: A P3-like ERP component reflecting the processing of response errors. Psychophysiology, 46, 531–538. doi:10.1111/j.1469-8986.2009.00790.x
Rushworth, M. F. S., Hadland, K. A., Paus, T., & Sipila, P. K. (2002a). Role of the human medial frontal cortex in task switching: A combined fMRI and TMS study. Journal of Neurophysiology, 87, 2577–2592. doi:10.1152/jn.00812.2001
Rushworth, M. F. S., Passingham, R. E., & Nobre, A. C. (2002b). Components of switching intentional set. Journal of Cognitive Neuroscience, 14, 1139–1150. doi:10.1162/089892902760807159
Sarter, M., Gehring, W. J., & Kozak, R. (2006). More attention must be paid: The neurobiology of attentional effort. Brain Research Reviews, 51, 145–160. doi:10.1016/j.brainresrev.2005.11.002
Scheffers, M. K., & Coles, M. G. H. (2000). Performance monitoring in a confusing world: Error-related brain activity, judgments of response accuracy, and types of errors. Journal of Experimental Psychology: Human Perception and Performance, 26, 141–151. doi:10.1037/0096-1523.26.1.141
Shalgi, S., Barkan, I., & Deouell, L. Y. (2009). On the positive side of error processing: Error-awareness positivity revisited. European Journal of Neuroscience, 29, 1522–1532. doi:10.1111/j.1460-9568.2009.06690.x
Shiu, L. P., & Chan, T. C. (2006). Unlearning a stimulus–response association. Psychological Research, 70, 193–199. doi:10.1007/s00426-004-0201-x
Steinhauser, M., & Yeung, N. (2010). Decision processes in human performance monitoring. Journal of Neuroscience, 30, 15643–15653. doi:10.1523/JNEUROSCI.1899-10.2010
Tanaka, H. (2009). Error positivity is related to attentional control of task switching. NeuroReport, 20, 820–824. doi:10.1097/WNR.0b013e32832bfc94
Tillman, C. M., & Wiens, S. (2011). Behavioral and ERP indices of response conflict Stroop and flanker tasks. Psychophysiology, 48, 1405–1411. doi:10.1111/j.1469-8986.2011.01203.x
Ullsperger, M., Harsay, H. A., Wessel, J. R., & Ridderinkhof, K. R. (2010). Conscious perception of errors and its relation to the anterior insula. Brain Structure and Function, 214, 629–643. doi:10.1007/s00429-010-0261-1
van Veen, V., & Carter, C. S. (2002). The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience, 14, 593–602. doi:10.1162/08989290260045837
van Veen, V., & Carter, C. S. (2006). Conflict and cognitive control in the brain. Current Directions in Psychological Science, 15, 237–240. doi:10.1111/j.1467-8721.2006.00443.x
Waszak, F., Hommel, B., & Allport, A. (2003). Task-switching and long-term priming: Role of episodic stimulus-task bindings in task-shift costs. Cognitive Psychology, 46, 361–413. doi:10.1016/S0010-0285(02)00520-0
Weissman, D. H., Giesbrecht, B., Song, A. W., Mangun, G. R., & Woldorff, M. G. (2003). Conflict monitoring in the human anterior cingulate cortex during selective attention to global and local object features. NeuroImage, 19, 1361–1368. doi:10.1016/S1053-8119(03)00167-8
Yeung, N., Botvinick, M. M., & Cohen, J. D. (2004). The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review, 111, 931–959. doi:10.1037/0033-295X.111.4.931
Yeung, N., & Cohen, J. D. (2006). The impact of cognitive deficits on conflict monitoring: Predictable dissociations between the error-related negativity and N2. Psychological Science, 17, 164–171. doi:10.1111/j.1467-928-.2006.01680.x
Yeung, N., Cohen, J. D., & Botvinick, M. M. (2011). Errors of interpretation and modeling: A reply to Grindband et al. NeuroImage, 57, 316–319. doi:10.1016/j.neuroimage.2011.04.029
Yeung, N., Ralph, J., & Nieuwenhuis, S. (2007). Drink alcohol and dim the lights: The impact of cognitive deficits on medial frontal cortex function. Cognitive, Affective, & Behavioral Neuroscience, 7, 347–355. doi:10.3758/CABN.7.4.3
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1.54 mb)
Rights and permissions
About this article
Cite this article
Schroder, H.S., Moran, T.P., Moser, J.S. et al. When the rules are reversed: Action-monitoring consequences of reversing stimulus–response mappings. Cogn Affect Behav Neurosci 12, 629–643 (2012). https://doi.org/10.3758/s13415-012-0105-y
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-012-0105-y