Abstract
As a group, cigarette smokers exhibit blunted subjective, behavioral, and neurobiological responses to nondrug incentives and rewards, relative to nonsmokers. Findings from recent studies suggest, however, that there are large individual differences in the devaluation of nondrug rewards among smokers. Moreover, this variability appears to have significant clinical implications, since reduced sensitivity to nondrug rewards is associated with poorer smoking cessation outcomes. Currently, little is known about the neurobiological mechanisms that underlie these individual differences in the responsiveness to nondrug rewards. Here, we tested the hypothesis that individual variability in reward devaluation among smokers is linked to the functioning of the striatum. Specifically, functional magnetic resonance imaging was used to examine variability in the neural response to monetary outcomes in nicotine-deprived smokers anticipating an opportunity to smoke—circumstances found to heighten the devaluation of nondrug rewards by smokers in prior work. We also investigated whether individual differences in reward-related brain activity in those expecting to have access to cigarettes were associated with the degree to which the same individuals subsequently were willing to resist smoking in order to earn additional money. Our key finding was that deprived smokers who exhibited the weakest response to rewards (i.e., monetary gains) in the ventral striatum were least willing to refrain from smoking for monetary reinforcement. These results provide evidence that outcome-related signals in the ventral striatum serve as a marker for clinically meaningful individual differences in reward-motivated behavior among nicotine-deprived smokers.
Similar content being viewed by others
Introduction
The vast majority of attempts to quit smoking cigarettes are unsuccessful (Piasecki, 2006). The reduced sensitivity to nondrug rewards (e.g., money) exhibited by cigarette smokers is one factor that likely contributes significantly to such failed attempts. Namely, relative to nonsmokers, smokers display blunted subjective, behavioral, and neurobiological responses to nondrug rewards (e.g., al-Adawi & Powell, 1997; Martin-Soelch, Missimer, Leenders, & Schultz, 2003; Rose et al., 2012). These biases may be related to neuroadaptations associated with chronic cigarette use, preexisting vulnerabilities in reward functioning, or both (George & Koob, 2010; Kalivas & Volkow, 2005; Muller et al., 2013; Nees et al., 2013; Schneider et al., 2012; Sweitzer, Donny, & Hariri, 2012). Regardless of origin, this relative insensitivity to nondrug rewards presumably diminishes the impact of potential sources of motivation for maintaining smoking abstinence and thus serves as an important obstacle to smoking cessation.
Recent findings indicate that the devaluation of nondrug rewards by smokers indeed has significant clinical implications (Lam et al., 2012; MacKillop et al., 2012; Mueller et al., 2009; Sheffer et al., 2012; Versace et al., 2012). For example, Sheffer et al. observed that greater discounting of delayed monetary rewards was associated with a significantly reduced likelihood of maintaining abstinence across the 28 weeks following treatment in a sample of quitting smokers who received cognitive-behavioral therapy with relapse prevention. Moreover, these innovative studies have revealed that quitting smokers also vary widely in the degree to which they are responsive to affective and motivational cues, with reduced sensitivity to such stimuli associated with poorer smoking cessation outcomes. For instance, Versace et al. found that scalp event-related potentials (ERPs) evoked by pleasant pictures predicted long-term abstinence in quitting smokers receiving behavioral counseling and pharmacological treatment. Specifically, smokers who displayed dampened brain responses (scalp ERPs) to the pleasant images were significantly less likely to be abstinent at 10, 12, and 24 weeks following their quit date than were those who exhibited more robust responses to the pictures. Collectively, these results highlight the clinical relevance of individual differences in the processing of reward-related information among smokers.
Presently, however, little is known about the specific neural mechanisms associated with the varying levels of sensitivity to reward-related information processing exhibited by smokers. Addressing this knowledge gap would provide insight into the cognitive and affective processes that underpin the devaluation of nondrug rewards in those at highest risk for exhibiting such an effect. This information, in turn, would have significant implications for the development of interventions aimed at increasing reward sensitivity—and receptivity to incentives for remaining abstinent—in smokers who are likely to have the most difficulty when attempting to quit. A primary goal of the present functional magnetic resonance imaging (fMRI) study, then, was to examine the neural substrates mediating variability in the devaluation of nondrug rewards by nicotine-deprived smokers. More precisely, we sought to test the hypothesis that such individual differences in reward-related information processing are linked to the functioning of the striatum. This prediction is based on extensive evidence that the dorsal and ventral striatum are key components of the brain circuitry supporting reward processing (Delgado, 2007; Haber & Knutson, 2010). Furthermore, both the dorsal and ventral striatum have been implicated in the devaluation of nondrug rewards by smokers as a group (Buhler et al., 2010; Dagher et al., 2001; Kobiella et al., 2013; Luo, Ainslie, Giragosian, & Monterosso, 2011; MacKillop et al., 2012;Martin-Solch et al., 2001; Martin-Solch et al., 2003; Peters et al., 2011; Rose et al., 2012). Thus, we aimed to extend such findings by testing our hypothesis that the magnitude of the striatal response to nondrug rewards (as measured by the blood oxygen level dependent signal to monetary losses and gains) varies across smokers—with some exhibiting greater responsiveness than others. Here, we will examine this activity in nicotine-deprived smokers who are, or are not, expecting to smoke, since anticipating the opportunity to smoke, itself, has been linked to a decrease in the striatal response to nondrug monetary reward (Wilson, Sayette, Delgado, & Fiez, 2008; Wilson, Sayette, & Fiez, 2004; Wilson, Smyth, & MacLean, 2014).
That said, the ultimate hypothesis to be tested here is that variation in the striatal processing of nondrug rewards for those expecting to smoke will predict the subsequent willingness to forego a cigarette in an effort to earn more money. This hypothesis was motivated by two lines of evidence. First, nonhuman animal studies demonstrate that individual differences in responsiveness to a natural reward cue predict individual differences in drug seeking and drug taking (Grigson, 1997; Grigson, Twining, Freet, Wheeler, & Geddes, 2009). Specifically, greater avoidance of a drug-paired saccharin reward cue in rats has been found to correlate positively with their rate of drug self-administration (Gomez, 2002), the total amount of drug they consume (e.g., Grigson & Twining, 2002; Twining, Bolan, & Grigson, 2009), and how vigorously they seek drugs following extended abstinence (Grigson & Twining, 2002). Moreover, avoidance of the otherwise palatable drug-paired saccharin cue also is associated with a full blunting (Grigson & Hajnal, 2007) or even a reversal (Wheeler et al., 2011) of dopamine in the ventral striatum (i.e., nucleus accumbens). Second, results from our recent work suggest that human smokers exhibit a conceptually related effect and that this effect appears to be associated with the ventral striatum. Specifically, in a recent study that incorporated fMRI and ecological momentary assessment methods, we found that there was tendency to devalue nondrug rewards (e.g., money) during a stimulated quit attempt when cigarettes were perceived to be accessible only among smokers with a relatively weak response to monetary outcomes in the ventral striatum (i.e., the ventral caudate nucleus); those with a comparatively strong response to monetary outcomes in the ventral striatum did not exhibit such an effect (Wilson et al., 2014). In the present study, we sought to directly investigate the clinical relevance of these initial findings by testing the hypothesis that nondrug incentives for abstinence would be least effective for nicotine-deprived smokers exhibiting the greatest reduction in reward-related activity in the striatum during the anticipation of smoking.
Method
Participants
Fifty-one right-handed, native English speaking cigarette smokers between the ages 18 of 45 completed the experiment, with 44 yielding usable data (1 participant was excluded due to technical error, and 6 participants were excluded due to excessive head motion during fMRI data collection). Self-identified ethnicity of the usable sample was as follows: 91 % Caucasian, 5 % Asian, 2 % African American, and 2 % Hispanic. Participants were recruited through radio and newspaper advertisements. In order to be eligible for the study, individuals had to report that they smoked at least 10 cigarettes per day for the past 12 months and that they were not currently planning to quit smoking or actively pursuing any form of smoking cessation treatment. Exclusionary criteria included cardiovascular or respiratory disease during the previous year, current use of psychiatric medications, current dependence on a substance other than nicotine based upon a brief structured interview (Sheehan et al., 1998), and current depression (>16 on the Center for Epidemiologic Studies Depression Scale; Radloff, 1977). Participants were assigned randomly to two smoking opportunity conditions (instructed-yes, instructed-no) during the experiment, as detailed below. Age, sex, ethnicity, cigarettes/day, and nicotine dependence (as assessed using the Fagerström Test for Nicotine Dependence; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) were similar across conditions (p values >.05). Table 1 reports select demographic characteristics and self-reported smoking urge for the full sample and for each group. All procedures were approved by the institutional review board of the Pennsylvania State University, and written informed consent was obtained from all participants. Individuals were paid up to US$140 for their participation.
Assessment of smoking urge and nicotine withdrawal
Participants verbally rated their current urge to smoke on a scale ranging from 0 (absolutely no urge to smoke at all) to 100 (strongest urge to smoke I’ve ever experienced) twice during the experimental session: (1) immediately prior to being placed in the scanner for fMRI data acquisition and (2) immediately before being removed from the scanner at the conclusion of fMRI data collection.
Participants in the instructed-yes condition also completed measures assessing smoking urge and nicotine withdrawal symptoms while completing the smoking lapse task, which is described further below. Regarding the former, participants completed the Questionnaire of Smoking Urges–Brief (QSU–Brief; Cox, Tiffany, & Christen, 2001) every 10 min during the task. The QSU–Brief consists of 10 self-descriptive items (e.g., “I have a desire for a cigarette right now”), each rated on a 7-point scale anchored by 1 (strongly disagree) and 7 (strongly agree). The questionnaire yields a total score capturing overall smoking urge that ranges from 10 to 70, as well as two subscale scores (each consisting of 5 items with possible scores ranging from 5 to 35) assessing urge associated with anticipated positive and negative reinforcement from smoking, respectively. Scores were obtained by summing all items (for the total score) or the relevant subset of items (for the two subscales), with higher scores reflecting greater urge in each case.
In order to assess symptoms of nicotine withdrawal, participants completed the Minnesota Nicotine Withdrawal Scale (MNWS; Hughes & Hatsukami, 1986) at the beginning and end of the lapse task. The version of the MNWS used in the present study included a total of eight items: one asking about craving for cigarettes and seven items asking about other common symptoms of nicotine withdrawal (e.g., irritability). Participants were asked to rate the intensity of each symptom at that moment on a 5-point scale anchored by 0 (none) and 4 (severe). As was suggested by Hughes and Hatsukami (1998), a total withdrawal severity score was calculated by summing the seven noncraving items. Possible scores thus ranged from 0 to 28, with higher scores reflecting more severe nicotine withdrawal symptoms.
Card-guessing task
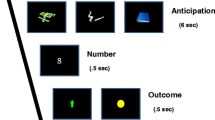
While in the scanner (see below), all participants performed a card-guessing task for monetary compensation (adapted from Delgado, Nystrom, Fissell, Noll, & Fiez, 2000). The paradigm was chosen because it has proven useful for characterizing within- and between-person variability in the striatal response to nondrug rewards in several previous studies (e.g., Delgado, 2007; Hariri et al., 2006; Wilson et al., 2014). Each trial of the task began with a 2-s choice period, during which a question mark appeared within a “card” (a rectangle) on the screen. Participants guessed whether the numerical value of the card was higher or lower than five, which they indicated by pressing one of two buttons. After the choice period, a number from 1 to 9 (excluding 5) was presented for 1 s, followed by feedback (also presented for 1 s) informing participants whether or not their guess was correct. Feedback consisted of either a green upward-pointing arrow (correct guess/monetary gain) or a red downward-pointing arrow (incorrect guess/monetary loss). Trials concluded with the presentation of a fixation cross for 12.5 s. Participants were informed that each correct guess led to the addition of $1.00 to the total payment they would receive, while each incorrect guess led to the loss of $0.50 from this total, and that they could earn up to $30 on the basis of their performance during the task. Unbeknownst to participants, outcomes were predetermined (50 % gain, 50 % loss) to ensure an equivalent experience across participants and were presented pseudorandomly.
Smoking lapse task
Participants in the instructed-yes condition completed a behavioral task modeling the willingness to refrain from smoking to obtain a nondrug incentive (McKee, 2009; McKee, Krishnan-Sarin, Shi, Mase, & O'Malley, 2006; McKee, Weinberger, Shi, Tetrault, & Coppola, 2012). (Participants in the instructed-no condition completed a risk task that is not a focus of the present study and will be reported elsewhere.) During the task, instructed-yes participants first were presented with a tray containing eight cigarettes of their preferred brand, a lighter, and an ashtray. Subsequently, they were informed that they would remain in the testing room for a total of 50 min and that they would earn $1 for each 5-min block of time that they delayed smoking, with the potential to earn up to $10 if they delayed smoking for the entire period. Participants were free, however, to initiate smoking at any point during the 50-min period, at which point they ceased earning additional money. The amount of time that smoking was postponed (range of 0–50 min) was the dependent measure of primary interest in the present study. As was described above, craving and nicotine withdrawal symptoms were assessed either every 10 min (craving) or at 0 and 50 min (nicotine withdrawal symptoms) during the delay period, using the QSU–Brief and MNWS, respectively. Analyses presented herein focus on ratings collected at the beginning and end of the task.
Procedure
Individuals who responded to recruitment advertisements underwent a preliminary telephone screening interview. Eligible participants then visited the lab for two sessions: an initial behavioral baseline session (scheduled to begin between 11:00 a.m. and 4:00 p.m. for all participants) and an fMRI experimental session (scheduled to begin between 10:00 a.m. and 2:00 p.m. for all participants). During the baseline session, participants provided an expired-air carbon monoxide (CO) sample, which was used to verify smoking status (>10 parts per million). Participants also completed a battery of interview and self-report assessments, including demographics and tobacco use questionnaires. After completing the baseline assessment, participants were scheduled for the 2-h experimental session (held on a separate day). They were instructed to abstain from smoking and from using nicotine-containing products for the 12 h preceding the experiment and that a CO sample would be obtained to verify compliance with these instructions. Participants also were instructed to refrain from consuming drugs or alcohol for the 24 h preceding the experiment.
Participants completed the experimental session within 3 weeks of the baseline session (M = 8.2 days separating sessions; range of 1–21 days between visits). Upon arrival for the experiment, participants reported the last time they had smoked a cigarette, and a second CO sample was obtained to check compliance with deprivation instructions. The latter had to be at least 50 % lower than the baseline sample, a cutoff that was established on the basis of prior experience with similar samples and procedures (e.g., Sayette, Loewenstein, Griffin, & Black, 2008; Wilson, Sayette, & Fiez, 2012). All 44 participants included in the analyses satisfied this criterion. Following CO verification, all participants were informed that they would not be able to smoke during the experiment and, therefore, would have to wait approximately 2 h before having the chance to consume cigarettes. This time frame has been used successfully in prior research to create a robust expectancy of not being able to smoke (Juliano & Brandon, 1998; Wilson, Sayette, Delgado, & Fiez, 2005; Wilson et al., 2012). Next, participants were informed that they would have the opportunity to earn additional money on the basis of their performance during the card-guessing task. After being given instructions regarding how to perform the task, participants verbally rated their urge to smoke from 0 to 100 and were placed inside the fMRI scanner. All participants completed an initial set of 60 interleaved trials of the card-guessing task (30 gain and 30 loss trials) after being told that they would not be able to smoke during the study.
After completing the initial set of 60 trials, participants were assigned randomly to one of two smoking opportunity conditions (instructed-yes or instructed-no). This within-participants expectancy manipulation allowed us to assess the effects of changes in smoking opportunity on reward processing within individuals (our primary aim), as well as to compare reward-related responses in those who were and who were not expecting to smoke.
Participants in the instructed-yes condition were given the following instructions by an experimenter:
You are about halfway done with the feedback task. Before you continue, I wanted to tell you about a mistake in the instructions that I previously gave you. When I told that you would not be able to smoke during the study, I was looking at the wrong information. Actually, you will have the chance to smoke during the study. Specifically, you will be removed from the scanner and will be given a brief break after you finish the feedback task, which will take about 16 more minutes to complete. You will be given an opportunity to smoke during the break. I apologize for the error.
Participants in the instructed-no condition were given the following instructions:
You are about halfway done with the feedback task. You will be removed from the scanner and given a brief break after you finish the feedback task, which will take about 16 more minutes to complete. As indicated earlier, you will not be permitted to smoke during the break. You will have to wait until the study is finished before having the opportunity to smoke.
For both conditions, instructions were delivered via an intercom while participants remained in the MRI scanner. After receiving instructions, all participants completed a second set of 60 interleaved trials of the card-guessing task (30 gain and 30 loss trials). Participants then rated their urge to smoke from 0 to 100 and were removed from the scanner.
After being removed from the scanner, participants in the instructed-yes condition were escorted to a testing room and were told that, as promised, they would be given a chance to smoke but that they would have the opportunity to earn extra money by delaying cigarette use. They then completed the smoking lapse task. Participants in the instructed-no condition completed a different paradigm assessing risk taking that will be presented elsewhere, as noted above. Finally, participants were debriefed and paid for their participation.
fMRI methods
Scanning was conducted at the Penn State Social, Life, and Engineering Sciences Imaging Center using a 3-Tesla Siemens Trio scanner (Siemens Medical Solutions, Erlangen, Germany). Prior to functional scanning, a high-resolution three-dimensional structural volume was acquired using a T1-weighted magnetization-prepared rapid gradient echo sequence. During functional scanning, 38-slice oblique-axial functional images (3 × 3 × 3 mm voxels) were acquired using a standard echo-planar imaging pulse sequence [TR = 2,000 ms, TE = 25 ms, FOV = 192 mm, flip angle = 79°].
Analytic strategy
BrainVoyager QX software (version 2.4.2; Brain Innovation, Maastricht, The Netherlands) and the NeuroElf toolbox (version 0.9c; www.neuroelf.net) for MATLAB (version 8.0; The MathWorks, Inc., Natick, MA) were used to preprocess and analyze the imaging data. The following preprocessing steps were employed prior to statistical analysis: motion correction (six-parameter rigid body transformation), slice scan time correction (trilinear/sinc interpolation), spatial smoothing using a three-dimensional Gaussian filter (4-mm full width at half maximum), voxel-wise linear detrending, and high-pass filtering of frequencies (three cycles per time course). Subsequently, structural and functional images were transformed to standard Talairach stereotaxic space (Talairach & Tournoux, 1988).
fMRI data were analyzed using a random-effects general linear model (GLM) with task-related regressors. Specifically, regressors of interest were created by convolving a delta function representing trial onset times for the two trial conditions presented during the card-guessing task (i.e., monetary gain and monetary loss) with a standard two-gamma hemodynamic response function. These regressors were entered into a GLM to obtain parameter estimates (i.e., beta weights) for each participant. As described above, participants completed two 60-trial sets of the card-guessing task that were separated by the delivery of instructions at the midpoint of the scan session (those in the instructed-yes condition were told that they would able to smoke soon, while those in the instructed-no condition were reminded that they would not be able to smoke during the study). Because we predicted that neural responses to monetary outcomes would change as a function of this smoking opportunity manipulation, data from each 60-trial set of the card-guessing task were modeled separately. On the basis of Monte Carlo simulations conducted using NeuroElf, it was determined that a combined per-voxel threshold of p < .001 and cluster-extent threshold of 9 or more contiguous voxels would yield a corrected family-wise error rate of p < .05. These threshold parameters were applied to all group-based statistical maps.
Our principal goal was to test the prediction that smokers who showed the largest attenuation in the striatal response to nondrug rewards during the anticipation of smoking would be the least willing to resist smoking in order to obtain a nondrug incentive. Accordingly, using an approach motivated by previous research (Fareri, Niznikiewicz, Lee, & Delgado, 2012), we first identified striatal regions of interest (ROIs) by conducting a contrast of monetary gain versus loss outcomes. (As is detailed below, this yielded ROIs in the ventral striatum bilaterally.) This contrast was performed using only data from the first half of the scan session (preceding the smoking opportunity manipulation), which generated an ROI mask that then was applied to data acquired during the second half of the scan session (following the smoking opportunity manipulation) for subsequent analyses. In order to quantify potential effects of the smoking opportunity manipulation while controlling for variability in outcome-related responses at baseline (Cohen, Cohen, West, & Aiken, 2003), residualized change scores in the ventral striatal response to outcomes were created by regressing postinstruction beta weights on preinstruction beta weights extracted from the ventral striatum, with the resulting residuals used in primary analyses. These residualized change scores were used to examine the association between outcome-related ventral striatal activity during the second half of the scan session and performance during the smoking lapse task in the instructed-yes group. Specifically, because the delay to smoking was distributed bimodally (as described in the Results section), independent samples t tests (two-tailed) were used to contrast expectancy-related changes in the striatal response to outcomes in those who did versus did not choose to smoke during the lapse task. We predicted that those who “lapsed” would exhibit a significantly larger decrease in the striatal response to monetary gains when informed that cigarettes soon would be accessible than would those who refrained from smoking during the lapse paradigm.
In addition to our main aim of establishing direct links between ventral striatal responsiveness during the card-guessing task and clinically relevant behavior outside of the scanner, a secondary objective of the study was to more broadly examine the effects of smoking opportunity on ventral striatal activity associated with the gain and loss of money. Toward this end, residualized change scores in the ventral striatal response to outcomes (calculated using the procedure described above) were entered into a second-level ANOVA with smoking opportunity (instructed-yes, instructed-no) as a between-participants factor, trial condition (monetary gain, monetary loss) as a within-participants factors, and participant as a random factor.
Results
Smoking urge and nicotine withdrawal
A 2 (smoking opportunity: instructed-yes, instructed-no) × 2 (time: preinstruction, postinstruction) ANOVA revealed a main effect of time, F(1, 42) = 13.47, p < .001. As is presented in Table 1, urge ratings rose over time for both groups. The smoking opportunity main effect and the smoking opportunity × time interaction were not significant (p values > .4).
Table 2 presents ratings of smoking urge (as assessed using the QSU–Brief) and symptoms of nicotine withdrawal (as assessed using the MNWS) for participants in the instructed-yes group during the smoking lapse task. As is shown, mean levels of smoking urge and nicotine withdrawal severity were higher in those who did versus did not smoke (as may be expected), but these differences failed to reach significance (p values > .1).
Identification of striatal ROIs
To isolate striatal ROIs sensitive to reward outcomes, we first conducted a contrast of monetary gain versus loss outcomes including only data from the preinstruction portion of the scan session, with the mask generated using this procedure subsequently applied to data from the postinstruction period of the scan session. This contrast yielded activation in several regions (Table 3) but was particularly robust in the ventral striatum bilaterally (Fig. 1a). With the exception of the medial frontal gyrus, which exhibited the opposite pattern, the response to gains was greater than the response to losses in the identified brain areas.
a Ventral striatal regions exhibiting a main effect of outcome. Brain slice is 1 mm above the anterior-commissure–posterior-commissure plane in Talairach stereotaxic space. Right: Residualized change in the ventral striatal response to monetary gains (b) and losses (c) for those who subsequently did (blue bars) and did not (red bars) smoke during the smoking lapse task. Bars indicate means, and error bars indicate +1 standard error of the mean. Asterisks indicate a significant difference between those who did versus did not smoke (*p < .05, **p < .01)
Striatal activation and willingness to resist smoking
The main objective of the study was to test the hypothesis that smokers’ willingness to resist smoking in order to obtain a nondrug incentive would be predicted by their striatal response to nondrug rewards during the anticipation of smoking. As was noted in the Method section, the delay to smoking during the lapse task was distributed bimodally in the sample: Five participants in the instructed-yes condition chose to smoke during the 50-min delay period of the lapse paradigm (M = 12.2-min delay; SD = 10.6), while 18 participants chose not to smoke during the 50-min task. (There were no differences in age, cigarettes smoked per day, or level of nicotine dependence [assessed via FTND] between those who did and did not smoke; p values > .1). Accordingly, analyses focused on contrasting residualized change in the response to outcomes in the ventral striatum following the smoking expectancy manipulation in instructed-yes participants who did, versus did not, smoke, using independent samples t tests, with a particular interest in characterizing subgroup differences in the response to rewards.
Consistent with our hypothesis, participants in the instructed-yes condition who chose to smoke during the lapse task exhibited a significantly lower response to monetary gains, relative to those who refrained from smoking during the task in both the left, t(21) = 2.55, p = .019, and right, t(21) = 2.97, p = .007, ventral striatum (Fig. 1b). Importantly, these effects remained significant when controlling for age, number of cigarettes smoked per day, self-reported smoking urge, level of nicotine dependence (assessed using the FTND), and nicotine withdrawal symptoms (assessed using the MNWS). There was no difference between those who did and did not smoke in the response to monetary losses in either the left, t(21) = 1.70, p = .103, or right, t(21) = 1.21, p = .238, ventral striatum (Fig. 1c).
To evaluate whether these effects were specific to the striatum, we examined the association between outcome-related activation and the delay to smoking during the lapse task for the additional brain regions identified in the contrast of monetary gain versus loss outcomes. As was expected, we did not observe significant group differences in any of the remaining brain areas (p values > .1).
Smoking opportunity and striatal activation
We conducted a 2 (smoking opportunity: instructed-yes, instructed-no) × 2 (trial condition: monetary gain, monetary loss) ANOVA to test whether the opportunity to smoke would decrease the ventral striatal response to monetary losses and gains. Contrary to predictions, this analysis failed to yield significant effects (p values > .05).
Discussion
The present study examined the neurobiological substrates of variability in the devaluation of nondrug rewards among nicotine-deprived cigarette smokers. Our primary goal was to test the hypothesis that such individual differences are associated with the functioning of the striatum and, more specifically, that reward-related striatal responses measured during the anticipation of an impending opportunity to smoke would predict the willingness to resist smoking for a nondrug incentive. Related to this aim, we found that deprived smokers who exhibited the weakest ventral striatal response to nondrug rewards (i.e., monetary gains) when expecting to have access to cigarettes were least willing to delay smoking for monetary reinforcement. As was predicted, this association was specific to the striatal response to monetary gains: Those who did and did not smoke during the smoking lapse task displayed similar responses to monetary losses in the ventral striatum. In addition, the willingness to resist smoking was not related to activation in other brain regions that also were sensitive to monetary outcomes, such as regions of the prefrontal and anterior cingulate cortices. In the present study, roughly 20 % of participants exhibited an attenuation of the striatal response to monetary gains and a subsequent disinclination to forgo cigarette use for money. To the extent that this reflects the proportion of deprived smokers who are relatively unresponsive to nondrug rewards more broadly, these effects may contribute to the maintenance of cigarette use and relapse for a considerable segment of the smoking population.
The present findings replicate recent research demonstrating that both the sensitivity to affective and motivational cues (Lam et al., 2012; Versace et al., 2012) and valuation of delayed rewards (Mueller et al., 2009; Sheffer et al., 2012) predict smoking cessation outcomes. Our results extend this by linking such effects to the ventral striatum, a brain region strongly implicated in reward-related processing and motivated behavior. In particular, converging findings from nonhuman animal (Schultz, Tremblay, & Hollerman, 2000) and human (Delgado, 2007; O'Doherty, 2004) research indicates that the magnitude of ventral striatal activity provides an index of the relative value of reward-related stimuli. Accordingly, one explanation for the observed pattern is that, for a subgroup of nicotine-deprived smokers, the incentive value associated with the prospect of smoking in the near future overshadowed the value ascribed to the money that was earned in the interim, as indicated by a blunting of the ventral striatal response to monetary gains.
Conceptually, the present findings parallel results from animal research examining the comparison of natural and drug-related rewards. Specifically, studies using both passive drug delivery and drug self-administration paradigms have demonstrated that rats will reduce the ingestion of appetitive gustatory stimuli after they have been paired with subsequent drug administration (Grigson et al., 2009). This pattern of behavior is thought to reflect, at least in part, an anticipatory contrast effect whereby the incentive value of the appetitive gustatory stimulus is diminished relative to the value of the drug administration it has come to predict (Grigson, 1997; Grigson et al., 2009). As was previously noted, research indicates that there is substantial individual variability in the degree to which rats devalue nondrug taste stimuli in the presence of cues associated with drug delivery and, further, that this heterogeneity is linked to important aspects of addictive behavior (Gomez, 2001, 2002; Grigson & Twining, 2002; Twining et al., 2009). The present study suggests that human drug users (i.e., nicotine-deprived cigarette smokers) exhibit similar behaviorally relevant individual differences in the responsiveness to nondrug rewards in the presence of cues signaling drug availability. An important challenge for future studies will be to further characterize the specific neurocognitive mechanisms that underlie this interindividual variability. In addition, cross-species research examining the extent to which the individual differences displayed by humans and nonhuman animals are mediated by similar underlying neurobiological processes would be valuable (see Broos et al., 2012, for an elegant example of such cross-species research). As with this human study, avoidance of the drug-paired cue was associated with an altered pattern of activity in the ventral striatum (nucleus accumbens) in the rat, and a greater alteration in this pattern of activity predicted a shorter latency to take the drug, greater load-up on cocaine, and faster acquisition of cocaine self-administration across trials (Wheeler et al., 2008).
Clinically, our results raise the possibility that, for certain nicotine-deprived smokers, nondrug rewards may be least effective at promoting smoking abstinence precisely when they are needed most—that is, when smokers must forgo the immediate reward associated with obtaining and consuming available cigarettes in lieu of delayed nondrug rewards (e.g., improved health; cf. Luijten, O'Connor, Rossiter, Franken, & Hester, 2013). Such availability-related shifts in reward sensitivity would have a particularly detrimental effect on incentive-based approaches to treating smoking, such as those that incorporate contingency management techniques (Stitzer & Petry, 2006). The present findings also indicate that the ventral striatal response to rewards offers a potentially useful metric for evaluating and improving smoking interventions. Of note, we found that activity in the ventral striatum provided information about clinically relevant behavior (i.e., the willingness to delay smoking for money) that was not captured by other measures, such as self-reported urge. This observation is consistent with recent findings suggesting that fMRI has the potential to offer insights into the substrates of behavior that may be obscured by the various constraints associated with self-report (Wilson et al., 2014). For instance, a recent fMRI study found that neural responses to health messages designed to promote smoking cessation in the medial prefrontal cortex predicted subsequent reductions in cigarette use above and beyond self-reported intentions to change smoking behavior (Falk, Berkman, Whalen, & Lieberman, 2011). Accordingly, ventral striatal responses to reward-related information may yield unique information that is relevant for facilitating the selection of strategies for reducing the devaluation of nondrug rewards in at-risk individuals (e.g., by manipulating factors such as reward magnitude and delay or tailoring of incentives based upon individual preference). More generally, results from this study are in accord with research demonstrating that responses in the striatum predict treatment outcomes and changes in drug consumption in those who use substances other than nicotine (e.g., cannabis, cocaine, and methamphetamine; see Cousijn et al., 2013; Martinez et al., 2011; Wang et al., 2012, respectively), highlighting the broad clinical relevance of striatal functioning.
While the present experiment focused on investigating individual differences in neural and behavioral responses to nondrug rewards in deprived smokers who were informed that cigarettes soon would be available, a secondary objective was to explore the broader effects of smoking opportunity on reward-related processing. Unlike in our previous study (Wilson et al., 2008), and contrary to predictions, deprived smokers who were anticipating a chance to smoke within minutes did not exhibit a smaller striatal response to rewards than those who were not expecting to have the opportunity to smoke in the near future. A key difference between our prior study and the present one is that the former employed a very direct manipulation of smoking expectancy, whereas the latter used a subtler approach. Regarding our earlier method, an explicit attempt was made to increase the salience of the manipulation by having the experimenter stand in front of a sign designating the room as a “smoking area for research purposes” while delivering smoking expectancy instructions (see Wilson et al., 2005). It is possible that the tactics employed in the present study were not as potent. Notwithstanding this potential limitation, results are consistent with the idea that the within-participants smoking opportunity manipulation was sufficient to identify a subgroup of deprived smokers who were particularly susceptible to expectancy-related changes in reward processing.
A second notable difference between our previous study and the present one is that the former employed a between-group design in which participants were informed that they could or could not smoke only once, prior to being placed in the fMRI scanner. In the present study, those in the instructed-no condition were told that they would not be able to smoke for an extended period of time both at the beginning of the study and at the midpoint of the fMRI session while in the scanner. Accordingly, whereas the instructed-no group in our previous study presumably had an opportunity to acclimate to the fact that they would not be able to smoke prior to performing the card-guessing task (e.g., during the minutes that passed as they were situated in the scanner and anatomical images were collected), instructed-no participants in the present study were made acutely aware of the fact that cigarettes were unavailable immediately prior to performing the second set of task blocks. The instructed-no group in the present study may have exhibited a change in reward sensitivity because the second delivery of instructions elicited frustration or negative affect, rather than simply serving as a reminder (Bogdan & Pizzagalli, 2006). In line with this interpretation, emerging evidence indicates that stress and negative affect do indeed reduce the responsiveness of brain reward regions, although the precise pattern of such effects has varied across studies (cf. Lighthall et al., 2012; Ossewaarde et al., 2011; Porcelli, Lewis, & Delgado, 2012). A limitation of the present study is that participants’ affective state was not assessed in a comprehensive manner during the experimental session. Accordingly, additional research is needed to examine the extent to which the diminished ventral striatal response to feedback observed in the instructed-no group was driven by increases in negative affect versus other influences (e.g., habituation).
The present study was considerably larger than Wilson et al. (2008) and, thereby, permitted an examination of individual differences in the effects of smoking expectancy on reward-related processing. Furthermore, while the number of individuals exhibiting such an effect in the present study was modest, the proportion of the sample that appeared to be at risk (roughly 1 in 5 deprived smokers) was far from trivial. Thus, the results highlight an effect that may contribute to the maintenance of cigarette use and relapse for a large number of smokers. It should also be noted that the subgroup of participants who “lapsed” in the present study is comparable in size to the subgroups of interest in recent studies using brain imaging methods to examine clinical outcomes in smokers (e.g., 8 quitters in Froeliger et al., 2010; 9 lapsers in Janes et al., 2010). Nevertheless, future research with larger samples would be useful.
In summary, the results from the present study indicate that individual differences in the ventral striatal response to nondrug rewards predict the degree to which such stimuli influence subsequent behavior in nicotine-deprived smokers, perhaps especially when measured under conditions of cigarette availability. Specifically, we found that the subset of deprived smokers who showed the largest decrease in the striatal response to rewards as a function of smoking expectancy were the least willing to subsequently refrain from smoking in order to obtain a monetary incentive. Such individuals are likely to have substantial difficulty when attempting to quit smoking. Further characterizing the processes that contribute to variability in the sensitivity to nondrug rewards among deprived smokers has important implications for the treatment of addiction, particularly in relation to the widespread use of nondrug incentives and rewards to motivate abstinence. In addition, investigation of these effects may help illuminate individual differences in the neurobiological and behavioral mechanisms that contribute to the maintenance of smoking, as well as those that underlie variability in the susceptibility to developing addiction to cigarettes and other substances.
References
al-Adawi, S., & Powell, J. (1997). The influence of smoking on reward responsiveness and cognitive functions: A natural experiment. Addiction, 92(12), 1773–1782.
Bogdan, R., & Pizzagalli, D. A. (2006). Acute stress reduces reward responsiveness: Implications for depression. Biological Psychiatry, 60(10), 1147–1154. doi:10.1016/j.biopsych.2006.03.037
Broos, N., Schmaal, L., Wiskerke, J., Kostelijk, L., Lam, T., Stoop, N., & Goudriaan, A. E. (2012). The relationship between impulsive choice and impulsive action: A cross-species translational study. PloS One, 7(5), e36781. doi:10.1371/journal.pone.0036781
Buhler, M., Vollstadt-Klein, S., Kobiella, A., Budde, H., Reed, L. J., Braus, D. F., & Smolka, M. N. (2010). Nicotine dependence is characterized by disordered reward processing in a network driving motivation. Biological Psychiatry, 67(8), 745–752. doi:10.1016/j.biopsych.2009.10.029
Cohen, J., Cohen, P., West, S. G., & Aiken, L. S. (2003). Applied multiple regression/correlation analysis for the behavioral sciences (3rd ed.). Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers.
Cousijn, J., Wiers, R. W., Ridderinkhof, K. R., van den Brink, W., Veltman, D. J., Porrino, L. J., & Goudriaan, A. E. (2013). Individual differences in decision making and reward processing predict changes in cannabis use: A prospective functional magnetic resonance imaging study. Addiction Biology, 18(6), 1013–1023. doi:10.1111/j.1369-1600.2012.00498.x
Cox, L. S., Tiffany, S. T., & Christen, A. G. (2001). Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research, 3(1), 7–16.
Dagher, A., Bleicher, C., Aston, J. A., Gunn, R. N., Clarke, P. B., & Cumming, P. (2001). Reduced dopamine D1 receptor binding in the ventral striatum of cigarette smokers. Synapse, 42(1), 48–53. doi:10.1002/syn.1098
Delgado, M. R. (2007). Reward-related responses in the human striatum. Annals of the New York Academy of Sciences, 1104, 70–88. doi:10.1196/annals.1390.002
Delgado, M. R., Nystrom, L. E., Fissell, C., Noll, D. C., & Fiez, J. A. (2000). Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology, 84(6), 3072–3077.
Falk, E. B., Berkman, E. T., Whalen, D., & Lieberman, M. D. (2011). Neural activity during health messaging predicts reductions in smoking above and beyond self-report. Health Psychology, 30(2), 177–185. doi:10.1037/a0022259
Fareri, D. S., Niznikiewicz, M. A., Lee, V. K., & Delgado, M. R. (2012). Social network modulation of reward-related signals. Journal of Neuroscience, 32(26), 9045–9052. doi:10.1523/jneurosci.0610-12.2012
Froeliger, B., Kozink, R. V., Rose, J. E., Behm, F. M., Salley, A. N., & McClernon, F. J. (2010). Hippocampal and striatal gray matter volume are associated with a smoking cessation treatment outcome: Results of an exploratory voxel-based morphometric analysis. Psychopharmacology, 210(4), 577–583. doi:10.1007/s00213-010-1862-3
George, O., & Koob, G. F. (2010). Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neuroscience and Biobehavioral Reviews, 35(2), 232–247. doi:10.1016/j.neubiorev.2010.05.002
Gomez, F. (2001). Induction of conditioned taste aversion with a self-administered substance in rats. Brain Research. Brain Research Protocols, 8(2), 137–142.
Gomez, F. (2002). Conditioned saccharin aversion induced by self-administered cocaine negatively correlates with the rate of cocaine self-administration in rats. Brain Research, 946(2), 214–220.
Grigson, P. S. (1997). Conditioned taste aversions and drugs of abuse: A reinterpretation. Behavioral Neuroscience, 111(1), 129–136.
Grigson, P. S., & Hajnal, A. (2007). Once is too much: Conditioned changes in accumbens dopamine following a single saccharin-morphine pairing. Behavioral Neuroscience, 121(6), 1234–1242. doi:10.1037/0735-7044.121.6.1234
Grigson, P. S., & Twining, R. C. (2002). Cocaine-induced suppression of saccharin intake: A model of drug-induced devaluation of natural rewards. Behavioral Neuroscience, 116(2), 321–333.
Grigson, P. S., Twining, R. C., Freet, C. S., Wheeler, R. A., & Geddes, R. I. (2009). Drug-induced suppression of conditioned stimulus intake: Reward, aversion, and addiction. In S. Reilly & T. R. Schachtman (Eds.), Conditioned taste aversion: Behavioral and neural processes. New York: Oxford University Press.
Haber, S. N., & Knutson, B. (2010). The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology, 35(1), 4–26. doi:10.1038/npp.2009.129
Hariri, A. R., Brown, S. M., Williamson, D. E., Flory, J. D., de Wit, H., & Manuck, S. B. (2006). Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. Journal of Neuroscience, 26(51), 13213–13217. doi:10.1523/jneurosci.3446-06.2006
Heatherton, T. F., Kozlowski, L. T., Frecker, R. C., & Fagerstrom, K. O. (1991). The fagerstrom test for nicotine dependence: A revision of the fagerstrom tolerance questionnaire. British Journal of Addiction, 86(9), 1119–1127.
Hughes, J. R., & Hatsukami, D. K. (1986). Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry, 43(3), 289–294.
Hughes, J. R., & Hatsukami, D. K. (1998). Errors in using tobacco withdrawal scale. Tobacco Control, 7(1), 92–93.
Janes, A. C., Pizzagalli, D. A., Richardt, S., deB Frederick, B., Chuzi, S., Pachas, G., & Kaufman, M. J. (2010). Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biological Psychiatry, 67(8), 722–729. doi:10.1016/j.biopsych.2009.12.034
Juliano, L. M., & Brandon, T. H. (1998). Reactivity to instructed smoking availability and environmental cues: Evidence with urge and reaction time. Experimental and Clinical Psychopharmacology, 6(1), 45–53.
Kalivas, P. W., & Volkow, N. D. (2005). The neural basis of addiction: A pathology of motivation and choice. The American Journal of Psychiatry, 162(8), 1403–1413. doi:10.1176/appi.ajp.162.8.1403
Kobiella, A., Ripke, S., Kroemer, N. B., Vollmert, C., Vollstadt-Klein, S., Ulshofer, D. E., & Smolka, M. N. (2013). Acute and chronic nicotine effects on behaviour and brain activation during intertemporal decision making. Addiction Biology. doi:10.1111/adb.12057
Lam, C. Y., Robinson, J. D., Versace, F., Minnix, J. A., Cui, Y., Carter, B. L., & Cinciripini, P. M. (2012). Affective reactivity during smoking cessation of never-quitters as compared with that of abstainers, relapsers, and continuing smokers. Experimental and Clinical Psychopharmacology, 20(2), 139–150. doi:10.1037/a0026109
Lighthall, N. R., Sakaki, M., Vasunilashorn, S., Nga, L., Somayajula, S., Chen, E. Y., & Mather, M. (2012). Gender differences in reward-related decision processing under stress. Social Cognitive and Affective Neuroscience, 7(4), 476–484. doi:10.1093/scan/nsr026
Luijten, M., O'Connor, D. A., Rossiter, S., Franken, I. H., & Hester, R. (2013). Effects of reward and punishment on brain activations associated with inhibitory control in cigarette smokers. Addiction. doi:10.1111/add.12276
Luo, S., Ainslie, G., Giragosian, L., & Monterosso, J. R. (2011). Striatal hyposensitivity to delayed rewards among cigarette smokers. Drug and Alcohol Dependence, 116(1–3), 18–23. doi:10.1016/j.drugalcdep.2010.11.012
MacKillop, J., Amlung, M. T., Wier, L. M., David, S. P., Ray, L. A., Bickel, W. K., & Sweet, L. H. (2012). The neuroeconomics of nicotine dependence: A preliminary functional magnetic resonance imaging study of delay discounting of monetary and cigarette rewards in smokers. Psychiatry Research, 202(1), 20–29. doi:10.1016/j.pscychresns.2011.10.003
Martinez, D., Carpenter, K. M., Liu, F., Slifstein, M., Broft, A., Friedman, A. C., & Nunes, E. (2011). Imaging dopamine transmission in cocaine dependence: Link between neurochemistry and response to treatment. The American Journal of Psychiatry, 168(6), 634–641. doi:10.1176/appi.ajp.2010.10050748
Martin-Soelch, C., Missimer, J., Leenders, K. L., & Schultz, W. (2003). Neural activity related to the processing of increasing monetary reward in smokers and nonsmokers. European Journal of Neuroscience, 18(3), 680–688.
Martin-Solch, C., Magyar, S., Kunig, G., Missimer, J., Schultz, W., & Leenders, K. L. (2001). Changes in brain activation associated with reward processing in smokers and nonsmokers. A positron emission tomography study. Experimental Brain Research, 139(3), 278–286.
McKee, S. A. (2009). Developing human laboratory models of smoking lapse behavior for medication screening. Addiction Biology, 14(1), 99–107. doi:10.1111/j.1369-1600.2008.00135.x
McKee, S. A., Krishnan-Sarin, S., Shi, J., Mase, T., & O'Malley, S. S. (2006). Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology, 189(2), 201–210. doi:10.1007/s00213-006-0551-8
McKee, S. A., Weinberger, A. H., Shi, J., Tetrault, J., & Coppola, S. (2012). Developing and validating a human laboratory model to screen medications for smoking cessation. Nicotine & Tobacco Research. doi:10.1093/ntr/nts090
Mueller, E. T., Landes, R. D., Kowal, B. P., Yi, R., Stitzer, M. L., Burnett, C. A., & Bickel, W. K. (2009). Delay of smoking gratification as a laboratory model of relapse: Effects of incentives for not smoking, and relationship with measures of executive function. Behavioral Pharmacology, 20(5–6), 461–473. doi:10.1097/FBP.0b013e3283305ec7
Muller, K. U., Mennigen, E., Ripke, S., Banaschewski, T., Barker, G. J., Buchel, C., . . . Smolka, M. N. (2013). Altered Reward Processing in Adolescents With Prenatal Exposure to Maternal Cigarette Smoking. JAMA Psychiatry, 1-10. doi: 10.1001/jamapsychiatry.2013.44
Nees, F., Witt, S. H., Lourdusamy, A., Vollstadt-Klein, S., Steiner, S., Poustka, L., & Flor, H. (2013). Genetic risk for nicotine dependence in the cholinergic system and activation of the brain reward system in healthy adolescents. Neuropsychopharmacology. doi:10.1038/npp.2013.131
O'Doherty, J. P. (2004). Reward representations and reward-related learning in the human brain: Insights from neuroimaging. Current Opinion in Neurobiology, 14(6), 769–776. doi:10.1016/j.conb.2004.10.016
Ossewaarde, L., Qin, S., Van Marle, H. J., van Wingen, G. A., Fernandez, G., & Hermans, E. J. (2011). Stress-induced reduction in reward-related prefrontal cortex function. NeuroImage, 55(1), 345–352. doi:10.1016/j.neuroimage.2010.11.068
Peters, J., Bromberg, U., Schneider, S., Brassen, S., Menz, M., Banaschewski, T., & Buchel, C. (2011). Lower ventral striatal activation during reward anticipation in adolescent smokers. The American Journal of Psychiatry, 168(5), 540–549. doi:10.1176/appi.ajp.2010.10071024
Piasecki, T. M. (2006). Relapse to smoking. Clinical Psychology Review, 26(2), 196–215. doi:10.1016/j.cpr.2005.11.007
Porcelli, A. J., Lewis, A. H., & Delgado, M. R. (2012). Acute stress influences neural circuits of reward processing. Frontiers in Neuroscience, 6, 157. doi:10.3389/fnins.2012.00157
Radloff, L. S. (1977). The CES-D Scale. Applied Psychological Measurement, 1(3), 385–401.
Rose, E. J., Ross, T. J., Salmeron, B. J., Lee, M., Shakleya, D. M., Huestis, M., & Stein, E. A. (2012). Chronic exposure to nicotine is associated with reduced reward-related activity in the striatum but not the midbrain. Biological Psychiatry, 71(3), 206–213. doi:10.1016/j.biopsych.2011.09.013
Sayette, M. A., Loewenstein, G., Griffin, K. M., & Black, J. J. (2008). Exploring the cold-to-hot empathy gap in smokers. Psychological Science, 19(9), 926–932. doi:10.1111/j.1467-9280.2008.02178.x
Schneider, S., Peters, J., Bromberg, U., Brassen, S., Miedl, S. F., Banaschewski, T., & Buchel, C. (2012). Risk taking and the adolescent reward system: A potential common link to substance abuse. The American Journal of Psychiatry, 169(1), 39–46. doi:10.1176/appi.ajp.2011.11030489
Schultz, W., Tremblay, L., & Hollerman, J. R. (2000). Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex, 10(3), 272–284.
Sheehan, D. V., Lecrubier, Y., Sheehan, K. H., Amorim, P., Janavs, J., Weiller, E., & Dunbar, G. C. (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, 59(Suppl 20), 22–57.
Sheffer, C., Mackillop, J., McGeary, J., Landes, R., Carter, L., Yi, R., & Bickel, W. (2012). Delay discounting, locus of control, and cognitive impulsiveness independently predict tobacco dependence treatment outcomes in a highly dependent, lower socioeconomic group of smokers. American Journal on Addictions, 21(3), 221–232. doi:10.1111/j.1521-0391.2012.00224.x
Stitzer, M., & Petry, N. (2006). Contingency management for treatment of substance abuse. Annual Review of Clinical Psychology, 2, 411–434. doi:10.1146/annurev.clinpsy.2.022305.095219
Sweitzer, M. M., Donny, E. C., & Hariri, A. R. (2012). Imaging genetics and the neurobiological basis of individual differences in vulnerability to addiction. Drug and Alcohol Dependence, 123(Suppl 1), S59–S71. doi:10.1016/j.drugalcdep.2012.01.017
Talairach, J., & Tournoux, P. (1988). Co-planar stereotaxic atlas of the human brain: An approach to medical cerebral imaging. Stuttgart, Germany: Thieme.
Twining, R. C., Bolan, M., & Grigson, P. S. (2009). Yoked delivery of cocaine is aversive and protects against the motivation for drug in rats. Behavioral Neuroscience, 123(4), 913–925. doi:10.1037/a0016498
Versace, F., Lam, C. Y., Engelmann, J. M., Robinson, J. D., Minnix, J. A., Brown, V. L., & Cinciripini, P. M. (2012). Beyond cue reactivity: Blunted brain responses to pleasant stimuli predict long-term smoking abstinence. Addiction Biology, 17(6), 991–1000. doi:10.1111/j.1369-1600.2011.00372.x
Wang, G. J., Smith, L., Volkow, N. D., Telang, F., Logan, J., Tomasi, D., & Fowler, J. S. (2012). Decreased dopamine activity predicts relapse in methamphetamine abusers. Molecular Psychiatry, 17(9), 918–925. doi:10.1038/mp.2011.86
Wheeler, R. A., Aragona, B. J., Fuhrmann, K. A., Jones, J. L., Day, J. J., Cacciapaglia, F., & Carelli, R. M. (2011). Cocaine cues drive opposing context-dependent shifts in reward processing and emotional state. Biological Psychiatry, 69(11), 1067–1074. doi:10.1016/j.biopsych.2011.02.014
Wheeler, R. A., Twining, R. C., Jones, J. L., Slater, J. M., Grigson, P. S., & Carelli, R. M. (2008). Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron, 57(5), 774–785. doi:10.1016/j.neuron.2008.01.024
Wilson, S. J., Sayette, M. A., Delgado, M. R., & Fiez, J. A. (2005). Instructed smoking expectancy modulates cue-elicited neural activity: A preliminary study. Nicotine and Tobacco Research, 7(4), 637–645.
Wilson, S. J., Sayette, M. A., Delgado, M. R., & Fiez, J. A. (2008). Effect of smoking opportunity on responses to monetary gain and loss in the caudate nucleus. Journal of Abnormal Psychology, 117(2), 428–434. doi:10.1037/0021-843X.117.2.428
Wilson, S. J., Sayette, M. A., & Fiez, J. A. (2004). Prefrontal responses to drug cues: A neurocognitive analysis. Nature Neuroscience, 7(3), 211–214.
Wilson, S. J., Sayette, M. A., & Fiez, J. A. (2012). Quitting-unmotivated and quitting-motivated cigarette smokers exhibit different patterns of cue-elicited brain activation when anticipating an opportunity to smoke. Journal of Abnormal Psychology, 121(1), 198–211. doi:10.1037/a0025112
Wilson, S. J., Smyth, J. M., & MacLean, R. R. (2014). Integrating ecological momentary assessment and functional brain imaging methods: New avenues for studying and treating tobacco dependence. Nicotine & Tobacco Research doi:10.1093/ntr/ntt129
Acknowledgments
Funding for this study was provided by NIDA Grant R03DA029675. Dr. Wilson’s research is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under BIRCWH award number K12HD055882, “Career Development Program in Women’s Health Research at Penn State.” The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We thank Theresa McKim and the staff of the Penn State Smoking Research Lab for their assistance with data collection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wilson, S.J., Delgado, M.R., McKee, S.A. et al. Weak ventral striatal responses to monetary outcomes predict an unwillingness to resist cigarette smoking. Cogn Affect Behav Neurosci 14, 1196–1207 (2014). https://doi.org/10.3758/s13415-014-0285-8
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-014-0285-8