Abstract
Rationale
Varenicline, a partial nicotinic agonist, is theorized to attenuate pre-quit smoking reinforcement and post-quit withdrawal and craving. However, the mechanisms of action have not been fully characterized, as most studies employ only retrospective self-report measures, hypothetical indices of reinforcing value, and/or nontreatment-seeking samples.
Objectives
The current research examined the impact of pre-quit varenicline (vs. placebo) on laboratory measures of smoking and food (vs. water) reinforcement and craving.
Methods
Participants were 162 treatment-seeking smokers enrolled in a randomized controlled trial of smoking cessation (clinicaltrials.gov ID: NCT03262662). Participants completed two laboratory sessions: a pre-treatment session, ~ 1 week prior to beginning varenicline or placebo, and an active treatment session, after ~ 3 weeks of treatment. At each session, participants completed a laboratory choice procedure; on each of 36 trials, a lit cigarette, food item, or cup of water was randomly presented. Participants reported level of craving and spent $0.01–0.25 to have a corresponding 5–95% chance to sample the cue.
Results
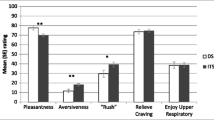
As predicted, spending was significantly higher on cigarette trials than water trials, and varenicline resulted in a greater between-session decline in spending on cigarette trials (but not water) than did placebo. Cigarette craving was enhanced in the presence of smoking cues compared to water, but neither average (tonic) cigarette craving nor cue-specific cigarette craving was significantly influenced by varenicline. Food spending and craving were generally unaffected by varenicline treatment.
Conclusions
These laboratory data from treatment-seeking smokers provide the strongest evidence to date that varenicline selectively attenuates smoking reinforcement prior to quitting.


Similar content being viewed by others
Notes
An additional 52 participants completed only the baseline session but were either ineligible for the study (n = 34), withdrew (n = 14 pre-intent-to-treat, n = 3 post-intent-to-treat), or were administratively withdrawn (n = 1) prior to the second lab session.
Questionnaires included the Minnesota Nicotine Withdrawal Scale (MNWS) (Hughes and Hatsukami 1986), the Mood form (Diener and Emmons 1985), the Nausea Profile- GI Distress Scale (Muth et al. 1996), and the Brief Questionnaire of Smoking Urges Brief (QSU-brief) (Cox & Tiffany 2001). These assessments measured withdrawal symptomatology, negative/positive affect, experiences of nausea, and craving, respectively. The data from those questionnaires were not analyzed in this manuscript.
During the Pre-Treatement session, participants also underwent training for completing ecological momentary assessments over the subsequent 9 weeks. This data is not presented in the manuscript.
We did observe an unanticipated group × food vs. water cue interaction, F(1, 157) = 4.6, p = .03, ηp2 = .03. This seemed to reflect that cigarette craving was slightly but significantly higher during water cues than food cues in the varenicline group, p < .01 but not the placebo group, p = .64; see Fig. 2).
Anonymous reviewers requested exploration of several moderators of treatment effects on spending and cigarette craving. These should be interpreted cautiously, given the large number of statistical tests and absence of a priori predictions.
1. Menthol vs. non-menthol cigarette smokers: as shown in Table 1, the varenicline and placebo groups were comparable in their representation of menthol vs. non-menthol smokers. Cigarette spending did not vary as a function of smoking menthol vs. non-menthol cigarettes, all p > .15. Although cigarette craving was generally higher among smokers of menthol cigarettes (M = 3.9) compared to non-menthol cigarettes (3.3), p = .01, treatment group did not interact with whether participants smoked menthol or non-menthol cigarettes, all p > .58.
2. CO reduction criterion met vs. not met: a comparable percentage of the varenicline and placebo groups met the 40% CO reduction criterion in both visits (68% and 63%, respectively, p = .50), but women were more likely than men to meet the CO reduction criterion (74% vs. 54%, p = .01). However, neither the main effect of CO reduction group nor any interactions were statistically significant for spending or cigarette craving, all p > .06.
3. Trial block (first half vs. second half of the task, among participants who smoked in the first half of each session): 80% of the sample (n = 130) smoked in the first half of each session, and this did not vary between the varenicline (77%) and placebo (84%) groups, p = .30. In this subsample, spending declined across trial blocks, p < .001, but trial block did not moderate any effects of treatment group on spending, all p > .10. Cigarette craving also declined across trial blocks, p < .001. A significant group × session × trial block interaction, p = .04 appeared to be driven primarily by an unexpectedly smaller (but still significant) decline in craving from the first half to the second half of the task among the placebo group during the pre-treatment session (0.8 units) compared to all other group × session conditions (1.1–1.4 units). Consequently, the largest treatment group difference in craving was during the second half of the pre-treatment session, which does not seem readily interpretable as anything except a chance finding. There was also a 5-way group × sex × session × trial block × food vs. water interaction for cigarette craving, p = .05; follow-up tests suggested that this complex, unpredicted interaction was driven primarily by subtle changes in cigarette craving between food and water cues among women. Cigarette craving was 0.1–0.2 units greater during water than food cues for the following conditions: varenicline group, trialblock 1 of both sessions, and placebo group, triablock 2 of session 1, p < .04. For the placebo group, the pattern flipped in triablock 2 of session 2, with modestly greater craving during food compared to water cues, p = .08. Cigarette craving did not vary in any of the remaining cells of the design. We do not believe that this unpredicted, complex interaction is worth detailing further.
4. Moderation analyses involving individual differences in quit motivation and medication adherence were not conducted due to limited variability in these constructs.
5. We declined to add data regarding changes in smoking behavior during the 3-week period between sessions, as changes in smoking rate and biochemical exposure are primary outcomes of the larger clinical trial in which this behavioral pharmacology study is embedded.
One potential concern regarding in vivo cues was raised when, anecdotally, several participants reported that olfactory smoking cues (i.e., smell of smoke) were present during some food and water trials, despite a negative pressure air filtration system designed to prevent carryover smoke. If this were to effect the findings of the current study, we would expect that it would dampen the observed effect size for cue-specific craving (cigarette vs. water cues), as olfactory smoking cues would ostensibly increase cigarette craving rated during water cues. However, we found no evidence that cue reactivity was diminished in the current study, and the effect size in the current study (ηp2 = .47) is comparable to, if not slightly higher than, effect sizes seen in other CBUCC studies (ηp2 = .45; Gass and Tiffany 2017; ηp2 = .31; Gass and Tiffany 2020), and to a study examining pre-quit varenicline on cue-specific craving in the natural environment rated across 5 weeks (ηp2 = .24–.61, Gass et al. 2012).
References
Ashare RL, Tang KZ, Mesaros AC, Blair IA, Leone F, Strasser AA (2012) Effects of 21 days of varenicline versus placebo on smoking behaviors and urges among non-treatment seeking smokers. J Psychopharmacol 26(10):1383–1390
Betts JM, Dowd AN, Forney M, Hetelekides E, Tiffany ST (2020) A meta-analysis of cue reactivity in tobacco cigarette smokers. Nicotine Tob Res, ntaa147, https://doi.org/10.1093/ntr/ntaa147.
Bohadana A, Freier-Dror Y, Peles V, Babai P, Izbicki G (2020) Extending varenicline preloading to 6 weeks facilitates smoking cessation: a single-site, randomised controlled trial. EClinicalMedicine 19:100228. https://doi.org/10.1016/j.eclinm.2019.11.021
Bouton ME (2019) Extinction of instrumental (operant) learning: interference, varieties of context, and mechanisms of contextual control. Psychopharmacology 236(1):7–19
Bouton ME, Winterbauer NE, Todd TP (2012) Relapse processes after the extinction of instrumental learning: renewal, resurgence, and reacquisition. Behav Process 90(1):130–141
Bouton ME, Maren S, McNally GP (2020) Behavioral and neurobiological mechanisms of Pavlovian and instrumental extinction learning. Physiol Rev: https://doi.org/10.1152/physrev.00016.2020.
Brandon TH, Drobes DJ, Unrod M, Heckman BW, Oliver JA, Roetzheim RC et al (2011) Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacology 218(2):391–403
Cahill K, Stead L, Lancaster T (2009) A preliminary benefit-risk assessment of varenicline in smoking cessation. Drug Safety 32(2):119–135
Cahill K, Stevens S, Perera R, Lancaster T (2013) Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane DB Sys Rev 5. https://doi.org/10.1002/14651858.CD009329.pub2
Cahill K, Lindson-Hawley N, Thomas KH, Fanshawe TR, Lancaster T (2016) Nicotine receptor partial agonists for smoking cessation. Cochrane DB Sys Rev 5. https://doi.org/10.1002/14651858.CD006103.pub7
Carter BL, Tiffany ST (2001) The cue-availability paradigm: the effects of cigarette availability on cue reactivity in smokers. Exp Clin Psychopharm 9(2):183–190
Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J et al (2005) Varenicline: an α4β2 nicotinic receptor partial agonist for smoking cessation. J Med Chem 48(10):3474–3477
Conklin CA, Tiffany ST (2002) Applying extinction research and theory to cue-exposure addiction treatments. Addiction 97(2):155–167
Diener E, Emmons RA (1985) The independence of positive and negative affect. J Pers Soc Psychol 47(5):1105–1117
DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW (2002) Patient adherence and medical treatment outcomes: a meta-analysis. Med Care 40:794–811
Dowd AN, Tiffany ST (2019) Comparison of tobacco and electronic cigarette reward value measured during a cue-reactivity task: an extension of the Choice Behavior Under Cued Conditions procedure. Nicotine Tob Res 21(10):1394–1400
Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y et al (2011) Effects of varenicline on smoking cue–triggered neural and craving responses. Arch Gen Psychiat 68(5):516
Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O’Malley SS (2011) A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology 215(4):655–663
Gass JC, Tiffany ST (2017) Craving and tobacco use: development of the Choice Behavior Under Cued Conditions (CBUCC) procedure. Psychol Addict Behav 31(3):276–283
Gass JC, Tiffany ST (2020) Assessment of the Choice Behavior Under Cued Conditions (CBUCC) paradigm as a measure of motivation to smoke under laboratory conditions. Addiction 115(2):302–312
Gass JC, Wray JM, Hawk LW, Mahoney MC, Tiffany ST (2012) Impact of varenicline on cue-specific craving assessed in the natural environment among treatment-seeking smokers. Psychopharmacology 223(1):107–116
Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB et al (2006) Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 296(1):47–55
Green RJ, Ray LA (2018) Effects of varenicline on subjective craving and relative reinforcing value of cigarettes. Drug Alcohol Depend 188:53–59
Hajek P, McRobbie HJ, Myers KE, Stapleton J, Dhanji AR (2011) Use of varenicline for 4 weeks before quitting smoking: decrease in ad lib smoking and increase in smoking cessation rates. Archives of Internal Medicine 171(8):770–777
Halperin AC, McAfee TA, Jack LM, Catz SL, McClure JB, Deprey TM et al (2009) Impact of symptoms experienced by varenicline users on tobacco treatment in a real world setting. Journal of Substance Abuse Treatment 36(4):428–434
Hawk LW Jr, Ashare RL, Lohnes SF, Schlienz NJ, Rhodes JD, Tiffany ST et al (2012) The effects of extended pre-quit varenicline treatment on smoking behavior and short-term abstinence: a randomized clinical trial. Clinical Pharmacology & Therapeutics 91(2):172–180
Hughes JR, Hatsukami D (1986) Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry 43(3):289–229
Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE et al (2006) Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation. JAMA - Journal of the American Medical Association 296(1):56–63
Kehoe EJ, White NE (2002) Extinction revisited: Similarities between extinction and reductions in US intensity in classical conditioning of the rabbit’s nictitating membrane response. Animal Learning & Behavior 30(2):96–111
Kotz D, Brown J, West R (2013) Predictive validity of the Motivation To Stop Scale (MTSS): a single-item measure of motivation to stop smoking. Drug Alcohol Depend 128(1-2):15–19
Kraemer HC, Wilson GT, Fairburn CG, Agras WS (2002) Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatr 59(10):877–883
Lattal KM, Lattal KA (2012) Facets of Pavlovian and operant extinction. Behavioural Processes 90(1):1–8
MacKinnon DP (2008) Introduction to statistical mediation analysis. Routledge, Philidelphia
McClure EA, Vandrey RG, Johnson MW, Stitzer ML (2013) Effects of varenicline on abstinence and smoking reward following a programmed lapse. Nicotine Tob Res 15(1):139–148
McKee SA, Smith PH, Kaufman M, Mazure CM, Weinberger AH (2016) Sex differences in varenicline efficacy for smoking cessation: a meta-analysis. Nicotine Tob Res 18(5):1002–1011
Motschman CA, Gass JC, Wray JM, Germeroth LJ, Schlienz NJ, Munoz DA et al (2016) Selection criteria limit generalizability of smoking pharmacotherapy studies differentially across clinical trials and laboratory studies: a systematic review on varenicline. Drug Alcohol Depend 169:180–189
Muth ER, Stern RM, Thayer JF, Koch KL (1996) Assessment of the multiple dimensions of nausea: The nausea profile (NP). J Psychosom Res 40(5):511–520
National Cancer Institute (US) Tobacco Research Implementation Group (1998) Tobacco research implementation plan: priorities for tobacco research beyond the year 2000. National Cancer Institute, National Institutes of Health
Poling J, Rounsaville B, Gonsai K, Severino K, Sofuoglu M (2010) The safety and efficacy of varenicline in cocaine using smokers maintained on methadone: a pilot study. Am J Addiction 19(5):401–408
Ray LA, Lunny K, Bujarski S, Moallem N, Krull JL, Miotto K (2013) The effects of varenicline on stress-induced and cue-induced craving for cigarettes. Drug Alcohol Depend 131(1–3):13 6–142
Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA et al (2007) Pharmacological profile of the α4β2nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 52(3):985–994
Rose JE, Behm FM, Westman EC (1998) Nicotine–mecamylamine treatment for smoking cessation: The role of pre-cessation therapy. Exp Clin Psychopharm 6(3):331
Sanderson-Cox L, Tiffany ST (2001) Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res 3(1):7–16
Schlienz NJ, Hawk LW, Tiffany ST, O’Connor RJ, Mahoney MC (2014) The impact of pre-cessation varenicline on behavioral economic indices of smoking reinforcement. Addict Behav 39(10):1484–1490
Tiffany ST, Drobes DJ (1991) The development and initial validation of a questionnaire on smoking urges. Brit J Addict 86(11):1467–1476
Trask S, Thrailkill EA, Bouton ME (2017) Occasion setting, inhibition, and the contextual control of extinction in Pavlovian and instrumental (operant) learning. Behav Process 137:64–72
Acknowledgments
The authors acknowledge the contributions of Jennifer Adams and Constance Duerr (project coordination), Win Phyo and Baltaj Sandhur (data collection), Louise Cooper and Denise Swiatek (pharmacy support), Nicolas Schlienz (logistics and technical support), and the community members who participated in this project. Pfizer provided the study medication and placebo for this study, but had no role in study design or data analysis or interpretation.
Funding
This research was supported in part by the National Cancer Institute (R01 CA206193); additional research infrastructure support was provided by the NIH National Center for Advancing Translational Sciences (UL1TR001412 and UL1TR002001). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the United States Federal Government or VA Healthcare System.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MCM has received study medications from Pfizer in support of randomized clinical trials and has previously served as a consultant to and speaker for Pfizer on the topic of smoking cessation; he also serves as medical director of the New York State Smokers Quit Line. There are no other financial disclosures.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lawson, S.C., Gass, J.C., Cooper, R.K. et al. The impact of three weeks of pre-quit varenicline on reinforcing value and craving for cigarettes in a laboratory choice procedure. Psychopharmacology 238, 599–609 (2021). https://doi.org/10.1007/s00213-020-05713-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-020-05713-7