Abstract
Severe sepsis remains both an important clinical challenge and an economic burden in intensive care. An estimated 750 000 cases occur each year in the US alone (300 cases per 100 000 population). Lower numbers are estimated for most European countries (e.g. Germany and Austria: 54–116 cases per year per 100 000).
Sepsis patients are generally treated in intensive care units (ICUs) where close supervision and intensive care treatment by a competent team with adequate equipment can be provided. Staffing costs represent from 40% to >60% of the total ICU budget. Because of the high proportion of fixed costs in ICU treatment, the total cost of ICU care is mainly dependent on the length of ICU stay (ICU-LOS). The average total cost per ICU day is estimated at approximately €1200 for countries with a highly developed healthcare system (based on various studies conducted between 1989 and 2001 and converted at 2003 currency rates).
Patients with infections and severe sepsis require a prolonged ICU-LOS, resulting in higher costs of treatment compared with other ICU patients. US cost-of-illness studies focusing on direct costs per sepsis patient have yielded estimates of €34 000, whereas European studies have given lower cost estimates, ranging from €23 000 to €29 000. Direct costs, however, make up only about 20–30% of the cost of illness of severe sepsis. Indirect costs associated with severe sepsis account for 70–80% of costs and arise mainly from productivity losses due to mortality.
Because of increasing healthcare cost pressures worldwide, economic issues have become important for the introduction of new innovations. This is evident when introducing new biotechnology products, such as drotrecogin-α (activated protein C), into specific therapy for severe sepsis. Data so far suggest that when drotrecogin-α treatment is targeted to those patients most likely to achieve the greatest benefit, the drug is cost effective by the standards of other well accepted life-saving interventions.
Similar content being viewed by others
Increasingly, decision makers in the healthcare industry are looking into the strength of scientific evidence on clinical practice and cost effectiveness when allocating resources or granting reimbursement for medical interventions. This process, called evidence-based medicine, is becoming more and more frequently applied in decision making in modern medicine and is now reaching areas long considered ‘untouchable’ by economic measures, such as intensive or critical care medicine. Nowadays, an intensive care unit (ICU) run according to modern standards represents a major economic burden for every hospital. This is particularly so for university hospitals because these institutions, as tertiary referral centres, have to absorb the majority of severely ill patients, such as patients with sepsis, severe sepsis and septic shock; in general, these patients are among the most expensive to treat.
The aims and objectives of this article are to overview the important economic aspects of sepsis and to review data on the cost effectiveness of current and new therapies for this disease. In order to inform a nonmedical audience on the medical background and clinical conditions of this disease, we begin this review with the definition and epidemiology of sepsis, followed by a short overview of the current range of treatment. To enable easier comparison between cost data from different studies, (euro 1 = $US1.15 = $Can1.59 = £0.71) [all currencies were translated into Euros based on July 2003 exchange rate].
1. Definition of Sepsis, Severe Sepsis and Septic Shock
The general definition of sepsis is the systemic inflammatory response to infection. Sepsis, severe sepsis and septic shock represent the increasingly severe stages of the same disease. Uniformly accepted definitions of sepsis, severe sepsis and septic shock have existed since 1992, when an expert panel from the American College of Chest Physicians (ACCP) and the Society of Critical Care Medicine (SCCM) produced a consensus statement on definitions for sepsis and the stages of associated inflammatory response.[1] Prior to this statement being produced, the imprecise definition of sepsis and its sequelae caused confusion for clinicians. Furthermore, clinical data published before this definition was established were often difficult to interpret because of the heterogeneity of critically ill patients included in most studies.
To describe systemic responses to inflammatory reactions, regardless of their aetiology, a systemic inflammatory response syndrome (SIRS) was defined.[1] SIRS may result from either infectious or non-infectious processes such as pancreatitis or surgical trauma. Sepsis was then defined by the ACCP/SCCM conference panel as SIRS resulting from a documented infection. Further definitions allow a classification of the systemic responses to infection according to their severity (table I). It has been demonstrated that mortality rates increase as the number of observed SIRS criteria increases and the disease progresses; 28-day mortality rates were 7%, 10% and 17% for patients with two, three and four SIRS criteria, respectively, and 16%, 20% and 46% for patients with sepsis, severe sepsis, and septic shock, respectively.[2]
The American College of Chest Physicians and the Society of Critical Care Medicine consensus conference definitions of sepsis, severe sepsis and septic shock[1]
The above definitions have recently been updated,[3] dismissing the SIRS criteria and proposing prediction/insult/response/organ dysfunction (PIRO) criteria. However, this new differentiation regarding sepsis criteria has not become relevant for cost analysis to date.
2. Epidemiology and Clinical Spectrum of Severe Sepsis
Although the above definitions do provide a framework for classifying patients, a persisting and unresolved problem for the clinical epidemiologist is the application of these definitions in both the actual clinical setting and in reporting. A major setback for the clinician is that the definitions are nonspecific. The criteria are, in part, retrospective, which can lead to initial misclassification of patients. In addition, previous treatment affects the presentation of the septic syndromes at the time of classification. It is for these reasons that the number of sepsis cases in the official health statistics is low compared with the estimated incidence.
Alongside clinical activities evaluating new therapies for the treatment of sepsis in the last few years, efforts have also been focused on sepsis epidemiology.
2.1 Epidemiology
Estimates of the prevalence and/or incidence of sepsis varies between studies, most likely reflecting the different methods and definitions used. In general, the incidence of sepsis has been found to be highest at the extremes of age, i.e. in neonates/infants and in the elderly.[4–8]
Angus et al.[4] aimed to provide an accurate national estimate of current sepsis incidence, outcome and costs in the US by constructing a research database from hospital discharge records. A coding system based on the International Classification of Diseases (9th edition) [ICD-9] was used to identify patients with severe sepsis. Based on these data, the authors calculated an estimated 750 000 cases (300 cases per 100 000 population and 2.26 cases per 100 hospital discharges) of severe sepsis in the US in 1995. Another US study of eight academic medical centres reported a sepsis rate of 2.0 ± 0.16 cases per 100 admissions, or 2.8 ± 0.17 cases per 1000 patient-days.[9] Mortality rates were approximately 30–40% in these studies.
Recent epidemiological studies from Europe have reported incidences of sepsis that are lower than those reported for the US; whether these are true differences or due to different methodological approaches is a matter of dispute. Studies from Germany[10] and Austria[11] have reported incidences of severe sepsis of 54–116 cases per 100 000 population. In a recent study conducted by the European Sepsis Group among patients with an ICU length of stay >24 hours, the crude incidence of infectious episodes was 19%; approximately 28% of infections were associated with sepsis, 24% with severe sepsis and 30% with septic shock (18% were not classified).[12]
There are various possible explanations for the discrepancies in findings. Correct categorisation of severe sepsis is difficult. In the US, categorisation was conducted retrospectively through ICD codes combined with clinical and physiological criteria, or similar methods.[4,9] While a good approach, it requires access to an extensive compilation of hospital-based medical information. Epidemiological studies in many other countries have used other methods that may underestimate the number of patients with sepsis treated in normal wards. One may also speculate that the way healthcare is organised and delivered in different countries may result in differences regarding the incidence of observed/diagnosed sepsis. For instance, in a healthcare system with limited capacity for maximum treatment (including intensive care), infectious diseases may often not be recognised as sepsis in the normal ward. Patients diagnosed with pneumonia, especially the elderly, could die from sepsis without the diagnosis of sepsis ever being made. This would result in a lower incidence of sepsis being diagnosed than in countries that have a higher availability of intensive care beds, such as the US. However, this is unlikely to fully explain any difference between the US and Germany, where readiness to use the complete treatment spectrum seems comparable.
Interestingly, the US studies showed that a substantial proportion of patients were treated outside the ICU; in Angus et al.[4] 32% of patients received treatment at a level below maximal capacity. Possible reasons why patients with severe sepsis may be treated in normal wards rather than the ICU include: lack of serious organ failure, e.g. mechanical ventilation is not necessary; ICU treatment is not indicated for other reasons, e.g. futile prognosis, no consent; ICU treatment is not offered, e.g. because of financial considerations; severity of the disease is underestimated; an ICU bed is not available in the hospital because of restricted capacity; it is not possible to transfer to a tertiary ICU unit, e.g. restricted regional ICU capacity.
2.2 Clinical Spectrum
A full description of the pathophysiology is beyond the scope of this article and is described in full elsewhere.[13,14] Initially sepsis is characterised by an activation of the immune system via an increase in inflammatory mediators, but as sepsis persists there is a shift toward a general immune depression with the clinical picture often including a loss of delayed hypersensitivity, an inability to clear infection and a predisposition to nosocomial infection.[13,14]
The common clinical manifestations of sepsis include alteration in body temperature (fever or hypothermia), tachypnoea or hyperventilation, tachycardia, leucocytosis, leucopenia, altered blood pressure (high or low blood pressure), thrombocytopenia and/or disseminated intravascular coagulopathy (DIC), and alterations in mental status.[1,15,16]
Vasodilatation and increased vasopermeability (leakage of fluid into interstitial space), together with microthrombi, may lead to changes in microcirculatory blood flow and thus to tissue hypoperfusion. Tissue oedema also aggravates hypoperfusion. Lactic acidosis and oliguria occur.
Hypoperfusion-related stasis in the splanchnic area may cause translocation of bacteria and bacterial toxins across the intestinal barrier, which contributes to the persistence and aggravation of the inflammatory response. As a consequence of prolonged hypoperfusion, organ dysfunction results, leading to multiple organ failure and completing the picture of severe sepsis.[17–19]
Most cases of sepsis occur in patients with underlying conditions that make them susceptible to infections, such as lesions of an anatomical barrier (surgery, multiple trauma, burns), neoplasms, renal or hepatic failure, diabetes mellitus, chemotherapies, intravenous drug use or AIDS.[5,12] However, sepsis and septic shock may also occur in patients without underlying disease. In such cases, highly virulent organisms, such as meningococci, are usually responsible. Alternatively, sepsis can occur during infections that are treated inadequately or not at all.
3. Treatment of Sepsis: Overview
In general, the treatment of severe sepsis and septic shock consists of measures aimed at the infectious process itself (antibacterials, surgical eradication of infectious focus) in combination with life-supportive care (haemodynamic stabilisation, circulatory support, organ support) and specific treatment against the septic response (table II).[20] The combination of highly complex therapies requires treatment in an ICU with an adequate structure that allows close supervision by a competent team, use of critical treatment pathways and protocols,[21] and availability of sophisticated technology for continuous monitoring and organ support.
3.1 Elimination of the Infectious Source
Whenever possible, the appropriate surgical intervention with the aim of irradiating the infectious focus has high priority. Potential sources of systemic infection spreadout (e.g. infected foreign materials, perforations, anastomotic leakage, peritonitis, etc.) must be aggressively treated once the patient is haemodynamically stabilised.[22] In patients with basic respiratory failure, a vicious cycle can take effect where nosocomial pneumonia induces sepsis and sepsis enhances pneumonia.[23] In 20–30% of patients, the septic focus cannot be found.[5,24]
It has been demonstrated that the mortality rate among patients with sepsis who receive prompt and appropriate antibacterial therapy is approximately 10–15% lower than among those who do not receive the adequate antibacterial immediately.[25] Broad-spectrum antibacterial treatment (based on recommendations for the local spectrum of micro-organisms) often has to be started without microbiological identification of the causative organism.[26,27] Most antibacterials are given in high intravenous doses for several days, with adjustment once the results of microbiological culture and sensitivity tests become available. Resistance to antibacterials, especially with use of broad spectrum agents, is a concern.[26] Previous administration of broad spectrum antibacterials has shown to be a considerable risk factor for later development of nosocomial pneumonia with resistant strains.[28]
3.2 Supportive Care
Fluid replacement is crucial for patients with severe sepsis to maintain adequate perfusion of vital organs.[13,29] The massive release of inflammatory mediators in sepsis causes capillary leakage and intracapillary fluid is lost into the interstitial space, causing severe systemic hypovolemia.
Early goal-directed therapy, with volume resuscitation to restore the balance between oxygen delivery and oxygen demand, appears to be crucial in severe sepsis. In a recent study, Rivers et al. demonstrated that early, aggressive volume therapy with infusions of colloid or crystalloid, vasoactive substances and red-cell transfusions to optimise cardiac pre- and afterload significantly improved the survival rate of patients with severe sepsis and septic shock; in-hospital mortality was reduced to 30.5% from 46.6% with standard therapy.[30]
If hypotension persists in septic shock after volume repletion (caused by low systemic vascular resistance, sometimes combined with cardiac depression), the use of vasoactive drugs, especially catecholamines, becomes necessary.[31–33]
Approximately 85% of patients with severe sepsis develop lung problems and require mechanical ventilation.[25] However, some ventilatory strategies such as alveolar overdistension and high tidal volumes can damage the lung, and use of lower tidal volumes has been associated with a substantial survival benefit.[34] Alveolar overdistension causes so-called biotrauma (i.e. pulmonary cytokine release), which adds to deterioration of lung function and aggravates multiple organ failure.[35–37] Ventilator-associated complications strongly correlate with the duration of invasive ventilation; thus, early weaning may improve outcome.
A relatively small number of patients with severe sepsis and septic shock develop acute renal failure and require renal replacement therapy (RRT), and early initiation has been shown to improve the clinical situation and to reduce mortality.[38–40] However, initial expectations that haemofiltration might become an effective clinical treatment in sepsis have not been fulfilled.[41–43]
Adequate enteral/parenteral nutritional support is essential in patients with sepsis because they always have a high energy turnover due to the underlying hypermetabolic, inflammatory process. Enteral nutrition provides some gut protection against ischaemic damage that allows translocation of intestinal micro-organisms into the systemic circulation, fuelling the inflammatory situation. Recently, a remarkable benefit in survival was demonstrated by keeping blood glucose levels within a narrow range of 80–120 mg/dL.[44] This tight metabolic regimen was able to reduce hospital mortality by 34% in critically ill patients, mostly with severe sepsis and multiple organ failure. However, the underlying mechanisms of this remarkable effect are not well understood yet and require further research efforts.
Dysfunction of the blood coagulation cascade is common in patients with sepsis but few develop the clinical picture of DIC.[25] At present, no standard therapy for DIC is used uniformly throughout Europe and the US, but fresh frozen plasma, platelet concentrates and immunoglobulins are employed, depending on the individual case and the personal judgement of the treating physician.[45,46] The optimal haematocrit level for blood supply is still a controversial topic.[47]
In severe sepsis it is essential to treat the first organ failure aggressively as it is the cumulative failure of one or more organs that finally may lead to death.[25] The lung, kidney, heart, blood, CNS, and liver are the organs potentially most affected. Unfortunately, not all the affected organs can be supported effectively. CNS and liver function can be monitored but often not effectively treated (except in rare circumstances where strategies such as liver transplantation are possible). For the heart, no specific therapy other than volume replacement and vasoactive support is available.
3.3 Specific Treatment of Severe Sepsis
Until recently, trialling of many new concepts for the specific treatment of sepsis ended in disappointment.[48,49] Various anti-inflammatory treatments, such as ibuprofen, interleukin(IL)-1 receptor antagonists, murine monoclonal antibodies against endotoxin and soluble tumour necrosis factor-α (TNF-α)-receptor fusion protein, have shown no benefit in the treatment of sepsis.[50–54] Neutralisation of TNF by a monoclonal antibody has been shown to improve survival in sepsis patients with high IL-6 levels,[55] but there are no confirmative data available yet.
While previous treatment strategies using high-dose corticosteroids resulted in a significant increase in mortality,[56,57] a new concept has recently been promoted, based on the hypothesis that septic shock may be associated with a relative adrenal insufficiency. In a study of 229 septic shock patients with a relative adrenal insufficiency (nonresponsive to a corticotropin test), 7 days’ treatment with low doses of either hydrocortisone or fludrocortisone significantly improved the 28-day survival rate compared with the placebo.[58] Unfortunately, the study results were weakened by statistical shortcomings. At present, a large new European multicentre study Corticosteroid Therapy of Septic Shock (CORTICUS) is under under way to test this promising concept.
A potentially important advance in the treatment of patients with severe sepsis was reported in 2001, when the results of a large multicentre trial on recombinant human-activated-protein-C (drotrecogin-α), an anticoagulant, were reported.[24] Drotrecogin-α is the first anti-inflammatory agent that has proved effective in sepsis. Administration of drotrecogin-α resulted in an absolute risk reduction of 6.1% for death (all patients); in patients with at least two organ failures there was an even greater reduction of 7.4%.[24] Interestingly, two other anticoagulants, antithrombin III[59] and tissue factor pathway inhibitor,[60] failed clinically as treatment for severe sepsis, possibly these two agents work at different sites in the coagulation cascade.
4. Cost of Intensive Care Treatment
Probably the biggest limitation of any economic analysis in healthcare is the difficulty of obtaining the actual costs associated with the therapeutic interventions of a given disease. Direct medical costs associated with severe sepsis primarily consist of hospital costs. The lack of standardised methods for determining the direct cost of ICU care,[61] combined with inadequate documentation of ICU costs,[62] reflect the basic difficulties encountered in undertaking cost-of-illness studies in intensive care. It is mainly for this reason that information on the cost of severe sepsis is scarce.
4.1 General Allocation of Cost
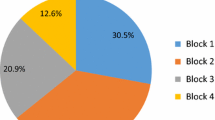
Intensive care is, in most cases, the section with the highest resource consumption within any hospital.[63] The allocation of costs for intensive care is usually done by the ‘top-down’ procedure, i.e. allocating section budgets and resource use to subunits and users, subdivided as far as possible. Edbrooke et al.[64] developed a ‘top-down’ procedure in which the major components were described by ‘cost blocks’ (see figure 1).
Proportion of different cost blocks within the total cost of intensive care. Median costs of £1087 (≊ €1538) per ICU patient-day (1995–96 values) were calculated from six university hospitals and five district general hospitals in the UK.[64]
In the previously mentioned US survey by Angus et al.[4] (section 2.1), it was found that only 51% of patients with severe sepsis were treated in the ICU, 17% were treated in a coronary care ward or ventilated in an intermediate care ward, with the remaining 32% treated in a normal ward. Hospital resource use and costs for the ICU patients were much higher than those for the non-ICU patients; hospital length of stay (LOS) 23.3 days versus 15.6 days, and cost per case €26 000 versus €12 000. (All currencies used in the different studies were translated into euro based on the July 2003 exchange rate).
In general, the difference between intensive care and normal ward care is the level of availability and consumption of resources. Intensive care medicine is extremely labour-intensive, requiring the continuous presence of competent medical and nursing staff to monitor and treat the critically ill patient at any time during the ICU stay. Thus, personnel costs (for nurses, physicians, technicians and assistants) consume from 45% to >60% of the total ICU budget.[64–67] Compared with the large proportion of personnel costs, other fixed costs (such as nonclinical support services, equipment, and rent and maintenance costs for building and properties) have a minor impact on the total costs of intensive care.[64] Variable costs including drugs, other consumables, and laboratory and diagnostic services, amounted to only 30% of total costs.
The workload of ICU staff cannot be allocated to the individual patient. Therefore, a large part of the resources consumed in the ICU are fixed costs (see figure 1 and table III). For this reason, ICU costs per patient-day on average are comparable between various countries with comparable salary structures in their healthcare systems (table III). In the seven countries with highly developed healthcare systems (i.e. excluding India), the average total cost per ICU day was approximately €1200. The large proportion of fixed costs also means that costs for an individual patient’s intensive care treatment correlate closely with the ICU LOS.
The distribution of intensive care treatment costs is generally rather skewed. Most ICU patients stay only for a short period of time (mean ICU-LOS: 3–4 days). On the other hand, the small number of critically ill patients requiring a prolonged ICU stay consume a large part of ICU resources.[69,75,79,80] Infections in general, and sepsis in particular, often require prolonged ICU treatment. Thus, predominantly because of the long ICU-LOS, the cost of treatment for patients with sepsis is considerably higher than treatment for other ICU patients,[81,82] and personnel costs account for a considerable proportion of these costs.
4.2 Direct Cost of Intensive Care
Direct treatment costs for the individual patient or DRG cannot be assessed by ‘top-down’ procedures. To assess patient-related resource consumption or direct costs for specific treatments, a ‘bottom-up’ procedure is needed. Such a procedure is extremely labour intensive if not supported by a patient-data management system (PDMS) through which all patient-related activities are automatically recorded. PDMS-based documentation systems have recently been successfully used to evaluate patient-related direct costs.[74,83]
The large proportion of ICU costs that are fixed staff costs reduces the effect of other expenses on total ICU resource consumption. Total direct ICU cost per patient per day varies to a relatively small extent. In 2000/2001, we performed a retrospective cost analysis in our surgical ICU that evaluated total direct ICU costs (personnel, procedures and consumables) assessed by means of a PDMS and exact cost catalogues of 1631 patients with an ICU-LOS >24 hours.[75] In patients with uncomplicated intensive care and an ICU-LOS of ≤7 days (average 2.6 ± 1.4 days) the total direct cost per day per patient was €862. In those patients needing intensive care treatment for >7 days (average 15.3 ± 9.6 days) the total direct cost per day per patient rose to €1011. As expected, it was the total ICU-LOS that made total cost of long-term ICU treatment so expensive, particularly since many of these ICU patients had severe sepsis. Another retrospective study from three ICUs in university hospitals in Germany showed comparable results.[74] The direct, patient-related, mean daily ICU cost for 385 patients with severe sepsis was €1318 per patient. Again, the large proportion that is personnel costs was evident (36%) [see figure 2].[74]
Distribution of costs for intensive care unit (ICU) treatment of severe sepsis. Direct ICU treatment costs for 385 patients with severe sepsis from three German university hospitals (1997–2000) [% of total ICU cost]. Average ICU length of stay was 16.6 days; average total direct ICU cost per patient-day was €1318[74]
In a study from a Norwegian university hospital[72] the mean cost per ICU day was €2601, based on total direct costs of staff, drugs and consumables, capital equipment and administrative overheads, and additional costs incurred in other hospital departments, such as operating theatres, physiotherapy, x-ray, etc. from 1997 to 1999. Patients with severe sepsis cost, on average, €2671 per ICU day. Direct patient-related costs for treating severe sepsis and septic shock in intensive care have been analysed retrospectively in an adult general ICU in a UK university hospital.[84] Over a period of 10 months (1995–96), 36 of 213 patients with sepsis were evaluated. The mortality rate among these patients was 53%. The median ICU-LOS was approximately 14 days longer than for patients without sepsis. Patient-related costs per case varied considerably, but the daily costs were consistent. The median daily cost for inpatients without sepsis was €650 per patient (interquartile range €560–790) whereas the median daily cost for patients with sepsis was €810 per patient (interquartile range €740–1100), with slight variations depending on the timing of onset of sepsis. Personnel costs ranged from 39% to 49% of the total cost per patient.
Angus et al.[85] reported a mean ICU cost of €23 870 for patients with severe sepsis, based on data collected prospectively from a cohort of 552 patients with sepsis in a multicentre international trial of drotrecogin-α (Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis [PROWESS]). Based on a mean ICU-LOS of 11.4 days, a mean daily ICU cost of €2090 (2000 values) per patient was calculated.
Compared with the staff costs related to excessive ICU-LOS, the costs for specific treatment procedures are minor. Two expensive technologies (in terms of initial investment), mechanical ventilation and continuous RRT, are frequently used in the standard treatment of patients with sepsis as for other ICU patients.
Mechanical ventilation is often regarded as a marker procedure of intensive care. Investment in modern ventilators is essential and considerable for any ICU; depending on the patient mix treated in the ICU, one ventilator per bed is mandatory (plus extra ventilators for substitution). The direct (running) costs for mechanical ventilation are difficult to estimate. Disposables needed for ventilation are minor expenses but nursing care and monitoring are mandatory. On the other hand, non-ventilated restless patients often need more nursing care than deeply ventilated patients. Approximately 85% of patients with sepsis require ventilatory support, typically for 7–14 days, and almost half meet the criteria for adult respiratory distress syndrome.[25]
For patients with severe sepsis developing acute renal failure, mortality rates range from 40–80%.[86] Thus, when RRT is required, the question of cost arises. RRT requires a high investment from the ICU; but depending on the spectrum of diagnoses treated in the ICU, only a small number of machines are needed. Using the modern, highly automated pumps, extra personnel costs are minor but the costs of consumables (filters, lines and especially fluids) are considerable (average €42.50 per patient-day, according to our own data).
A cost analysis for RRT was carried out by Korkeila et al.[87] They evaluated 62 patients (of 3447 patients admitted to the ICU during 1 year) requiring RRT for non-endstage renal failure and followed the patients 5 years after release from the hospital to compute overall mortality and estimate quality of life. The cost of RRT per patient surviving >6 months was estimated at €69 600 (1992–93 values).
From the many studies examining the cost of antibacterial therapies in the ICU, we present here one example to illustrate that medication costs are relatively minor expenses. Scott et al. determined the costs of antibacterial regimens currently used for serious infections in three large New Zealand hospitals.[88] Total costs (drugs, consumables and labour costs for preparation, administration and monitoring) per patient-treatment day varied from €4.46 (1997 values) for gentamicin to €65.85 for tazobactam. It was estimated that nephrotoxicity could add €12–22 per day to the cost of aminoglycoside treatment.
Compared with increased hospital LOS due to bacterial resistance to the antibacterial regimen, drug-acquisition costs are of minor economic importance.[89] Antibacterial resistance is of greater importance today because of escalating resistance rates, but studies to systematically evaluate the economic effects of drug resistance have not been performed.
4.3 Total Hospital Costs for Severe Sepsis
Few cost studies deal with total hospital costs for severe sepsis. A retrospective study from Canada analysed the hospital cost for treating 100 patients with severe sepsis and/or septic shock for 28 days.[90] The mean total hospital cost per patient was €8100 (2000 values), with an average of €710 per hospital day. ICU costs accounted for 38% of the total cost.
Data from 99 ICUs in Italy showed that the total hospital cost for treating severe sepsis and/or septic shock was €21 572 (2000 values) per patient, compared with €11 590 for patients without sepsis (based on total costs of €1033 per day in the ICU and €300 per day in a normal ward). Again, the excess costs for treating sepsis were mostly due to a longer ICU-LOS (average 18.7 days vs 7.1 days).[91]
Angus et al.[4] estimated the resource use for treating severe sepsis in the US through DRG-based reimbursement of hospitals and using an average cost-to-charge ratio. The average cost per case of sepsis 1995 values was estimated to be €19 200 (mean hospital LOS: 19.6 days). Non-survivors generated higher costs than survivors (€22 500 vs €17 900).
In an earlier study, Chalfin et al.[92] reported mean total hospital charges of €33 300 (1985–88 values) per survivor for patients with sepsis, while the mean total charges for non-survivors amounted to €42 800. In contrast, in a recent report based on a multicentre international study of drotrecogin-α,
Angus et al.[85] calculated mean total hospital costs of €33 300 (2000 values) for patients with severe sepsis, with survivors generating higher costs than non-survivors (€36 800 vs 24 900).
5. Cost Effectiveness of Intensive Care Treatment and Alternatives
5.1 Intensive Care
A prospective cost-effectiveness analysis in seven ICUs of teaching hospitals in France was performed by Sznajder et al.[71] The mean LOS of 176 patients with at least one organ failure was 9 days (median 4 days), and the hospital mortality rate was 29%. For these patients, the mean total cost per ICU stay (measured bottom-up) was €12 400, with substantial variations (SD ± €16 400) according to different diagnoses. Costs were higher for non-survivors than for survivors. From these data, a mean daily cost of €1380 (1996 values) per patient can be calculated. The incremental cost-effectiveness ratio was €1000 per life-year saved for intensive care versus no intensive care.
In a recent study from Norway by Flaaten and Kvale[72] the cost effectiveness of intensive care treatment was estimated at €684 (converted to 2001 values) per life-year saved, based on 18 months’ survival data. For patients with severe sepsis a cost-effectiveness ratio of €2785 per life-year saved was reported.
The cost effectiveness of ventilator support was addressed by Hamel et al.[93] Data from the Study to Understand the Prognosis for Outcomes and Risks of Treatments (SUPPORT) trial were used to estimate the value of this technology in monetary terms. 1005 patients with pneumonia or adult respiratory distress syndrome were evaluated for their outcome (mortality) as well as their functional status and quality of life following hospitalisation. Depending on the patient’s initial risk of death, the incremental cost effectiveness of mechanical ventilation varied from €25 200 to €95 600 per QALY (comparing provision of ventilator support and aggressive care versus withholding these intensive measures) [1998 values]. In particular, for patients with <50% probability of surviving at least 2 months, the incremental cost per QALY was >€87 000, i.e. 3-fold higher than for patients with higher survival chances. These costs were calculated based on hospital fiscal data and Medicare data, from a society perspective rather than hospital expenditures. From the hospital view these are charges, which may or may not reflect true resource use.
Even a prolonged stay in the ICU, which produces high costs, is not a valid argument for restricting intensive care treatment. Heyland et al.[69] demonstrated that 44% of patients with a prolonged ICU-LOS of >14 days survived for at least 1 year. Our own data demonstrate that patients with an ICU-LOS of >20 days have a hospital survival rate of 74%.[75]
In summary, based on current severity scoring systems, a reliable survival prediction for individual ICU patients, with or without sepsis, cannot be made at admission to the ICU. Decisions to restrict treatment may be taken by the ICU team on grounds of unresponsiveness to all therapeutic efforts rather than on the basis of a general guideline to limit treatment for probability of survival, age or economic reasons.
Few analyses of the cost effectiveness of antibacterial therapies have been carried out to date. However, Rodloff et al.[94] compared the cost effectiveness of an initial imipenem regimen with other antibacterials for the treatment of nosocomial pneumonia. Both regimens showed equal clinical efficacy, but the imipenem treatment resulted in significant savings due to a faster recovery and a reduced duration of therapy. This observation is most interesting as it emphasises the duration of treatment. In intensive care medicine, with its high fixed costs, any reduction in treatment requirements and LOS may considerably reduce costs. This point was emphasised by Nathwani et al.[95] who analysed the treatment of methicillin-resistant staphylococcal infection, comparing linezolid with vancomycin. Although no significant difference in clinical outcome or mortality was observed, linezolid administration reduced the duration of intravenous therapy compared with vancomycin, and increased the likelihood of hospital discharge during the first week, thus increasing the cost effectiveness of antibacterial treatment. Dresser et al.[96] compared the cost effectiveness of gatifloxacin versus ceftriaxone with a macrolide for community-acquired pneumonia (CAP). Clinical efficacy and LOS were similar with both treatments. However, there were only two incidences of CAP that did not respond to gatifloxacin therapy, whereas nine nonresponders were observed in the ceftriaxone group. These treatment failures added one incremental day to the antibacterial-related LOS, resulting in a cost-effectiveness ratio of €4550 per treatment success for gatifloxacin versus €6130 per treatment success for ceftriaxone (1998 values). In this analysis the cost of hospitalisation was once again the key cost factor.
No published data were found concerning the cost effectiveness of RRT for acute renal failure in the ICU. Furthermore, no reports on the cost effectiveness of other organ-support therapies, such as vasoactive or anticoagulative treatment, were retrieved by our search, indicating that a large part of standard ICU therapy has not been evaluated for economic efficiency. This lack of cost-effectiveness data on routine therapeutic measures in the ICU could be due to a lack of data on the clinical efficacy of many compounds used in the ICU. This demonstrates the predominantly empirical approach of many intensivists and accentuates the need for a more evidence-based approach in the future. In addition, because of the complex interactions of various intensive care therapies, it is often difficult, if not impossible, to evaluate the efficacy of single therapeutic measures within the context of the entire treatment process.
5.2 Alternatives to Intensive Care
The ICU direct costs per day are generally three to seven times higher than for non-ICU care.[97,98] However, no cost-effectiveness analyses comparing treatment of patients with severe sepsis in the normal ward with treatment in the ICU have been carried out, most likely for the reason that treatment of severe sepsis with organ failures simply requires full intensive care.
Early transfer to a ‘step-down unit’ after ICU treatment has been shown to reduce overall treatment cost. Norris et al.[97] suggested net savings (without drug costs) of approximately €1040 (1992) per patient-day if intensive care was substituted by regular care on a normal ward. This study only focused on the cost of substitution and did not look for outcomes. Similar results were found by Singer et al.[68] Such considerations should stimulate intensivists to discharge patients from their ICU as soon as possible. However, discharging patients too early and under emergency circumstances (e.g. when beds or sufficient staff are not available during weekends or at night) has proven to increase the risk for patients.[99] Health benefits for the patient should have priority over economic reasons. In contrast to the above findings, Keenan et al.[100] found no evidence in a systematic review of the literature that a ‘step-down-unit’ represents a cost-effective alternative to the ICU.
One could speculate that maximal intensive care therapy might not be indicated for patients, with a low probability of survival. Besides the ethical impact of such concepts, the main obstacle is the impossibility of identifying, with acceptable certainty, who has with a minimal chance of survival. No existing severity scores (e.g. Acute Physiology and Chronic Health Evaluation [APACHE], Simplified Acute Physiology Score [SAPS], Mortality Probability Model [MPM]) can predict fatal outcome for individuals with acceptable precision.[101]
6. Possibilities for Cost Containment in Intensive Care
There are various potentially effective strategies to reduce resource use in intensive care, the relevance of which depends largely on local conditions. Such strategies include:
-
increasing awareness of economic consequences
-
optimising internal organisation in the ICU
-
optimising the network within the hospital
-
prioritising avoidance of complications
-
profiting from early discharge from the ICU.
By discussing the relative cost of diagnostic and therapeutic procedures, a considerable reduction in resource use may be achieved.[102]
Introducing protocols (e.g. for weaning from mechanical ventilation), defining internal procedures (e.g. for diagnostic strategies), and optimising therapeutic processes (e.g. by optimising antibacterial therapy) may result in considerable cost reductions.[103,104] In particular, the ICU-LOS may be reduced, which contributes significantly to reducing expenditure. Also the intensity of nursing care is relevant when evaluating the economic consequences of changing treatment and management options. Even if a patient requires ‘one to one’ nursing care, decisions can still be made regarding whether or not the nurse performs additional non-bedside duties.
The ICU is involved in a complex network of interdisciplinary activities and thus can be affected by processes outside of the unit. In other words, it is not sufficient to optimise processes within the unit; interdisciplinary co-operation must also be optimised (e.g. external consultations, discharge/transfer from the ICU), which can be a difficult task to achieve in a hospital. Critical pathways have been assumed to improve such interdisciplinary procedures. However, official, external pathways (in contrast to internal procedures) may not be useful within an intensive care environment. Berenholtz et al.[105] found that for 78% of a patient’s ICU stay, the patient was either not eligible for a critical pathway or that the patient’s clinical course was ‘off’ pathway.
Preventing complications, particularly nosocomial infections, will always reduce the cost of treatment. There is extensive evidence to show that when treatment is complicated by nosocomial infections the LOS and treatment costs increase considerably, resulting in excessive resource consumption.[82,106]
Given the high treatment cost of intensive care, every reduction in LOS will contribute significantly to lower total resource use. However, along with restricting or reducing intensive care processes, the capacity for and quality of post-ICU treatment in the hospital and external rehabilitation must be considered. The structure and capacity of a healthcare system play an important role here. If, for instance, ICU capacities are extremely limited, premature discharge from the unit will often be unavoidable. If readmission of the patient as a consequence of subsequent deterioration is required, longer and more expensive treatment may result. Although potentially economically desirable, early discharge from the ICU can only be performed if optimal care for the patient is nevertheless ensured; minimal risk for the patient remains first priority.
In conclusion, there is certainly a large potential for cost containment in intensive care. However, because of the high level of interdisciplinary activities within the ICU, there are no simple, ICU-specific solutions that can be easily implemented. The ‘easy’ way of reducing ICU expenditure, e.g. by reducing personnel, may ultimately produce the opposite effect. Discussions in the US on so-called ‘closed’ ICUs have increased awareness that a competent team of nurses and physicians providing continuous intensive care (24-hour coverage) is more clinically efficient and may even reduce overall costs compared with a system of personnel being called in from other areas of the hospital.[107–109]
7. Cost Effectiveness of New Therapies
Recently developed biotechnology products are beginning to fill some major therapeutic gaps. However, for disorders where no treatment was previously possible, these new drugs may introduce a new need for health service expenditure. For example, drotrecogin-α is a biotechnology drug that is effective in the treatment of severe sepsis, and its introduction has significantly lowered mortality rates.[24] There is a risk that such new and expensive products might be denied entry into the healthcare system, merely because they add to the already high treatment costs of patients with sepsis, even if they prove equally or more cost effective than other life saving measures that are used routinely.
A first study addressing the cost effectiveness of drotrecogin-α was published recently by Manns et al.[110] The cost effectiveness of drotrecogin-α was compared with conventional therapy for patients with severe sepsis. The economic analysis performed involved all patients and subgroups defined by age and severity of illness, respectively. The probabilities of transition between clinical states and the estimates of resource use were derived from a population-based cohort of patients with severe sepsis, using effectiveness data from the PROWESS study.[24] The cost per life-year gained by treating all patients with drotrecogin-α was €24 300 [2001 values]. Treating patients with an APACHE II score of ≥25 was more cost effective (€21 300 per life-year gained), whereas drotrecogin-α treatment was less cost effective for those patients with a lower APACHE II score (€31 000 per life-year gained); a lower APACHE II score indicates less severe disease. For patients with an APACHE II score of ≥25, the cost per life-year gained with drotrecogin-α increased with age from €14 200 for patients aged <40 years to €24 400 for those aged ≥80 years.
In a report by Angus et al.[85] the cost effectiveness of drotrecogin-α was estimated on the basis of the PROWESS study data collected prospectively as part of this international multicentre trial. Drotrecogin-α increased the cost of care by €8500 (2000 values) per patient and increased survival by 0.061 lives saved per patient treated, resulting in a cost of €139 000 per life saved compared with conventional care. Projected to a lifetime time horizon, drotrecogin-α increased the cost of care by €13 900 and increased survival by 0.33 QALYs per treated patient, yielding a cost of €41 750 per QALY. When limited to patients with an APACHE II score of ≥25, the cost effectiveness of drotrecogin-α improved to €23 800 per QALY; it was not considered cost effective for patients with an APACHE II score of <25.
The cost effectiveness of drotrecogin-α was evaluated in a German ICU cost setting by Neilson et al.[111], based on the clinical outcome and resource use data of the PROWESS trial. Using a decision-analysis model, the cost effectiveness of drotrecogin-α as an adjunct to standard therapy was estimated. The additional average cost (2001 values) was €7400 per patient treated with drotrecogin-α compared with standard therapy. Adjusting life expectancy for the potential effects of co-morbidity, ICU-LOS and severe sepsis, resulted in 9.9 years per additional survivor (0.59 life-years per patient treated with drotrecogin-α). The cost effectiveness of drotrecogin-α for all patients was calculated at €14 100 per life-year gained. A similar analysis was performed in Austria and Switzerland[112] and yielded comparable results. The cost effectiveness of drotrecogin-α was calculated at €15 100 and €14 700 (2001 values), respectively per life-year gained for all patients.
In summary, under various model assumptions (all based on the data of the PROWESS study) regarding the per-patient cost of drotrecogin-α, the pattern of resource use, the survival benefit and country-specific ICU cost settings, the cost effectiveness of drotrecogin-α was comparable to other generally accepted life-saving interventions in Europe[111,112] and the US.[113] Studies report that cost-effectiveness is more favourable for more severely ill patients i.e. APACHE II score ≥25 or 2 or more organ dysfunctions (the latter being the currently approved indication in Europe). Although indirect costs make up the majority of the total costs of sepsis (see section 8) these studies considered only healthcare costs. Manns et al.[110] included indirect costs in a sensitivity analysis, but concluded that the cost effectiveness of drotrecogin-α was not sensitive to inclusion of the indirect costs.
As shown in the cost-effectiveness analyses discussed in this section, the decisive factors in the models are the age of the surviving patients (i.e. expected life-years) and survival benefit (i.e. percent of absolute risk reduction in mortality with drotrecogin-α treatment). In other words, the severity of illness as well as the age of the patients treated will considerably influence long-term survival and thus cost effectiveness. This means that the cost effectiveness of treatment with drotrecogin-α will decrease as the age of the treated patient population increases. On the other hand, as the survival benefit of drotrecogin-α (absolute risk reduction in mortality) increases with the increasing severity of sepsis (as demonstrated in the PROWESS trial), the cost effectiveness will increase as the severity of the disease (measured as APACHE II score or in number of organ dysfunctions) increases.
Thus, translation of the results from the PROWESS study into the ‘real ICU world’ setting depends much on the actual population of patients with sepsis treated with drotrecogin-α, as the age distribution as well as the severity of the disease in such patient groups determines the actual outcome. As an example, following European Commission labelling, which has approved the use of drotrecogin-α for patients with sepsis with two or more organ failures, may result in a worse cost effectiveness than following the US FDA labelling, which has approved drotrecogin-α for use in adult patients with severe sepsis (sepsis associated with acute organ dysfunction) who have a high risk of death (e.g. as determined by APACHE II >25). For the patient population defined by the US FDA, the absolute risk reduction with drotrecogin-α (hospital mortality at 28 days) is 12.8%, whereas for the population with two or more organ dysfunctions the absolute risk reduction is 7.4%.[114]
The two corresponding survivor populations from these subpopulations of patients with sepsis may also differ considerably in age from the all-patient survivor group of the PROWESS trial. For example, the age distribution for patients with two or more organ dysfunctions who were treated with drotrecogin-α, in combination with the absolute risk reduction of 7.4%, resulted in an improved life expectancy of 12 years for sepsis survivors (versus 9.9 years for all patients who were treated with drotrecogin-α) in the PROWESS trial.[111] In combination, the differences in key parameters between these two populations of patients with sepsis result in an increased cost effectiveness of drotrecogin-α for patients with two or more organ failures of €10 200 per life-year saved, compared with €14 100 for the all-patient survivor population in the drotrecogin-α group.[111] Similarly, the cost effectiveness for patients with two or more organ failures improved from €15 100 to €11 000 per life-year saved in Austria and from €14 700 to €11 100 per life-year saved in Switzerland.[112]
An interesting new perspective has recently been introduced by Teres et al.[115] They retrospectively analysed data from 50 ICUs participating in the US American IMPACT project (1998–9) comprising 2434 patients with severe sepsis on ICU admission. It was found that differences in LOS (both ICU and hospital) between survivors and non-survivors were related to the severity of illness. For sepsis patients in the middle range of severity of illness, non-survivors had a longer ICU-LOS but a shorter hospital LOS than survivors. In the upper range of severity of illness, survivors had a longer ICU-LOS and hospital-LOS compared with patients with severity of illness in the lower ranges. Thus, when considering the economic consequences of new innovative treatments, it may be relevant to consider who will be the recipients of such therapy. A net benefit may be limited to a portion of the patient population with a certain degree of severity of illness, and the cost-effectiveness ratio might be much less favourable for other groups of patients such as potential survivors that require prolonged treatment. Thus, if a more severely ill population were treated more aggressively, in-hospital resource consumption and post-discharge costs may increase considerably.
Large multinational trials are generally designed to demonstrate the clinical efficacy of new innovative treatment. These trials are not suitable for predicting the cost effectiveness of such new treatments since ‘real life’ situations are different from the specific circumstances of controlled clinical trials. One potential way around this would be to establish a time-limited introduction phase during which new treatments have to prove their cost effectiveness. During such introduction phases, well defined follow-up analyses might provide valid information on the primary indication (e.g. comorbidities), the treated patient mix (e.g. age, severity of illness), the outcomes (e.g. LOS, complications, survival rate), and economic parameters (e.g. cost savings or additional cost). On the other hand, with current significant restrictions of healthcare expenditure in general, any increase in resource consumption may limit the acceptance of new innovative therapeutic concepts, even if they have been shown to be cost effective.
8. Cost-Of-Illness Estimates
Cost-of-illness estimates for severe sepsis are hampered by the fact that very limited information is available on patients after they have left the ICU and the hospital. Questions of life expectancy and QOL after a sepsis episode remain largely unanswered, making indirect cost estimates somewhat speculative.
The previously mentioned study of Angus et al.[4] also included a cost-of-illness estimate of severe sepsis. The hospital cost per patient with severe sepsis was estimated to be €19 200, translating into a total hospital cost of €14.5 billion for the US in 1995. The incidence of severe sepsis, and its associated costs, were projected to grow as the US population expands and ages.
In a recent study by Schmid et al.,[10] severe sepsis was found to impose annual costs (1998 values) of between €3.6 and 7.9 billion to the German society. Based on an incidence estimate of between 44 000 and 95 000 cases per year, calculation of direct costs per patient yielded €23 300 on average. Direct costs, however, were found to make up only around 28% of the cost of illness of severe sepsis in Germany. The total indirect costs ranged from €2.6 to 5.7 billion. Productivity loss due to premature death accounted for the largest part of these indirect costs. A previous publication[116] also estimated incidence and annual cost of sepsis in Germany, but was solely based on literature, followed by a model calculation. Their findings were comparable with regard to total direct costs (€0.85–2.35 billion) [1996 values] and per-patient indirect costs, but the estimate of incidence (125 000–300 000 sepsis cases per year in Germany) was considerably higher.
A study in Austria yielded total costs for severe sepsis of €676–958 million annually (2000 values).[11] The mean total direct ICU costs per sepsis patient were €28 600, based on an incidence estimate of 6700–9500 cases per year. Of the total costs of severe sepsis in Austria, 69% were due to productivity loss resulting from premature death, whereas direct costs comprised 28% of the total.
Based on the above estimates for the cost of illness of severe sepsis in Europe and the US, sepsis might be considered an expensive medical problem from a societal perspective. With increasing life expectancy in the populations of most developed countries, the economic burden of sepsis may become even more prominent in the next few decades.
9. Conclusions
This century appears set to become the century of biotechnological advance, particularly in the area of healthcare. Unfortunately, the general restrictions on healthcare resources make the introduction of new interventions difficult. New and expensive therapies based on biotechnology represent an unwelcome dilemma for most healthcare systems featuring aging populations and heightened public expectations. Under these circumstances, new therapies no longer receive an unambiguous welcome by the medical communities and there is an increasing need to justify costs alongside clearly demonstrated clinical benefits.
At present, the modern ICU represents a major economic burden to society. Since intensive care will continue to consume a disproportionately high amount of healthcare resource in the future, there will be a growing focus on cost containment and on cost effective use of ICU resources. For this reason, cost-effectiveness assessments will represent an increasingly important tool in future discussions on the economy of new ICU therapies and treatment of sepsis patients. In this discussion, it should be kept in mind that staffing costs represent a considerable part of expenditures, since treating critically ill patients is extremely personnel intensive; this is, to some degree, inevitable if quality of care is to be maintained.
Nevertheless, every new biotechnology-based therapy brings about a renewed discussion on the appropriateness of its use, as demonstrated by the treatment of severe sepsis with drotrecogin-α, discussed in section 7.
New therapies cannot be expected to offer benefits without added costs. The cost-benefit equation will become increasingly more difficult to assess and we should enter the iterative process of combining the clinical learning curve of a new therapy with continuous economic assessment of this therapy. This is also required to correct early pricing and reimbursement decisions if later dictated by clinical and economic reality.
References
Bone RC, Balk RA, Cerra FE, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101: 1656–62
Rangel-Frausto M, Pittet D, Costigan M, et al. The natural history of the systemic inflammatory response syndrome (SIRS): a prospective study. JAMA 1995; 273 (2): 117–23
Levy MM, Fink PM, Marshall JC, et al. SCCMIESICMlACCP/ ATS/SIS International Sepsis Definition Conference. Intensive Care Med 2003; 29: 530–8
Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit CareMed 2001; 29: 1303–10
Brun-Buisson C, Doyon F, Carlet J, et al. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults: a multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. JAMA 1995; 274: 968–74
Baine WE, Yu W, Summe JP. The epidemiology of hospitalization of elderly Americans for septicaemia or bacteraemia in 1991–1998: application of medicare claims data. Ann Epidemiol 2001; 11: 118–26
McBean M, Rajamani S. Increasing rates of hospitalization due to septicemia in the US elderly population, 1986–1997. J Infect Dis 2001; 183: 596–603
Watson RS, Carcillo JA, Linde-Zwirble WT, et al. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med 2003; 167: 695–701
Sands KE, Bates DW, Lanken PN, et al. Epidemiology of sepsis syndrome in 8 academic medical centers. Academic Medical Center Consortium Sepsis Project Working Group. JAMA 1997; 278: 234–40
Schmid A, Burchardi H, Clouth J, et al. Burden of illness imposed by severe sepsis in Germany. Eur J Health Econ 2002; 3: 77–82
Schmid A, Schneider H, Adolf A, et al. Economic burden of illness imposed by severe sepsis in Austria. Wien Klin Wochenschr 2002; 114: 697–701
Alberti C, Brun-Buisson C, Burchardi H, et al. Epidemiology of sepsis and infection in lCU patients from an international multicentrecohort study. Intensive CareMed 2002; 28: 108–21
Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 2003; 348: 138–50
Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: understanding the role of innate and acquired imminity. Shock 2001; 16: 83–96
Bone RC, Fisher CJ, Clemmer TP, et al. Sepsis syndrome: a valid clinical entity. Crit Care Med 1989; 17: 389–93
Balk RA. Severe sepsis and septic shock. Crit Care Clin 2000; 16: 179–92
Zanetti G, Baumgartner JD, Glauser MP. Sepsis and septic shock. Schweiz Med Wochenschr 1997; 127: 489–99
Horn KD. Evolving strategies in the treatment of sepsis and systemic inflammatory response syndrome (SIRS). QJM 1998; 91: 265–77
Rackow EC, Astiz ME. Pathophysiology and treatment of septic shock. JAMA 1991; 266: 548–54
Guidelines for the management of severe sepsis and septic shock. The International Sepsis Forum. Intensive Care Med 2001; 27 Suppl. 1: S1–134
Brilli RJ, Spevetz A, Branson RD, et al. Critical care delivery in the intensive care unit: defining clinical roles and best practice model. Crit Care Med 2001; 29: 2007–19
Jimenez MF, Marshall JC. Source control in the management of sepsis. Intensive Care Med 2001; 27: S49–62
Hubmayr RD, Burchardi H, Elliot M, et al. Statement of the 4th international consensus conference in critical care on ICUacquired pneumonia: Chicago (IL), May 2002. Intensive Care Med 2002; 28: 1521–36
Bernard GR, Vincent JL, Laterre PF, et al. Fificacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 2001; 344: 699–709
Wheeler AP, Bernard GR. Treating patients with severe sepsis. N Engl J Med 1999; 340: 207–14
Hammond JMJ, Potgieter PD. Current disease management strategies in the treatment of serious infections. Clin Drug Invest 1998; 15: 9–17
Bodmann KF, Vogel F. Antibacterial therapy of sepsis (Antimikrobielle Therapie der Sepsis). Chemotherapie J 2001; 10: 43–56
Chastre J, Fagon JY, Bornet-Lesco M, et al. Evaluation of bronchoscopic techniques for the diagnosis of nosocomial pneumonia. Am J Respir Crit Care Med 1995; 152: 231–40
Bone RC The sepsis syndrome: definition and general approach to management. Clin ChestMed 1996; 17: 175–181
Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345: 1368–77
Martin C, Saux P, Eon B, et al. Septic shock: a goal-directed therapy using volume loading, dobutamine and/or norepinephrine. Acta Anaesthesiol Scand 1990; 34: 413–7
Task Force of the American College of Critical Care Medicine: Practice parameters for hemodynamic support of sepsis in adult patients in sepsis. Crit Care Med 1999; 27: 639–60
Meier-Hellmann A, Sakka S, Reinhart K. Supportive therapy of the sepsis syndrome. Clin Chern Lab Med 1999; 37: 333–9
Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342: 1301–8
Dreyfuss D, Soler P, Saumon G. Mechanical ventilation-induced pulmonary edema: interaction with previous lung alterations. Crit Care Med 1995; 151: 1568–78
Tremblay LN, Slutsky AS. Ventilator-induced injury: from barotrauma to biotrauma. Proc Assoc Am Physicians 1998; 110: 482–8
Ranieri VM, Suter PM, Tortorella C, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 1999; 282: 54–61
Cole L, Bellomo R, Journois D, et al. High-volumehaemofiltration in human septic shock. Intensive Care Med 2001; 27: 978–86
Gettings LG, Reynolds HN, Scalea T. Outcome in post-traumatic acute renal failure when continuous renal replacement therapy is applied early vs. late. Intensive Care Med 1999; 25: 805–13
Ronco C, Bellomo R, Homel P, et al. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet 2000; 356: 26–30
Bellomo R. Continuous hemofiltration as blood purification in sepsis. New Horiz 1995; 3: 732–7
De Vriese AS, Vanholder RC, Pascual M, et al. Can inflammatory cytokines be removed efficiently by continuous renal replacement therapies? Intensive Care Med 1999; 25: 903–10
van Bommel EF. Should continuous renal replacement therapy be used for ‘ non-renal’ indications in critically ill patients with shock? Resuscitation 1997; 33: 257–70
Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med 2001; 345: 1359–67
Vervloet MG, Thijs LG, Hack CE, Schuster HP. Disturbances of blood coagulation and fibrinolysis (Störungen der Blutgerinnung und Fibrinolyse). In: Schuster H-P, Werdan K, editors. Intensive therapy with sepsis and multi-organ failure (Intensivtherapie bei Sepsis und Multiorganversagen). 3rd edition, Berlin: Springer-Verlag, 2000
Horn KD. Evolving strategies in the treatment of sepsis and systemic inflammatory response syndrome (SIRS). QJM 1998; 91: 265–77
Hebert PC, Well G, Blajchman MA, et al. A multicenter randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med 1999; 340: 409–17
Zeni F, Freeman B, Natanson C. Anti-inflammatory therapies to treat sepsis and septic shock: a reassessment. Crit Care Med 1997; 25: 1095–100
Marshall JC. Clinical trials of mediator-directed therapy in sepsis: what have we learned? Intensive Care Med 2000; 26 Suppl. 1: S75–83
Bernard GR, Wheeler AP, Russell JA, et al. The effects of ibuprofen on the physiology and survival of patients with sepsis. The Ibuprofen in Sepsis Study Group. N Engl J Med 1997; 336: 912–8
Fisher Jr CJ, Dhainaut JF, Opal SM, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome: results from a randomized, doubleblind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA 1994; 271: 1836–43
Opal SM, Fisher Jr CJ, Dhainaut JF, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med 1997; 25: 1115–24
Angus DC, Birmingham MC, Balk RA, et al. E5 murine monoclonal antiendotoxin antibody in gram-negative sepsis: a randomized controlled trial. E5 Study Investigators. JAMA 2000; 283: 1723–30
Abraham E, Laterre PF, Garbino J, et al. Lenercept (p55 tumor necrosis factor receptor fusion protein) in severe sepsis and early septic shock: a randomized, double-blind, placebo- controlled, multicenter phase III trial with 1,342 patients. Crit Care Med 2001; 29: 503–10
Panacek EA, Marshall J, Fischkoff S, et al. Neutralization of the TNF by a monoclonal antibody improves survival and reduces organ dysfunction in human sepsis: results of the monarcs trial [abstract]. Chest 2000; 118: 885
Cronin L, Cook DJ, Carlet J, et al. Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Moo 1995; 23: 1430–9
Lefering R, Neugebauer EA. Steroid controversy in sepsis and septic shock: a meta-analysis. Crit Care Moo 1995; 23: 1294–303
Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 2002; 288: 862–71
Warren BL, Eid A, Singer P, et al. Caring for the critically ill patient: high-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA 2001; 286: 1869–78
Reuters, MSN Money. Chiron Corporation: key developments [online]. Available from URL: http://news.moneycentral.msn.com/ticker/sigdev.asp?Symbol=ehir [Accessed 2004May 24]
Gyldmark M. A review of costs studies in intensive care units: problems with the cost concept. Crit Care Med 1995; 23: 964–72
Bone RC. Economic analysis of the intensive care unit: a dilemma [editorial]. Crit Care Med 1995; 23: 805
Chalfin DB, Cohen IL, Lambrinos J. The economics and cost-effectiveness of critical care medicine. Intensive Care Med 1995; 21: 952–61
Edbrooke D, Hibbert C, Ridley S, et al. The development of a method for comparative costing of individual intensive care units. The Intensive Care Working Group on Costing. Anaesthesia 1999; 54: 110–20
Edbrooke DL, Stevens VG, Hibbert CL, et al. A new method of accurately identifying costs of individual patients in intensive care: the initial results. Intensive Care Med 1997; 23: 645–50
Elliott D. Costing intensive care services: a review of study methods, results and limitations. Aust Crit Care 1997; 10: 55–63
Noseworthy TW, Konopad E, Shustack A, et al. Cost accounting of adult intensive care: methods and human and capital inputs. Crit Care Med 1996; 24: 1168–72
Singer M, Myers S, Hall G, et al. The cost of intensive care: a comparison on one unit between 1988 and 1991. Intensive Care Med 1994; 20: 542–9
Heyland DK, Konopad E, Noseworthy TW, et al. Is it ‘worthwhile’ to continue treating patients with a prolonged stay (>14 days) in the ICU?: an economic evaluation. Chest 1998; 114: 192–8
Dickie H, Vedio A, Dundas R, et al. Relationship between TISS and ICU cost. Intensive Care Med 1998; 24: 1009–17
Sznajder M, Aegerter P, Launois R, et al. A cost-effectiveness analysis of stays in intensive care units. Intensive Care Med 2001; 27: 146–53
Plaaten H, Kvale R. Cost of intensive care in a Norwegian University hospital 1997–1999. Crit Care 2003; 7: 72–8
Graf J, Graf C, Koch KC, et al. Cost analysis and outcome estimation using the “Therapeutic Intervention Scoring System” (Kostenanalyse und Prognoseabschatzung internistischer Intensivpatienten mittels des “Therapeutic Intervention Scoring System” [fISS und 11SS-28]). Med Klin 2002; 98: 123–32
Moerer O, Schmid A, Hofmann M, et al. Direct costs of severe sepsis in three German intensive care units based on retrospective electronic patient record analysis of resource use. Intensive Care Med 2002; 28: 1440–6
Neilson AR. Moerer O. Burchardi H. Schneider H. A new concept for DRG-based reimbursement of services in German intensive care units: results of a pilot study. Intensive Care Medicine 2004- June; 30 (6): 1220–3
Garcia S, Ruza F, Alvarado F, et al. Analysis of costs in a pediatric ICU. Intensive Care Med 1997; 23: 218–25
de Keizer NF, Bonsel GJ, Al MJ, et al. The relation between TISS and real paediatric ICU costs: a case study with generalizable methodology. Intensive Care Med 1998; 24: 1062–9
Parikh CR, Karnad DR. Quality, cost, and outcome of intensive care in a public hospital in Bombay, India. Crit Care Med 1999; 27: 1754–9
Oye RK, Bellamy PE. Patterns of resource consumption in medical intensive care. Chest 1991; 99: 685–9
Surgenor SD, Corwin HL, Henry SA, et al. The cost of providing intensive care to Diagnosis Related Groups. Clin Intensive Care 2001; 12: 161–7
Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients: excess length of stay, extra costs, and attributable mortality. JAMA 1994; 271: 1598–601
Moerer O, Hein S, Schürgers D, et al. Cost of infections in the ICU: a matched pairs study [abstract]. Intensive Care Med 2000; 26: A453
Rathgeber J, Schillings H, Kersten J, et al. Quality management and individual resource use recording in intensive care using the Göttinger information system for intensive care and surgery (GISI) [Qualitätsmanagerrent und individuelle Leistungserfassung in der Intensivmedizin durch das Göttinger Informationssystem für Intensivmedizin und OP (GISI)]. Anasthesiol Intensivmed Notfallmed Schmerzther 1998; 33: 58–63
Edbrooke DL, Hibbert CL, Kingsley JM, et al. The patient-related costs of care for sepsis patients in a United Kingdom adult general intensive care unit. Crit Care Med 1999; 27: 1760–7
Angus DC, Linde-Zwirbel WT, Clermont G, et al. Cost-effectiveness of drotrecogin alfa (activated) in the treatment of sepsis. Crit Care Med 2003; 31: 1–11
Schwilk B, Wiedeck H, Stein B, et al. Epidemiology of acute renal failure and outcome of haemodiafiltration in intensive care. Intensive Care Med 1997; 23: 1204–11
Korkeila M, Ruokonen E, Takala J. Cost of care, long term prognosis and quality of life in patients requiring renal replacement therapy during intensive care. Intensive Care Med 2000; 26: 1824–31
Scott WG, Scott HM, Henderson S, et al. Cost comparison of antibacterial therapies for serious infections: a New Zealand 3-hospital study. Pharmacoeconomics 1999; 16: 183–92
Martin SJ, Sahloff EG, Close SJ. Evaluation and cost assessment of fluoroquinolones in comminity-acquired respiratory infections. Expert Opin Pharmacother 2002; 3: 1251–66
Letarte J, Longo CJ, Pelletier J, et al. Patient characteristics and costs of severe sepsis and septic shock in Quebec. J Crit Care 2002; 17 (1): 39–49
Lucioni C, Mazzi S, Currado I. Sepsis costs in Italy. Intensive Care Med 2001; 27 Suppl. 2: S 284
Chalfin DB, Holbein EB, Fein AM, et al. Cost-effectiveness of monoclonal antibodies to gram-negative endotoxin in the treatment of gram-negative sepsis in ICU patients. JAMA 1993; 269: 249–54
Hamel MB, Philips RS, Davies RB, et al. Outcomes and costeffectiveness of ventilator support and aggressive care for patients with acute respiratory failure due to pneumonia or adult respiratory distress syndrome. Am J Med 2000; 109: 614–20
Radloff AC, Laubenthal HJ, Bastian A, et al. Comparative investigation of cost-effectiveness of initial treatment of nosocomial pneumonia with Imipenem/Cilastatin [Vergleichende Untersuchung zum Kosten-/EffektivitätsVerhältnis einer initialen Therapie mit Imipenem/Cilastatin bei der nosokomialen Pneumonie]. Anasthesiol Intensivmed Notfallmed Schmerzther 1996; 31: 172–80
Nathwani D. Impact of methicillin-resistant Staphylococcus aureus infection on key economic outcomes: does reducing the length of stay matter? J Antimicrob Chemother 2003; 51 Suppl. 2: 1137–44
Dresser LD, Niederman MS, Paladino JA. Cost-effectiveness of gatifloxacin versus ceftriaxone with a macrolide for the treatment of community-acquired pneumonia. Chest 2001; 119: 1439–48
Norris C, Jacobs P, Rapoport J, et al. ICU and non-ICU cost per day. Can J Anaesth 1995; 42: 192–6
Rapoport J, Teres D, Lerreshow S, et al. A method for assessing the clinical performance and cost-effectiveness of intensive care units: a multicenter inception cohort study. Crit Care Med 1994; 22: 1385–91
Goldfrad C, Rowan K. Consequences of discharges from intensive care at night. Lancet 2000; 355: 1138–42
Keenan SP, Massel D, Inman KJ, et al. A systematic review of the cost-effectiveness of noncardiac transitional care units. Chest 1998; 113: 172–7
Keenan SP, Doig GS, Martin CM, et al. Assessing the efficiency of the admission process to a critical care unit: does the literature allow the use of benchmarking? Intensive Care Med 1997; 23: 574–80
Blackstone ME, Miller RS, Hodgson AJ, et al. Lowering hospital charges in the trauma intensive care unit while maintaining quality of care by increasing resident and attending physician awareness. J Trauma 1995; 39: 1041–4
Kern H, Kox WJ. Impact of standard procedures and clinical standards on cost-effectiveness and intensive care unit performance in adult patients after cardiac surgery. Intensive Care Med 1999; 25: 1367–73
Marx WH, DeMaintenon NL, Mooney KF, et al. Cost reduction and outcome improvement in the intensive care unit. J Trauma 1999; 46: 625–9
Berenholtz S, Pronovost P, Lipsett P, et al. Assessing the effectiveness of critical pathways on reducing resource utilization in the surgical intensive care unit. Intensive Care Med 2001; 27: 1029–36
Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients: excess length of stay, extra costs, and attributable mortality. JAMA 1994; 271: 1598–601
Manthous CA, Amoateng-Adjepong Y, Al Kharrat T, et al. Effects of a medical intensivist on patient care in a community teaching hospital. Mayo Clin Proc 1997; 72: 391–9
Hanson CW, Deutschman CS, Anderson HL, et al. Effects of an organized critical care service on outcomes and resource utilization: a cohort study. Crit Care Med 1999; 27: 270–4
Burchardi H, Moerer O. Twenty-four hour presence of physicians in the ICU. Crit Care 2001; 5: 131–7
Manns BJ, Lee H, Doig CJ, et al. An economic evaluation of activated protein C treatment for severe sepsis. N Engl J Med 2002; 347: 993–1000
Neilson AR, Burchardi H, Chinn C, et al. Cost-effectiveness of drotrecogin alfa (activated) for the treatment of severe sepsis in Germany. J Crit Care 2003; 18 (4): 217–27
Neilson AR, Schnieder H, Finnern HW, et al. Comparing the cost-effectiveness of drotrecogin alfa (activated) in three European countries. Intensive Care Med 2003; 29 (Suppl. 1) S92: abstract 350
Dasta JF, Cooper LM. Impact of drotrecogin alfa (activated) on resource use and implications for reimbursement. Pharmacotherapy 2002; 22: 216S-22S
Vincent JL, Abraham E, Annane D, et al. Reducing mortality in sepsis: new directions. Crit Care 2002; 6 Suppl. 3: Sl-S18
Teres D, Rapoport J, Lemshow S, et al. Effects of severity of illness on resource use by survivors and non-survivors of severe sepsis at intensive care unit admission. Crit Care Med 2002; 30: 2413–9
Rychlik R, Pfeil B. The socio-economic relevance of sepsis in Germany [Soziookonomische Relevanz der Sepsis in Deutschland]. Gesundh Okon Qual Manag 2000; 5: 67–72
Acknowledgements
Heinz Schneider is an employee of HealthEcon AG, Basel Switzerland. Neither of the authors has a financial interest in this review nor have they received any financial support in preparing it.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burchardi, H., Schneider, H. Economic Aspects of Severe Sepsis. PharmacoEconomics 22, 793–813 (2004). https://doi.org/10.2165/00019053-200422120-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-200422120-00003