Abstract
Objective
To analyse the influence of covariates on the apparent clearance (CL) of tacrolimus in paediatric liver transplant recipients being converted from cyclosporin to tacrolimus.
Design
Retrospective modelling study.
Patients and participants
18 children, 13 girls and 5 boys, aged 4 months to 16 years (median 9.1 years) who required conversion to tacrolimus because of acute or chronic rejection or cyclosporin toxicity.
Methods
287 whole-blood tacrolimus concentrations from therapeutic drug monitoring were used to build a nonlinear mixed-effects population model (NON-MEM program) for the apparent clearance of tacrolimus. Variables considered were age, total bodyweight (TBW), body surface area (BSA), time after initiation of treatment (T), gender, haematocrit (Hct), albumin (Alb), aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (γGT), alkaline Phosphatase (ALP), bilirubin (BIL), Creatinine clearance (CLcr) and dosage of concomitant corticosteroids (EST).
Results
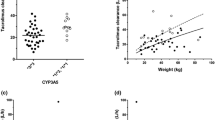
TBW, T, BIL and ALT were the covariates that displayed a significant influence on CL according to the final regression model: CL (L/h) = 10.4(TBW/70)¾ · e-000032 T· e-0.057 BIL. (1- 0.079 ALT). With this model, the estimates of the coefficients of variation were 24.3% and 29.5% for interpatient variability in CL and residual variability, respectively.
Conclusions
The proposed model for tacrolimus CL can be applied for a priori dosage calculations, although the results should be used with caution because of the unexplained variability in the CL. We therefore recommended close monitoring of tacrolimus whole blood concentrations, especially within the first months of treatment. The best use of the model would be its application in dosage adjustment based on therapeutic drug monitoring and the Bayesian approach.






Similar content being viewed by others
References
Cox K, Freese D. Tacrolimus (FK506): the pros and cons of its use as an immunosuppressant in pediatric liver transplantation. Clin Invest Med 1996; 19: 389–92
US Multicenter FK506 Liver Study Group. A comparison of tacrolimus (FK506) and cyclosporin for immunosuppression in liver transplantation. N Engl J Med 1994; 331: 1110–5
European FK506 Multicenter Liver Study Group. Randomized trial comparing tacrolimus (FK506) and cyclosporin in prevention of liver allograft rejection. Lancet 1994; 344: 423–8
McDiarmid SV, Busutil RW, Ascher EL, et al. FK506 (tacrolimus) compared with cyclosporin for primary immunosuppression after pediatrie liver transplantation: results of the US multicenter trial. Transplantation 1995; 59: 530–6
McDiarmid SV. The use of tacrolimus in pediatrie liver transplantation. J Pediatr Gastroenterol Nutr 1998; 26: 90–102
Egawa H, Esquivel CO, So SK, et al. FK506 conversion treatment in pediatrie liver transplantation. Transplantation 1994; 57: 1169–73
Jusko W, Piekoszewski W, Klintmalm G. Pharmacokinetics of tacrolimus in liver transplant patients. Clin Pharmacol Ther 1995; 57: 281–90
Yasuhara M, Hashida T, Toraguchi M. Pharmacokinetics and pharmacodynamics of FK506 in pediatrie patients receiving living-related donor liver transplantations. Transplant Proc 1995; 27: 1108–10
Venkataramanan R, Swaminathan A, Prasad T, et al. Clinical pharmacokinetics of tacrolimus. Clin Pharmacokinet 1995; 29: 404–30
Möller A, Iwasaki K, Kawamura A, et al. The disposition of 14C-labelled tacrolimus after intravenous and oral administration in healthy human subjects. Drug Metab Dispos 1999; 27: 633–6
Haycock GB, Chir B, Schwartz G, et al. Geometric method for measuring body surface: a height-weight formula validated in infants, children and adults. J Pediatr 1978; 93: 62–6
Schwartz G, Haycock GB, Edelmann CM, et al. A simple estimate of glomerular filtration rate in children derived from body weight and plasma creatine. Pediatrics 1976; 58: 259–63
Beal SL, Sheiner LB. Nonmem user’s guide. San Francisco: University of California, 1992
Undre NA, Schafer A, European Tacrolimus Multicentre Renal Study Group. Factors affecting the pharmacokinetics of tacrolimus in the first year after renal transplantation. Transplant Proc 1998; 30: 1261–3
Holford NHG. A size standard for pharmacokinetics. Clin Pharmacokinet 1996; 30: 329–32
West GB, Brown JH, Enquist BJ. The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science 1999; 284: 1677–9
Jusko WJ, Thomson AW, Fung J, et al. Consensus document: therapeutic monitoring of tacrolimus. Ther Drug Monit 1995; 17: 606–14
Peters DH, Fitton A, Plosker GL, et al. Tacrolimus: a review of its pharmacology and therapeutic potential in hepatic and renal transplantation. Drugs 1993; 46: 746–94
Winkler M, Ringe B, Baumann J, et al. Plasma vs whole blood for therapeutic drug monitoring of patients receiving FK506 for immunosuppression. Clin Chem 1994; 40: 2247–53
Wallemacq PE, Furlan V, Moller A, et al. Pharmacokinetics of tacrolimus (FK506) in paediatric liver transplant recipients. Eur J Drug Metab Pharmacokinet 1998; 23: 367–70
Jain AB, Abu-Elmagd K, Abdallah H, et al. Pharmacokinetics of FK506 in liver transplant recipients after continuous intravenous infusion. J Clin Pharmacol 1993; 33: 606–11
Satomura K, Ozaki N, Okajima H, et al. Pharmacokinetics of FK506 in living-related liver transplantation. Transplant Proc 1996; 28: 1005
Gruber SA, Hewitt JM, Sorenson AL, et al. Pharmacokinetics of FK506 after intravenous and oral administration in patients awaiting renal transplantation. J Clin Pharmacol 1994; 34: 859–64
Fitzsimmons WE, Bekersky I, Dressier D, et al. Demographic considerations in tacrolimus pharmacokinetics. Transplant Proc 1998; 30: 1359–64
Boswell GW, Bekersky I, Fay J, et al. Tacrolimus pharmacokinetics in BMT patients. Bone Marrow Transplant 1998; 21: 23–8
Sewing KF. Pharmacokinetics, dosing principles, and blood concentration monitoring of FK506. Transplant Proc 1994; 26: 3267–9
Pou L, Brunet M, Bilbao I, et al. Therapeutic drug monitoring of tacrolimus in liver transplantation, phase III FK506 multicenter Spanish study group: a two-year follow-up. Ther Drug Monit 1998; 20: 602–6
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sánchez, M.J.G., Manzanares, C., Santos-Buelga, D. et al. Covariate Effects on the Apparent Clearance of Tacrolimus in Paediatric Liver Transplant Patients Undergoing Conversion Therapy. Clin Pharmacokinet 40, 63–71 (2001). https://doi.org/10.2165/00003088-200140010-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200140010-00005