Abstract
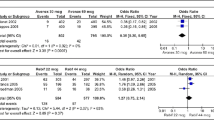
Biopharmaceuticals can induce antibodies, which interact with and neutralize the therapeutic effect of such drugs and are therefore termed neutralizing antibodies (NAbs). In the treatment of multiple sclerosis, NAbs against interferon (IFN)-β and natalizumab have been recognized. The prevalence of NAbs against different IFNβ preparations varies widely, mainly depending on the product but also on other factors such as amino acid sequence variations, glycosylation, formulation, route and frequency of application, dose, duration of treatment and patient characteristics (human leukocyte antigen [HLA] status). IFNβ-1a given intramuscularly induces significantly less NAbs than any other IFNβ formulation. The longitudinal development of NAbs also differs between IFNβ preparations, with higher reversion rates in IFNβ-1b-treated compared with IFNβ-1a-treated patients. The negative effect of NAbs on various outcome measures is very consistent across many studies, specifically when observation periods are longer than 2 years. NAbs against natalizumab occur less frequently (6%) and, like NAbs against IFNβ, they are associated with a loss of clinical and radiological efficacy of the drug.



Similar content being viewed by others
References
Walsh G. Biopharmaceuticals: biochemistry and biotechnology. 2nd ed. Chichester: Wiley & Sons, 2003
Schellekens H. Bioequivalence and the immunogenicity of biopharmaceuticals. Nat Rev Drug Discov 2002; 1: 457–62
Nagata S, Taira H, Hall A, et al. Synthesis in E. coli of a polypeptide with human leukocyte interferon activity. Nature 1980; 284: 316–20
Borden EC, Rinehart JJ, Storer BE, et al. Biological and clinical effects of interferon-beta ser at two doses. J Interferon Res 1990; 10: 559–70
Larocca AP, Leung SC, Marcus SG, et al. Evaluation of neutralizing antibodies in patients treated with recombinant interferon-beta ser. J Interferon Res 1989; 9Suppl. 1: S51–60
Grunberg SM, Kempf RA, Venturi CL, et al. Phase I study of recombinant beta-interferon given by four-hour infusion. Cancer Res 1987; 47: 1174–8
IFNB multiple sclerosis study group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis: I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 1993; 43: 655–61
IFNB multiple sclerosis study group, University of British Columbia MS/MRI analysis group. Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. Neurology 1995; 45: 1277–85
PRISMS. Randomised double-blind placebo-controlled study of interferon β-1a in relapsing/remitting multiple sclerosis. Lancet 1998; 352: 1498–504
Jacobs LD, Cookfair DL, Rudick RA, et al. A phase III trial of intramuscular recombinant interferon beta as treatment for exacerbating-remitting multiple sclerosis: design and conduct of study and baseline characteristics of patients. Multiple Sclerosis Collaborative Research Group (MSCRG). Mult Scler 1995; 1: 118–35
Jacobs LD, Beck RW, Simon JH, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. N Engl J Med 2000; 343: 898–904
Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006; 354: 899–910
Stuve O, Marra CM, Jerome KR, et al. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol 2006; 59: 743–7
Stuve O, Marra CM, Bar-Or A, et al. Altered CD4+/CD8+ T-cell ratios in cerebrospinal fluid of natalizumab-treated patients with multiple sclerosis. Arch Neurol 2006; 63: 1383–7
Calabresi PA, Giovannoni G, Confavreux C, et al. The incidence and significance of anti-natalizumab antibodies: results from AFFIRM and SENTINEL. Neurology 2007; 69: 1391–403
Casadevall N, Nataf J, Viron B, et al. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N Engl J Med 2002; 346: 469–75
Deisenhammer F, Schellekens H, Bertolotto A. Measurement of neutralizing antibodies to interferon beta in patients with multiple sclerosis. J Neurol 2004; 251Suppl. 2: II31–9
The IFNB Multiple Sclerosis Study Group, The University of British Columbia MS/MRI Analysis Group. Neutralizing antibodies during treatment of multiple sclerosis with interferon beta-1b: experience during the first three years. Neurology 1996; 47: 889–94
Bertolotto A, Deisenhammer F, Gallo P, et al. Immunogenicity of interferon beta: differences among products. J Neurol 2004; 251Suppl. 2:II15–24
Ross C, Clemmesen KM, Svenson M, et al. Immunogenicity of interferon-beta in multiple sclerosis patients: influence of preparation, dosage, dose frequency, and route of administration. Danish Multiple Sclerosis Study Group. Ann Neurol 2000; 48: 706–12
Kappos L, Clanet M, Sandberg-Wollheim M, et al. Neutralizing antibodies and efficacy of interferon beta-1a: a 4-year controlled study. Neurology 2005; 65: 40–7
Giovannoni G, Barbarash O, Casset-Semanaz F, et al. Immunogenicity and tolerability of an investigational formulation of interferon-beta1a: 24- and 48-week interim analyses of a 2-year, single-arm, historically controlled,phase IIIb study in adults with multiple sclerosis. Clin Ther 2007; 29: 1128–45
Petkau AJ, White RA, Ebers GC, et al. Longitudinal analyses of the effects of neutralizing antibodies on interferon beta-1b in relapsing-remitting multiple sclerosis. Mult Scler 2004; 10: 126–38
Polman C, Kappos L, White R, et al. Neutralizing antibodies during treatment of secondary progressive MS with interferon beta-1b. Neurology 2003; 60: 37–43
Panitch H, Miller A, Paty D, et al. Interferon beta-1b in secondary progressive MS: results from a 3-year controlled study. Neurology 2004; 63: 1788–95
Kappos L, Freedman MS, Polman CH, et al. Effect of early versus delayed interferon beta-1b treatment on disability after a first clinical event suggestive of multiple sclerosis: a 3-year follow-up analysis of the BENEFIT study. Lancet 2007; 370: 389–97
Frank JA, Richert N, Bash C, et al. Interferon-beta-1b slows progression of atrophy in RRMS: three-year follow-up in NAb− and NAb+ patients. Neurology 2004; 62: 719–25
Francis GS, Rice GP, Alsop JC. Interferon beta-1a in MS: results following development of neutralizing antibodies in PRISMS. Neurology 2005; 65: 48–55
Secondary Progressive Efficacy Clinical Trial of Recombinant Interferon beta 1a in MS (SPECTRIMS) Study Group. Randomized controlled trial of interferon-beta-1a in secondary progressive MS clinical results. Neurology 2001; 56: 1496–504
Clanet M, Radue EW, Kappos L, et al. A randomized, double-blind, dose-comparison study of weekly interferon beta-1a in relapsing MS. Neurology 2002; 59: 1507–17
Durelli L, Verdun E, Barbero P, et al. Independent Comparison of Interferon (INCOMIN) Trial Study Group. Every-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis: results of a 2-year prospective randomised multicentre study (INCOMIN). Lancet 2002; 359: 1453–60
Panitch H, Goodin DS, Francis G, et al. Randomized, comparative study of interferon beta-1a treatment regimens in MS: the EVIDENCE Trial. Neurology 2002; 59: 1496–506
Malucchi S, Sala A, Gilli F, et al. Neutralizing antibodies reduce the efficacy of taIFN during treatment of multiple sclerosis. Neurology 2004; 62: 2031–7
Perini P, Calabrese M, Biasi G, et al. The clinical impact of interferon beta antibodies in relapsing-remitting MS. J Neurol 2004; 251: 305–9
Sorensen PS, Koch-Henriksen N, Ross C, et al. Appearance and disappearance of neutralizing antibodies during interferon-beta therapy. Neurology 2005; 65: 33–9
Gneiss C, Tripp P, Reichartseder F, et al. Differing immunogenic potentials of interferon beta preparations in multiple sclerosis patients. Mult Scler 2006; 12: 731–7
Sominanda A, Rot U, Suoniemi M, et al. Interferon beta preparations for the treatment of multiple sclerosis patients differ in neutralizing antibody seroprevalence and immunogenicity. Mult Scler 2007; 13: 208–14
Farrell R, Kapoor R, Leary S, et al. Neutralizing antiinterferon beta antibodies are associated with reduced side effects and delayed impact on efficacy of interferon-beta. Mult Scler 2008; 14(2): 212–8
Malucchi S, Gilli F, Caldano M, et al. Predictive markers for response to interferon therapy in patients with multiple sclerosis. Neurology 2008; 70 (13 Pt 2): 1119–27
Rudick RA, Simonian NA, Alam JA, et al. Incidence and significance of neutralizing antibodies to interferon beta-1a in multiple sclerosis. Multiple Sclerosis Collaborative Research Group (MSCRG). Neurology 1998; 50: 1266–72
Li DK, Zhao GJ, Paty DW. Randomized controlled trial of interferon-beta-1a in secondary progressive MS: MRI results. Neurology 2001; 56: 1505–13
European Study Group on Interferon. Placebo-controlled multicentre randomised trial of interferon β-1b in treatment of secondary progressive multiple sclerosis. Lancet 1998; 352: 1491–7
Leary SM, Thompson AJ. Interferon beta-1a in primary progressive multiple sclerosis. J Neurol Sci 2003; 206: 215–6
Rice GPA, Paszner B, Oger J, et al. The evolution of neutralizing antibodies in multiple sclerosis patients treated with interferon β-1b. Neurology 1999; 52: 1277–9
Gneiss C, Reindl M, Lutterotti A, et al. Interferon-beta: the neutralizing antibody (NAb) titre predicts reversion to NAb negativity. Mult Scler 2004; 10: 507–10
Bellomi F, Scagnolari C, Tomassini V, et al. Fate of neutralizing and binding antibodies to IFN beta in MS patients treated with IFN beta for 6 years. J Neurol Sci 2003; 215: 3–8
Gneiss C, Reindl M, Berger T, et al. Epitope specificity of neutralizing antibodies against IFN-beta. J Interferon Cytokine Res 2004; 24: 283–90
Gneiss C, Tripp P, Ehling R, et al. Interferon-beta antibodies have a higher affinity in patients with neutralizing antibodies compared to patients with non-neutralizing antibodies. J Neuroimmunol 2006; 174: 174–9
Rio J, Nos C, Tintore M, et al. Assessment of different treatment failure criteria in a cohort of relapsing-remitting multiple sclerosis patients treated with interferon beta: implications for clinical trials. Ann Neurol 2002; 52: 400–6
Vollmer T. The natural history of relapses in multiple sclerosis. J Neurol Sci 2007; 256Suppl. 1: S5–13
Goodin DS. Disease-modifying therapy in multiple sclerosis: update and clinical implications. Neurology 2008; 71: S8–13
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001; 50: 121–7
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–52
Rudick RA, Cutter G, Reingold S. The multiple sclerosis functional composite: a new clinical outcome measure for multiple sclerosis trials. Mult Scler 2002; 8: 359–65
Confavreux C, Vukusic S, Moreau T, et al. Relapses and progression of disability in multiple sclerosis. N Engl J Med 2000; 343: 1430–8
Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG) [published erratum appears in Ann Neurol 1996 Sep; 40 (3): 480]. Ann Neurol 1996; 39: 285–94
Noseworthy JH. Clinical scoring methods for multiple sclerosis. Ann Neurol 1994; 36 Suppl.: S80–5
Li DKB, Paty DW,UBC MS/MRI Analysis Research Group, et al. Magnetic resonance imaging results of the PRISMS trial: a randomized, double-blind, placebo-controlled study of interferon-β1a in relapsing-remitting multiple sclerosis. Ann Neurol 1999; 46: 197–206
Paty DW, Li DK,UBC MS/MRI Study Group, et al. Interferon beta-1b is effective in relapsing-remittingmultiple sclerosis: II. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 1993; 43: 662–7
Miller DH, Soon D, Fernando KT, et al. MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS. Neurology 2007; 68: 1390–401
Goodin DS. Magnetic resonance imaging as a surrogate outcome measure of disability in multiple sclerosis: have we been overly harsh in our assessment? Ann Neurol 2006; 59: 597–605
Khoury SJ, Guttmann CR, Orav EJ, et al. Longitudinal MRI in multiple sclerosis: correlation between disability and lesion burden. Neurology 1994; 44: 2120–4
Filippi M, Paty DW, Kappos L, et al. Correlations between changes in disability and T2-weighted brain MRI activity in multiple sclerosis: a follow-up study. Neurology 1995; 45: 255–60
Molyneux PD, Barker GJ, Barkhof F, et al. Clinical-MRI correlations in a European trial of interferon beta-1b in secondary progressive MS. Neurology 2001; 57: 2191–7
McFarland HF, Barkhof F, Antel J, et al. The role of MRI as a surrogate outcome measure in multiple sclerosis. Mult Scler 2002; 8: 40–51
Ransohoff RM. Cellular responses to interferons and other cytokines: the JAK-STAT paradigm. N Engl J Med 1998; 338: 616–8
Williams BR. Transcriptional regulation of interferon-stimulated genes. Eur J Biochem 1991; 200: 1–11
Rani MR, Ransohoff RM. Alternative and accessory pathways in the regulation of IFN-beta-mediated gene expression. J Interferon Cytokine Res 2005; 25: 788–98
Deisenhammer F, Reindl M, Harvey J, et al. Bioavailability of interferon beta 1b in MS patients with and without neutralizing antibodies. Neurology 1999; 52: 1239–43
Cook SD, Quinless JR, Jotkowitz A, et al. Serum IFN neutralizing antibodies and neopterin levels in a cross-section of MS patients. Neurology 2001; 57: 1080–4
Vallittu AM, Halminen M, Peltoniemi J, et al. Neutralizing antibodies reduce MxA protein induction in interferon-beta-1a-treated MS patients. Neurology 2002; 58: 1786–90
Pachner AR, Narayan K, Price N, et al. MxA gene expression analysis as an interferon-beta bioactivity measurement in patients with multiple sclerosis and the identification of antibody-mediated decreased bioactivity. Mol Diagn 2004; 7: 17–25
Bertolotto A, Gilli F, Sala A, et al. Persistent neutralizing antibodies abolish the interferon beta bioavailability in MS patients. Neurology 2003; 60: 634–9
Gilli F, Bertolotto A, Sala A, et al. Neutralizing antibodies against IFN-beta in multiple sclerosis: antagonization of IFN-beta mediated suppression of MMPs. Brain 2004; 127: 259–68
Sorensen PS, Tscherning T, Mathiesen HK, et al. Neutralizing antibodies hamper IFNbeta bioactivity and treatment effect on MRI in patients with MS. Neurology 2006; 67: 1681–3
Gilli F, Marnetto F, Caldano M, et al. Biological markers of interferon-beta therapy: comparison among interferon-stimulated genes MxA, TRAIL and XAF-1. Mult Scler 2006; 12: 47–57
Pachner AR, Narayan K, Pak E. Multiplex analysis of expression of three IFNbeta-induced genes in antibody-positive MS patients. Neurology 2006; 66: 444–6
Santos R, Weinstock-Guttman B, Tamano-Blanco M, et al. Dynamics of interferon-beta modulated mRNA biomarkers in multiple sclerosis patients with anti-interferon-beta neutralizing antibodies. J Neuroimmunol 2006; 176: 125–33
Scagnolari C, Duda P, Bagnato F, et al. Pharmacodynamics of interferon beta in multiple sclerosis patients with or without serum neutralizing antibodies. J Neurol 2007; 254: 597–604
Sominanda A, Hillert J, Fogdell-Hahn A. In vivo bioactivity of interferon-beta in multiple sclerosis patients with neutralising antibodies is titre-dependent. J Neurol Neurosurg Psychiatry 2008; 79: 57–62
Kawade Y. An analysis of neutralization reaction of interferon by antibody: a proposal on the expression of neutralization titer. J Interferon Res 1980; 1: 61–70
Salmon P, Le Cotonnec JY, Galazka A, et al. Pharmaco-kinetics and pharmacodynamics of recombinant human interferon-beta in healthy male volunteers. J Interferon Cytokine Res 1996; 16: 759–64
Giovannoni G. Neutralizing antibodies during treatment of secondary progressive MS with interferon beta-1b. Neurology 2003; 61: 1025
Sorensen PS, Ross C, Clemmesen KM, et al. Clinical importance of neutralising antibodies against interferon beta in patients with relapsing-remitting multiple sclerosis. Lancet 2003; 362: 1184–91
Boz C, Oger J, Gibbs E, et al. Reduced effectiveness of long-term interferon-beta treatment on relapses in neutralizing antibody-positive multiple sclerosis patients: a Canadian multiple sclerosis clinic-based study. Mult Scler 2007; 13: 1127–37
Sorensen PS, Deisenhammer F, Duda P, et al. Guidelines on use of anti-IFN-beta antibody measurements in multiple sclerosis: report of an EFNS Task Force on IFN-beta antibodies in multiple sclerosis. Eur J Neurol 2005; 12: 817–27
Cohen JA, Cutter GR, Fischer JS, et al. Benefit of interferon beta-1a on MSFC progression in secondary progressive MS. Neurology 2002; 59: 679–87
Kracke A, Von Wussow P, Al-Masri AN, et al. Mx proteins in blood leukocytes for monitoring interferon beta-1b therapy in patients with MS. Neurology 2000; 54: 193–9
Giovannoni G, Lai M, Kidd D, et al. Daily urinary neopterin excretion as an immunological marker of disease activity in multiple sclerosis. Brain 1997; 120: 1–13
Deisenhammer F, Mayringer I, Harvey J, et al. A comparative study of the relative bioavailability of different interferon beta preparations. Neurology 2000; 54: 2055–60
Baumhackl U, Eibl G, Ganzinger U, et al. Prevalence of multiple sclerosis in Austria results of a nationwide survey. Neuroepidemiology 2002; 21: 226–34
Khan OA, Dhib-Jalbut SS. Neutralizing antibodies to interferon beta-1a and interferon beta-1b in MS patients are cross-reactive. Neurology 1998; 51: 1698–702
Wiendl H, Toyka KV, Rieckmann P, et al. Basic and escalating immunomodulatory treatments in multiple sclerosis: current therapeutic recommendations. Multiple Sclerosis Therapy Consensus Group (MSTCG). J Neurol 2008; 255: 1449–63
Goodin DS, Frohman EM, Hurwitz B, et al. Neutralizing antibodies to interferon beta: assessment of their clinical and radiographic impact. An evidence report: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2007; 68: 977–84
Teitelbaum D, Brenner T, Abramsky O, et al. Antibodies to glatiramer acetate do not interfere with its biological functions and therapeutic efficacy. Mult Scler 2003; 9: 592–9
Farina C, Vargas V, Heydari N, et al. Treatment with glatiramer acetate induces specific IgG4 antibodies in multiple sclerosis patients. J Neuroimmunol 2002; 123: 188–92
Salama HH, Hong J, Zang YC, et al. Blocking effects of serum reactive antibodies induced by glatiramer acetate treatment in multiple sclerosis. Brain 2003; 126: 2638–47
Acknowledgements
I am extremely grateful to all my excellent co-workers in the Neuroimmunology Laboratory, Innsbruck Medical University, especially the chief technician Ingrid Gstrein, who helped to start the NAb work in the first place, as well as Dagmar Rudzki and Martina Hölzl, who currently perform the NAb tests.
No sources of funding were used to assist in the preparation of this review. Dr Deisenhammer has participated in meetings sponsored by or received honoraria for acting as an advisor for Biogen Idec, Merck Serono, Bayer and Sanofi. His institution has received financial support for participation in randomized controlled trials of INFβ-1b (Betaferon,® Bayer Schering Pharma), INFβ-1a (Avonex,® Biogen Idec; Rebif,® Merck Serono), glatiramer acetate (Copaxone,® Teva Pharmaceuticals) and natalizumab (Tysabri,® Biogen Idec) in multiple sclerosis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deisenhammer, F. Neutralizing Antibodies to Interferon-β and other Immunological Treatments for Multiple Sclerosis. CNS Drugs 23, 379–396 (2009). https://doi.org/10.2165/00023210-200923050-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00023210-200923050-00003