Abstract
The development of antidrug antibodies (ADAs) is a major problem in several recombinant protein therapies used in the treatment of multiple sclerosis (MS). The etiology of ADAs is multifaceted. The predisposition for a breakdown of immune tolerance is probably genetically determined, and many factors may contribute to the immunogenicity, including structural properties, formation of aggregates, and presence of contaminants and impurities from the industrial manufacturing process. ADAs may have a neutralizing capacity and can reduce or abrogate the bioactivity and therapeutic efficacy of the drug and cause safety issues. Interferon (IFN)-β was the first drug approved for the treatment of MS, and—although it is generally recognized that neutralizing antibodies (NAbs) appear and potentially have a negative effect on therapeutic efficacy—the use of routine measurements of NAbs and the interpretation of the presence of NAbs has been debated at length. NAbs appear after 9–18 months of therapy in up to 40% of patients treated with IFNβ, and the frequency and titers of NAbs depend on the IFNβ preparation. Although all pivotal clinical trials of approved IFNβ products in MS exhibited a detrimental effect of NAbs after prolonged therapy, some subsequent studies did not observe clinical effects from NAbs, which led to the claim that NAbs did not matter. However, it is now largely agreed that persistently high titers of NAbs indicate an abrogation of the biological response and, hence, an absence of therapeutic efficacy, and this observation should lead to a change of therapy. Low and medium titers are ambiguous, and treatment decisions should be guided by determination of in vivo messenger RNA myxovirus resistance protein A induction after IFNβ administration and clinical disease activity. During treatment with glatiramer acetate, ADAs occur frequently but do not appear to adversely affect treatment efficacy or result in adverse events. ADAs occur in approximately 5% of patients treated with natalizumab within 6 months of therapy, and persistent NAbs are associated with a lack of efficacy and acute infusion-related reactions and should instigate a change of therapy. When using the anti-CD20 monoclonal antibodies ocrelizumab and ofatumumab in the treatment of MS, it is not necessary to test for NAbs as these occur very infrequently. Alemtuzumab is immunogenic, but routine measurements of ADAs are not recommended as the antibodies in the pivotal 2-year trials at the population level did not influence lymphocyte depletion or repopulation, efficacy, or safety. However, in some individuals, NAbs led to poor lymphocyte depletion.

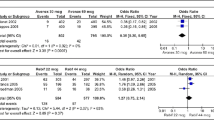
Reproduced from Sorensen et al. [5] with permission

Reproduced from Sorensen [53] with permission. MRI magnetic resonance imaging

Reproduced from Sorensen [53] with permission. neg negative, pos positive

Reproduced from Sorensen [125] with permission. MS multiple sclerosis

Reproduced from Jensen et al. [152] with permission
Similar content being viewed by others
Change history
25 July 2022
A Correction to this paper has been published: https://doi.org/10.1007/s40263-022-00942-0
References
Schellekens H, Casadevall N. Immunogenicity of recombinant human proteins: causes and consequences. J Neurol. 2004;251(Suppl 2):II4–9.
Schellekens H. Bioequivalence and the immunogenicity of biopharmaceuticals. Nat Rev Drug Discov. 2002;1(6):457–62.
Cohen BA, Oger J, Gagnon A, Giovannoni G. The implications of immunogenicity for protein-based multiple sclerosis therapies. J Neurol Sci. 2008;275(1–2):7–17.
Sorensen PS, Ross C, Clemmesen KM, Bendtzen K, Frederiksen JL, Jensen K, et al. Clinical importance of neutralising antibodies against interferon beta in patients with relapsing-remitting multiple sclerosis. Lancet. 2003;362(9391):1184–91.
Sorensen PS. Safety concerns and risk management of multiple sclerosis therapies. Acta Neurol Scand. 2017;136(3):168–86.
Sorensen PS, Koch-Henriksen N, Ross C, Clemmesen KM, Bendtzen K. Appearance and disappearance of neutralizing antibodies during interferon-beta therapy. Neurology. 2005;65(1):33–9.
Sauerborn M, Brinks V, Jiskoot W, Schellekens H. Immunological mechanism underlying the immune response to recombinant human protein therapeutics. Trends Pharmacol Sci. 2010;31(2):53–9.
Ross C, Clemmesen KM, Svenson M, Sorensen PS, Koch-Henriksen N, Skovgaard GL, et al. Immunogenicity of interferon-beta in multiple sclerosis patients: influence of preparation, dosage, dose frequency, and route of administration. Danish Multiple Sclerosis Study Group. Ann Neurol. 2000;48(5):706–12.
Hoffmann S, Cepok S, Grummel V, Lehmann-Horn K, Hackermuller J, Stadler PF, et al. HLA-DRB1*0401 and HLA-DRB1*0408 are strongly associated with the development of antibodies against interferon-beta therapy in multiple sclerosis. AmJ Hum Genet. 2008;83(2):219–27.
Andlauer TFM, Link J, Martin D, Ryner M, Hermanrud C, Grummel V, et al. Treatment- and population-specific genetic risk factors for anti-drug antibodies against interferon-beta: a GWAS. BMC Med. 2020;18(1):298.
Hassler S, Bachelet D, Duhaze J, Szely N, Gleizes A, Hacein-Bey Abina S, et al. Clinicogenomic factors of biotherapy immunogenicity in autoimmune disease: a prospective multicohort study of the ABIRISK consortium. PLoS Med. 2020;17(10): e1003348.
McKay F, Schibeci S, Heard R, Stewart G, Booth D. Analysis of neutralizing antibodies to therapeutic interferon-beta in multiple sclerosis patients: a comparison of three methods in a large Australasian cohort. J Immunol Methods. 2006;310(1–2):20–9.
Sorensen PS, Lisby S, Grove R, Derosier F, Shackelford S, Havrdova E, et al. Safety and efficacy of ofatumumab in relapsing-remitting multiple sclerosis: a phase II study. Neurology. 2014;2014(82):573–81.
Jensen PE, Sellebjerg F, Sondergaard HB, Sorensen PS. Correlation between anti-interferon-beta binding and neutralizing antibodies in interferon-beta-treated multiple sclerosis patients. Eur J Neurol. 2012;19:1311–7.
Sorensen PS, Ross C, Bendtzen K, Koch-Henriksen N. Neutralising antibodies against interferon beta in multiple sclerosis. Lancet. 2004;363(9403):168–9.
Farrell R, Kapoor R, Leary SM, Rudge P, Thompson AJ, Miller DH, et al. Neutralizing anti-interferon beta antibodies are associated with reduced side effects and delayed impact on efficacy of Interferon-beta. Mult Scler. 2008;14(2):212–8.
Calabresi PA, Giovannoni G, Confavreux C, Galetta SL, Havrdova E, Hutchinson M, et al. The incidence and significance of anti-natalizumab antibodies: results from AFFIRM and SENTINEL. Neurology. 2007;69(14):1391–403.
Duquette P, Girard M, Despault L, Dubois R, Knobler RL, Lublin FD, et al. Interferon beta-1B is effective in relapsing-remitting multiple-sclerosis-clinical-results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43(4):655–61.
Bertolotto A, Malucchi S, Milano E, Castello A, Capobianco M, Mutani R. Interferon beta neutralizing antibodies in multiple sclerosis: neutralizing activity and cross-reactivity with three different preparations. Immunopharmacology. 2000;48(2):95–100.
Khan OA, Dhib-Jalbut SS. Neutralizing antibodies to interferon beta-1a and interferon beta-1b in MS patients are cross-reactive. Neurology. 1998;51(6):1698–702.
Enevold C, Oturai AB, Sorensen PS, Ryder LP, Koch-Henriksen N, Bendtzen K. Polymorphisms of innate pattern recognition receptors, response to interferon-beta and development of neutralizing antibodies in multiple sclerosis patients. Mult Scler. 2010;16(8):942–9.
Buck D, Cepok S, Hoffmann S, Grummel V, Jochim A, Berthele A, et al. Influence of the HLA-DRB1 genotype on antibody development to interferon beta in multiple sclerosis. Arch Neurol. 2011;68(4):480–7.
Buck D, Andlauer TF, Igl W, Wicklein EM, Muhlau M, Weber F, et al. Effect of HLA-DRB1 alleles and genetic variants on the development of neutralizing antibodies to interferon beta in the BEYOND and BENEFIT trials. Mult Scler. 2019;25(4):565–73.
Waddington KE, Papadaki A, Coelewij L, Adriani M, Nytrova P, Kubala Havrdova E, et al. Using serum metabolomics to predict development of anti-drug antibodies in multiple sclerosis patients treated with IFNbeta. Front Immunol. 2020;11:1527.
Hedstrom AK, Ryner M, Fink K, Fogdell-Hahn A, Alfredsson L, Olsson T, et al. Smoking and risk of treatment-induced neutralizing antibodies to interferon beta-1a. Mult Scler. 2014;20(4):445–50.
Perini P, Calabrese M, Biasi G, Gallo P. The clinical impact of interferon beta antibodies in relapsing-remitting MS. J Neurol. 2004;251(3):305–9.
Kremenchutzky M. Long-term evolution of anti-INFbeta antibodies in IFNbeta-treated MS patients: the London, Canada, MS Clinic experience. Neurology. 2003;61(9 Suppl 5):S29–30.
Pachner A, Narayan K, Price N, Hurd M, Dail D. MxA gene expression analysis as an interferon-beta bioactivity measurement in patients with multiple sclerosis and the identification of antibody-mediated decreased bioactivity. Mol Diagn. 2003;7(1):17–25.
Mayr M, Berek K, Deisenhammer F. Evolution of interferon-beta binding antibodies in MS patients may predict development of neutralizing antibodies. Eur J Neurol. 2003;10(4):462–4.
Slavikova M, Schmeisser H, Kontsekova E, Mateicka F, Borecky L, Kontsek P. Incidence of autoantibodies against type I and type II interferons in a cohort of systemic lupus erythematosus patients in Slovakia. J Interferon Cytokine Res. 2003;23(3):143–7.
Monzani F, Meucci G, Caraccio N, Saviozzi M, Casolaro A, Moscato G, et al. Discordant effect of IFN-beta1a therapy on anti-IFN antibodies and thyroid disease development in patients with multiple sclerosis. J Interferon Cytokine Res. 2002;22(7):773–81.
Antonelli G, Simeoni E, Bagnato F, Pozzilli C, Turriziani O, Tesoro R, et al. Further study on the specificity and incidence of neutralizing antibodies to interferon (IFN) in relapsing remitting multiple sclerosis patients treated with IFN beta-1a or IFN beta-1b. J Neurol Sci. 1999;168(2):131–6.
Pungor E Jr, Files JG, Gabe JD, Do LT, Foley WP, Gray JL, et al. A novel bioassay for the determination of neutralizing antibodies to IFN-beta1b. J Interferon Cytokine Res. 1998;18(12):1025–30.
Kivisakk P, Alm GV, Fredrikson S, Link H. Neutralizing and binding anti-interferon-beta (IFN-beta) antibodies. A comparison between IFN-beta-1a and IFN-beta-1b treatment in multiple sclerosis. Eur J Neurol. 2000;7(1):27–34.
Deisenhammer F, Reindl M, Harvey J, Gasse T, Dilitz E, Berger T. Bioavailability of interferon beta 1b in MS patients with and without neutralizing antibodies. Neurology. 1999;52(6):1239–43.
Lawrence N, Oger J, Aziz T, Palace J, Vincent A. A sensitive radioimmunoprecipitation assay for assessing the clinical relevance of antibodies to IFN beta. J Neurol Neurosurg Psychiatry. 2003;74(9):1236–9.
Zang YC, Yang D, Hong J, Tejada-Simon MV, Rivera VM, Zhang JZ. Immunoregulation and blocking antibodies induced by interferon beta treatment in MS. Neurology. 2000;55(3):397–404.
Kageshita T, Yamamoto A, Yamazaki N, Ishihara K, Ono T. Low frequency of neutralizing antibodies against natural interferon-beta during adjuvant therapy for Japanese patients with melanoma. J Dermatol Sci. 1999;19(3):208–12.
Rudick RA, Simonian NA, Alam JA, Campion M, Scaramucci JO, Jones W, et al. Incidence and significance of neutralizing antibodies to interferon beta-1a in multiple sclerosis. Multiple Sclerosis Collaborative Research Group (MSCRG). Neurology. 1998;50(5):1266–72.
Abdul Ahad AK, Galazka AR, Revel M, Biffoni M, Borden EC. Incidence of antibodies to interferon-beta in patients treated with recombinant human interferon-beta 1a from mammalian cells. Cytokines Cell MolTher. 1997;3(1):27–32.
Fierlbeck G, Schreiner T, Schaber B, Walser A, Rassner G. Neutralizing interferon beta antibodies in melanoma patients treated with recombinant and natural interferon beta. Cancer Immunol Immunother. 1994;39(4):263–8.
Prummer O, Bunjes D, Wiesneth M, Hertenstein B, Arnold R, Porzsolt F, et al. Antibodies to interferon-alpha: a novel type of autoantibody occurring after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1996;17(4):617–23.
Dummer R, Muller W, Nestle F, Wiede J, Dues J, Lechner W, et al. Formation of neutralizing antibodies against natural interferon-beta, but not against recombinant interferon-gamma during adjuvant therapy for high-risk malignant melanoma patients. Cancer. 1991;67(9):2300–4.
Redlich PN, Grossberg SE. Analysis of antigenic domains on natural and recombinant human IFN-beta by the inhibition of biologic activities with monoclonal antibodies. J Immunol. 1989;143(6):1887–93.
Larocca AP, Leung SC, Marcus SG, Colby CB, Borden EC. Evaluation of neutralizing antibodies in patients treated with recombinant interferon-beta ser. J Interferon Res. 1989;9(Suppl 1):S51–60.
Kob M, Harvey J, Schautzer F, Kascha S, Bibl D, Egg R, et al. A novel and rapid assay for the detection of neutralizing antibodies against interferon-beta. Mult Scler. 2003;9(1):32–5.
Bertolotto A, Gilli F, Sala A, Capobianco M, Malucchi S, Milano E, et al. Persistent neutralizing antibodies abolish the interferon beta bioavailability in MS patients. Neurology. 2003;60(4):634–9.
Polman C, Kappos L, White R, Dahlke F, Beckmann K, Pozzilli C, et al. Neutralizing antibodies during treatment of secondary progressive MS with interferon beta-1b. Neurology. 2003;60(1):37–43.
Vallittu AM, Eralinna JP, Ilonen J, Salmi AA, Waris M. MxA protein assay for optimal monitoring of IFN-beta bioactivity in the treatment of MS patients. Acta Neurol Scand. 2008;118:12–7.
Grossberg SE, Kawade Y, Grossberg LD. The neutralization of interferons by antibody III. The constant antibody bioassay, a highly sensitive quantitative detector of low antibody levels. J Interferon Cytokine Res. 2009;29:93–104.
Grossberg SE, Kawade Y, Kohase M, Klein JP. The neutralization of interferons by antibody. II. Neutralizing antibody unitage and its relationship to bioassay sensitivity: the tenfold reduction unit. J Interferon Cytokine Res. 2001;21(9):743–55.
Herndon RM, Rudick RA, Munschauer FE III, Mass MK, Salazar AM, Coats ME, et al. Eight-year immunogenicity and safety of interferon beta-1a-Avonex treatment in patients with multiple sclerosis. Mult Scler. 2005;11(4):409–19.
Sorensen PS. Neutralizing antibodies against interferon-Beta. Ther Adv Neurol Disord. 2008;1(2):62–78.
Link J, Ramanujam R, Auer M, Ryner M, Hassler S, Bachelet D, et al. Clinical practice of analysis of anti-drug antibodies against interferon beta and natalizumab in multiple sclerosis patients in Europe: a descriptive study of test results. PLoS ONE. 2017;12(2): e0170395.
Lam R, Farrell R, Aziz T, Gibbs E, Giovannoni G, Grossberg SE, et al. Validating parameters of a luciferase reporter gene assay to measure neutralizing antibodies to IFN[beta] in multiple sclerosis patients. J Immunol Methods. 2008;336(2):113–8.
Farrell R, Espasandin M, Lakdawala N, Creeke P, Worthington V, Giovannoni G. Incorporation of an interferon-beta neutralizing antibody assay into routine clinical practice. Mult Scler. 2011;17(11):1333–40.
Farrell RA, Giovannoni G. Measuring and management of anti-interferon beta antibodies in subjects with multiple sclerosis. Mult Scler. 2007;13(5):567–77.
Sominanda A, Lundkvist M, Fogdell-Hahn A, Hemmer B, Hartung HP, Hillert J, et al. Inhibition of endogenous interferon beta by neutralizing antibodies against recombinant interferon beta. Arch Neurol. 2010;67(9):1095–101.
Hermanrud C, Ryner M, Luft T, Jensen PE, Ingenhoven K, Rat D, et al. Development and validation of cell-based luciferase reporter gene assays for measuring neutralizing anti-drug antibodies against interferon beta. J Immunol Methods. 2016;430:1–9.
Bellomi F, Bramanti P, Trojano M, Scagnolari C, Muto A, Sessa E, et al. Neutralizing and binding antibodies to interferon beta in patients with multiple sclerosis: a comparison of assay results from three italian centres. J Immunoassay Immunochem. 2009;30(1):40–50.
Hesse D, Sellebjerg F, Sorensen PS. Absence of MxA induction by interferon beta in patients with MS reflects complete loss of bioactivity. Neurology. 2009;73(5):372–7.
Deisenhammer F, Reindl M, Berger T. Immunoglobulin subclasses in patients with neutralizing and nonneutralizing antibodies against IFN-beta1b. J Interferon Cytokine Res. 2001;21(3):167–71.
Ross C, Svenson M, Clemmesen KM, Sorensen PS, Koch-Henriksen N, Bendtzen K. Measuring and evaluating interferon-beta-induced antibodies in patients with multiple sclerosis. Mult Scler. 2006;2006(12):39–46.
Gneiss C, Brugger M, Millonig A, Fogdell-Hahn A, Rudzki D, Hillert J, et al. Comparative study of four different assays for the detection of binding antibodies against interferon-{beta}. Mult Scler. 2008;14(6):830–6.
Gneiss C, Tripp P, Reichartseder F, Egg R, Ehling R, Lutterotti A, et al. Differing immunogenic potentials of interferon beta preparations in multiple sclerosis patients. Mult Scler. 2006;12(6):731–7.
Hegen H, Millonig A, Bertolotto A, Comabella M, Giovanonni G, Guger M, et al. Early detection of neutralizing antibodies to interferon-beta in multiple sclerosis patients: binding antibodies predict neutralizing antibody development. Mult Scler. 2014;20(5):577–87.
Gneiss C, Tripp P, Ehling R, Khalil M, Lutterotti A, Egg R, et al. Interferon-beta antibodies have a higher affinity in patients with neutralizing antibodies compared to patients with non-neutralizing antibodies. J Neuroimmunol. 2006;174(1–2):174–9.
Placebo-controlled multicentre randomised trial of interferon beta-1b in treatment of secondary progressive multiple sclerosis. European Study Group on interferon beta-1b in secondary progressive MS. Lancet. 1998;352(9139):1491–7.
Jacobs LD, Beck RW, Simon JH, Kinkel RP, Brownscheidle CM, Murray TJ, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N Engl J Med. 2000;343(13):898–904.
PRISMS Study Group. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet. 1998;352(9139):1498–504.
SPECTRIMS. Randomized controlled trial of interferon-beta-1a in secondary progressive MS: clinical results. Neurology. 2001;56(11):1496–504.
Giovannoni G, Barbarash O, Casset-Semanaz F, King J, Metz L, Pardo G, et al. Safety and immunogenicity of a new formulation of interferon {beta}-1a (Rebif(R) New Formulation) in a Phase IIIb study in patients with relapsing multiple sclerosis: 96-week results. Mult Scler. 2008;15(2):219–28.
Clanet M, Radue EW, Kappos L, Hartung HP, Hohlfeld R, Sandberg-Wollheim M, et al. A randomized, double-blind, dose-comparison study of weekly interferon beta-1a in relapsing MS. Neurology. 2002;59(10):1507–17.
Mikol DD, Barkhof F, Chang P, Coyle PK, Jeffery DR, Schwid SR, et al. Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs Glatiramer Acetate in Relapsing MS Disease [REGARD] study): a multicentre, randomised, parallel, open-label trial. Lancet Neurol. 2008;7(10):903–14.
Panitch H, Miller A, Paty D, Weinshenker B. Interferon beta-1b in secondary progressive MS: results from a 3-year controlled study. Neurology. 2004;63(10):1788–95.
Panitch H, Goodin DS, Francis G, Chang P, Coyle PK, O’Connor P, et al. Randomized, comparative study of interferon beta-1a treatment regimens in MS: the EVIDENCE trial. Neurology. 2002;59(10):1496–506.
Durelli L, Verdun E, Barbero P, Bergui M, Versino E, Ghezzi A, et al. Every-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis: results of a 2-year prospective randomised multicentre study (INCOMIN). Lancet. 2002;359(9316):1453–60.
Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, Richert JR, Salazar AM, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol. 1996;39(3):285–94.
Minagara A, Murray TJ. Efficacy and tolerability of intramuscular interferon beta-1a compared with subcutaneous interferon beta-1a in relapsing MS: results from PROOF. Curr Med Res Opin. 2008;24(4):1049–55.
Prince HE, Lape-Nixon M, Audette C, Van HK. Identification of interferon-beta antibodies in a reference laboratory setting: findings for 1144 consecutive sera. J Neuroimmunol. 2007;190(1–2):165–9.
Sominanda A, Rot U, Suoniemi M, Deisenhammer F, Hillert J, Fogdell-Hahn A. Interferon beta preparations for the treatment of multiple sclerosis patients differ in neutralizing antibody seroprevalence and immunogenicity. Mult Scler. 2007;13(2):208–14.
Perini P, Facchinetti A, Bulian P, Massaro AR, Pascalis DD, Bertolotto A, et al. Interferon-beta (INF-beta) antibodies in interferon-beta1a- and interferon-beta1b-treated multiple sclerosis patients. Prevalence, kinetics, cross-reactivity, and factors enhancing interferon-beta immunogenicity in vivo. Eur Cytokine Netw. 2001;12(1):56–61.
Magyari M, Koch-Henriksen N, Laursen B, Sorensen PS. Gender effects on treatment response to interferon-beta in multiple sclerosis. Acta Neurol Scand. 2014;130(6):374–9.
Hegen H, Auer M, Deisenhammer F. Pharmacokinetic considerations in the treatment of multiple sclerosis with interferon-beta. Expert Opin Drug Metab Toxicol. 2015;11(12):1803–19.
Nafissi S, Azimi A, Amini-Harandi A, Salami S, Shahkarami MA, Heshmat R. Comparing efficacy and side effects of a weekly intramuscular biogeneric/biosimilar interferon beta-1a with Avonex in relapsing remitting multiple sclerosis: a double blind randomized clinical trial. Clin Neurol Neurosurg. 2012;114(7):986–9.
Shokrollahi Barough M, Ashtari F, Sadat Akhavi M, Asghari N, Mosayebi G, Mirmohammadkhani M, et al. Neutralizing antibody production against Rebif(R) and ReciGen(R) in relapsing-remitting multiple sclerosis (RRMS) patients and its association with patient’s disability. Int Immunopharmacol. 2018;62:109–13.
Govindappa K, Sathish J, Park K, Kirkham J, Pirmohamed M. Development of interferon beta-neutralising antibodies in multiple sclerosis–a systematic review and meta-analysis. Eur J Clin Pharmacol. 2015;71(11):1287–98.
Bachelet D, Hassler S, Mbogning C, Link J, Ryner M, Ramanujam R, et al. Occurrence of anti-drug antibodies against interferon-beta and natalizumab in multiple sclerosis: a collaborative cohort analysis. PLoS ONE. 2016;11(11): e0162752.
Kalluri SR, Grummel V, Hracsko Z, Pongratz V, Pernpeintner V, Gasperi C, et al. Interferon-beta specific T cells are associated with the development of neutralizing antibodies in interferon-beta treated multiple sclerosis patients. J Autoimmun. 2018;88:83–90.
Pozzilli C, Antonini G, Bagnato F, Mainero C, Tomassini V, Onesti E, et al. Monthly corticosteroids decrease neutralizing antibodies to IFNbeta1 b: a randomized trial in multiple sclerosis. J Neurol. 2002;249(1):50–6.
Fernandez O, Guerrero M, Mayorga C, Munoz L, Lean A, Luque G, et al. Combination therapy with interferon beta-1b and azathioprine in secondary progressive multiple sclerosis. A two-year pilot study. J Neurol. 2002;249(8):1058–62.
Calabresi PA, Wilterdink JL, Rogg JM, Mills P, Webb A, Whartenby KA. An open-label trial of combination therapy with interferon beta-1a and oral methotrexate in MS. Neurology. 2002;58(2):314–7.
Durelli L, Ricci A. Anti-interferon antibodies in multiple sclerosis. Molecular basis and their impact on clinical efficacy. Front Biosci. 2004;9:2192–204.
Rice GP, Paszner B, Oger J, Lesaux J, Paty D, Ebers G. The evolution of neutralizing antibodies in multiple sclerosis patients treated with interferon beta-1b. Neurology. 1999;52(6):1277–9.
Hartung HP, Freedman MS, Polman CH, Edan G, Kappos L, Miller DH, et al. Interferon {beta}-1b-neutralizing antibodies 5 years after clinically isolated syndrome. Neurology. 2011;77(9):835–43.
Dujmovic I, Hegen H, Paz P, Croze E, Deisenhammer F. Persistency of neutralizing anti-interferon-beta antibodies in patients with multiple sclerosis treated with subcutaneous interferon-beta depends on antibody titers, IgG subclasses, and affinity maturation. J Interferon Cytokine Res. 2017;37(7):317–24.
Hegen H, Schleiser M, Gneiss C, Dipauli F, Ehling R, Kuenz B, et al. Persistency of neutralizing antibodies depends on titer and interferon-beta preparation. Mult Scler. 2012;18:610–5.
Durelli L, Barbero P, Cucci A, Ferrero B, Ricci A, Contessa G, et al. Neutralizing antibodies in multiple sclerosis patients treated with 375 micrograms interferon-beta-1b. Expert Opin Biol Ther. 2009;9(4):387–97.
Petersen B, Bendtzen K, Koch-Henriksen N, Ravnborg M, Ross C, Sorensen PS. Persistence of neutralizing antibodies after discontinuation of IFNbeta therapy in patients with relapsing-remitting multiple sclerosis. Mult Scler. 2006;12(3):247–52.
Reske D, Walser A, Haupt WF, Petereit HF. Long-term persisting interferon beta-1b neutralizing antibodies after discontinuation of treatment. Acta Neurol Scand. 2004;109(1):66–70.
van der Voort LF, Gilli F, Bertolotto A, Knol DL, Uitdehaag BM, Polman CH, et al. Clinical effect of neutralizing antibodies to interferon beta that persist long after cessation of therapy for multiple sclerosis. Arch Neurol. 2010;67(4):402–7.
Shapiro AM, Jack CS, LaPierre Y, Arbour N, Bar-Or A, Antel JP. Potential for interferon beta-induced serum antibodies in multiple sclerosis to inhibit endogenous interferon-regulated chemokine/cytokine responses within the central nervous system. Arch Neurol. 2006;63(9):1296–9.
Dunn N, Fogdell-Hahn A, Hillert J, Spelman T. Long-term consequences of high titer neutralizing antibodies to interferon-beta in multiple sclerosis. Front Immunol. 2020;11: 583560.
Hesse D, Frederiksen JL, Koch-Henriksen N, Schreiber K, Stenager E, Heltberg A, et al. Methylprednisolone does not restore biological response in multiple sclerosis patients with neutralizing antibodies against interferon-beta. Eur J Neurol. 2009;16(1):43–7.
Ravnborg M, Bendtzen K, Christensen O, Jensen P, Hesse D, Tovey M, et al. Treatment with azathioprine and cyclic methylprednisolone has little or no effect on bioactivity in anti-interferon beta antibody-positive patients with multiple sclerosis. Mult Scler. 2009;15(3):323–8.
Millonig A, Rudzki D, Holzl M, Ehling R, Gneiss C, Kunz B, et al. High-dose intravenous interferon beta in patients with neutralizing antibodies (HINABS): a pilot study. Mult Scler. 2009;15(8):977–83.
Marta M, Baker D, Creeke P, Pryce G, Gnanapavan S, Giovannoni G. Antigen-specific tolerization in human autoimmunity: inhibition of interferon-beta1a anti-drug antibodies in multiple sclerosis: a case report. Mult Scler Relat Disord. 2021;1(56): 103284.
Duquette P, Girard M, Dubois R, Kobler RL, Lublin F, Kelley L, et al. Neutralizing antibodies during treatment of multiple sclerosis with interferon beta-1b: Experience during the first three years. Neurology. 1996;47(4):889–94.
Pachner AR, Bertolotto A, Deisenhammer F. Measurement of MxA mRNA or protein as a biomarker of IFNbeta bioactivity: detection of antibody-mediated decreased bioactivity (ADB). Neurology. 2003;61(9 Suppl 5):S24–6.
Pachner AR, Dail D, Pak E, Narayan K. The importance of measuring IFNbeta bioactivity: monitoring in MS patients and the effect of anti-IFNbeta antibodies. J Neuroimmunol. 2005;166(1–2):180–8.
Deisenhammer F, Mayringer I, Harvey J, Dilitz E, Gasse T, Stadlbauer D, et al. A comparative study of the relative bioavailability of different interferon beta preparations. Neurology. 2000;54(11):2055–60.
Sellebjerg F, Datta P, Larsen J, Rieneck K, Alsing I, Oturai A, et al. Gene expression analysis of interferon-{beta} treatment in multiple sclerosis. Mult Scler. 2008;14(5):615–21.
Sorensen PS, Tscherning T, Mathiesen HK, Langkilde AR, Ross C, Ravnborg M, et al. Neutralizing antibodies hamper IFNbeta bioactivity and treatment effect on MRI in patients with MS. Neurology. 2006;67(9):1681–3.
Wandinger KP, Lunemann JD, Wengert O, Bellmann-Strobl J, Aktas O, Weber A, et al. TNF-related apoptosis inducing ligand (TRAIL) as a potential response marker for interferon-beta treatment in multiple sclerosis. Lancet. 2003;361(9374):2036–43.
Sellebjerg F, Krakauer M, Hesse D, Ryder LP, Alsing I, Jensen PE, et al. Identification of new sensitive biomarkers for the in vivo response to interferon-beta treatment in multiple sclerosis using DNA-array evaluation. Eur J Neurol. 2009;16:1291–8.
Pachner AR, Warth JD, Pace A, Goelz S. Effect of neutralizing antibodies on biomarker responses to interferon beta: the INSIGHT study. Neurology. 2009;73(18):1493–500.
Francis GS, Rice GP, Alsop JC. Interferon beta-1a in MS: results following development of neutralizing antibodies in PRISMS. Neurology. 2005;65(1):48–55.
Kappos L, Clanet M, Sandberg-Wollheim M, Radue EW, Hartung HP, Hohlfeld R, et al. Neutralizing antibodies and efficacy of interferon beta-1a: a 4-year controlled study. Neurology. 2005;65(1):40–7.
Paolicelli D, D’Onghia M, Pellegrini F, Direnzo V, Iaffaldano P, Lavolpe V, et al. The impact of neutralizing antibodies on the risk of disease worsening in interferon beta-treated relapsing multiple sclerosis: a 5 year post-marketing study. J Neurol. 2013;260(6):1562–8.
Malucchi S, Sala A, Gilli F, Bottero R, Di Sapio A, Capobianco M, et al. Neutralizing antibodies reduce the efficacy of betaIFN during treatment of multiple sclerosis. Neurology. 2004;62(11):2031–7.
Pachner AR, Cadavid D, Wolansky L, Skurnick J. Effect of anti-IFN{beta} antibodies on MRI lesions of MS patients in the BECOME study. Neurology. 2009;73(18):1485–92.
Goodin DS, Hurwitz B, Noronha A. Neutralizing antibodies to interferon beta-1b are not associated with disease worsening in multiple sclerosis. J Int Med Res. 2007;35(2):173–87.
Goodin DS, Hartung HP, O’Connor P, Filippi M, Arnason B, Comi G, et al. Neutralizing antibodies to interferon beta-1b multiple sclerosis: a clinico-radiographic paradox in the BEYOND trial. Mult Scler. 2012;18(2):181–95.
Sorensen PS. Effects of neutralizing antibodies to interferon beta in multiple sclerosis: a logical paradox. Mult Scler. 2012;18(2):131–2.
Sorensen PS. Neutralising antibodies to interferon-beta-measurement, clinical relevance, and management. J Neurol. 2006;253(Suppl 6):vi16–vi22.
Sorensen PS, Koch-Henriksen N, Flachs E, Bendtzen K. Is the treatment effect of IFN-{beta} restored after the disappearance of neutralizing antibodies? Mult Scler. 2008;14(6):837–42.
Hesse D, Sorensen PS. Using measurements of neutralizing antibodies: the challenge of IFN-beta therapy. Eur J Neurol. 2007;14(8):850–9.
Sominanda A, Hillert J, Fogdell-Hahn A. In vivo bioactivity of interferon-beta in multiple sclerosis patients with neutralising antibodies is titre-dependent. J Neurol Neurosurg Psychiatry. 2008;79(1):57–62.
Sorensen PS, Koch-Henriksen N, Bendtzen K. Are ex vivo neutralising antibodies against IFN-{beta} always detrimental to therapeutic efficacy in multiple sclerosis? Mult Scler. 2007;13(5):616–21.
Group PS. PRISMS-4: Long-term efficacy of interferon-beta-1a in relapsing MS. Neurology. 2001;56(12):1628–36.
Sorensen PS. Management of patients with neutralizing antibodies against interferon-beta: stop IFN-beta therapy or wait for the antibodies to go away? Eur J Neurol. 2009;16(1):1–2.
Sørensen PS, Deisenhammer F, Duda P, Hohlfeld R, Myhr KM, Palace J, Polman C, Pozzilli C, Ross C; EFNS Task Force on Anti-IFN-beta Antibodies in Multiple Sclerosis. Guidelines on use of anti-interferon-beta antibody measurements in multiple sclerosis—report of an EFNS Task Force on IFN-beta antibodies in multiple sclerosis. Eur J Neurol. 2005;12(11):817–27.
Goodin DS, Frohman EM, Hurwitz B, O’Connor PW, Oger JJ, Reder AT, et al. Neutralizing antibodies to interferon beta: assessment of their clinical and radiographic impact: an evidence report: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2007;68(13):977–84.
Sorensen PS, Bertolotto A. Re: neutralizing antibodies to interferon beta: assessment of their clinical and radiographic impact: an evidence report: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2007;69(15):1552.
Polman CH, Bertolotto A, Deisenhammer F, Giovannoni G, Hartung HP, Hemmer B, et al. Recommendations for clinical use of data on neutralising antibodies to interferon-beta therapy in multiple sclerosis. Lancet Neurol. 2010;9(7):740–50.
Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group [see comments]. Neurology. 1995;1995(45):1268–76.
Brenner T, Arnon R, Sela M, Abramsky O, Meiner Z, Riven-Kreitman R, et al. Humoral and cellular immune responses to Copolymer 1 in multiple sclerosis patients treated with Copaxone. J Neuroimmunol. 2001;115(1–2):152–60.
Salama HH, Hong J, Zang YC, El Mongui A, Zhang J. Blocking effects of serum reactive antibodies induced by glatiramer acetate treatment in multiple sclerosis. Brain. 2003;126(12):2638–47.
Teitelbaum D, Brenner T, Abramsky O, Aharoni R, Sela M, Arnon R. Antibodies to glatiramer acetate do not interfere with its biological functions and therapeutic efficacy. Mult Scler. 2003;9(6):592–9.
Basile E, Gibbs E, Aziz T, Oger J. During 3 years treatment of primary progressive multiple sclerosis with glatiramer acetate, specific antibodies switch from IgG1 to IgG4. J Neuroimmunol. 2006;177(1–2):161–6.
Karussis D, Teitelbaum D, Sicsic C, Brenner T. Long-term treatment of multiple sclerosis with glatiramer acetate: natural history of the subtypes of anti-glatiramer acetate antibodies and their correlation with clinical efficacy. J Neuroimmunol. 2010;220(1–2):125–30.
Avila S, Guerrero-Garcia JJ, Becerril-Villanueva E, Perez-Sanchez G, Pavon L, Rojas-Mayorquin AE, et al. A differential sex-specific pattern of IgG2 and IgG4 subclasses of anti-drug antibodies (ADAs) induced by glatiramer acetate in relapsing-remitting multiple sclerosis patients. Mult Scler Relat Disord. 2019;34:92–9.
Selmaj K, Barkhof F, Belova AN, Wolf C, van den Tweel ER, Oberye JJ, et al. Switching from branded to generic glatiramer acetate: 15-month GATE trial extension results. Mult Scler. 2017;23(14):1909–17.
Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910.
Rudick RA, Stuart WH, Calabresi PA, Confavreux C, Galetta SL, Radue EW, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):911–23.
Sorensen PS, Hyldgaard Jensen PE, Haghikia A, Lundkvist M, Vedeler C, Sellebjerg F, et al. Occurrence of antibodies against natalizumab in relapsing multiple sclerosis patients treated with natalizumab. Mult Scler. 2011;17(9):1074–8.
Sangalli F, Moiola L, Bucello S, Annovazzi P, Rizzo A, Radaelli M, et al. Efficacy and tolerability of natalizumab in relapsing-remitting multiple sclerosis patients: a post-marketing observational study. Neurol Sci. 2010;31(Suppl 3):299–302.
Putzki N, Yaldizli O, Buhler R, Schwegler G, Curtius D, Tettenborn B. Natalizumab reduces clinical and MRI activity in multiple sclerosis patients with high disease activity: results from a multicenter study in Switzerland. Eur Neurol. 2010;63(2):101–6.
Hedstrom AK, Alfredsson L, Lundkvist Ryner M, Fogdell-Hahn A, Hillert J, Olsson T. Smokers run increased risk of developing anti-natalizumab antibodies. Mult Scler. 2014;20(8):1081–5.
Sorensen PS, Koch-Henriksen N, Jensen PE. Neutralizing antibodies against interferon-{beta} do not predispose antibodies against natalizumab. Neurology. 2011;76(8):759–60.
Trojano M, Ramio-Torrenta L, Grimaldi LM, Lubetzki C, Schippling S, Evans KC, et al. A randomized study of natalizumab dosing regimens for relapsing-remitting multiple sclerosis. Mult Scler. 2021;27(14):2240–53.
Jensen PE, Koch-Henriksen N, Sellebjerg F, Sorensen PS. Prediction of antibody persistency from antibody titres to natalizumab. Mult Scler. 2012;18(10):1493–9.
Hellwig K, Schimrigk S, Fischer M, Haghikia A, Muller T, Chan A, et al. Allergic and nonallergic delayed infusion reactions during natalizumab therapy. Arch Neurol. 2008;65(5):656–8.
Phillips JT, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. Infusion-related hypersensitivity reactions during natalizumab treatment. Neurology. 2006;67(9):1717–8.
Killestein J, Jasperse B, Liedorp M, Seewann A, Polman C. Very late delayed-allergic reaction to natalizumab not associated with neutralizing antibodies. Mult Scler. 2009;15(4):525–6.
Krumbholz M, Pellkofer H, Gold R, Hoffmann LA, Hohlfeld R, Kumpfel T. Delayed allergic reaction to natalizumab associated with early formation of neutralizing antibodies. Arch Neurol. 2007;64(9):1331–3.
Blinkenberg M, Soelberg SP. Monoclonal antibodies for relapsing multiple sclerosis: a review of recently marketed and late-stage agents. CNS Drugs. 2017;31(5):357–71.
Salzer J, Svenningsson R, Alping P, Novakova L, Bjorck A, Fink K, et al. Rituximab in multiple sclerosis: A retrospective observational study on safety and efficacy. Neurology. 2016;87(20):2074–81.
Faustini F, Dunn N, Kharlamova N, Ryner M, Bruchfeld A, Malmstrom V, et al. First exposure to rituximab is associated to high rate of anti-drug antibodies in systemic lupus erythematosus but not in ANCA-associated vasculitis. Arthritis Res Ther. 2021;23(1):211.
Strand V, Balsa A, Al-Saleh J, Barile-Fabris L, Horiuchi T, Takeuchi T, et al. Immunogenicity of biologics in chronic inflammatory diseases: a systematic review. BioDrugs. 2017;31(4):299–316.
Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676–88.
Bar-Or A, Calabresi PA, Arnlod D, Markowitz C, Shafer S, Kasper LH, et al. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol. 2008;63(3):395–400.
Hawker K, O’Connor P, Freedman MS, Calabresi PA, Antel J, Simon J, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66(4):460–71.
Dunn N, Juto A, Ryner M, Manouchehrinia A, Piccoli L, Fink K, et al. Rituximab in multiple sclerosis: frequency and clinical relevance of anti-drug antibodies. Mult Scler. 2018;24(9):1224–33.
Sorensen PS, Blinkenberg M. The potential role for ocrelizumab in the treatment of multiple sclerosis: current evidence and future prospects. Ther Adv Neurol Disord. 2016;9(1):44–52.
Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221–34.
Kappos L, Li D, Calabresi PA, O’Connor P, Bar-Or A, Barkhof F, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet. 2011;378(9805):1779–87.
Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209–20.
Hauser SL, Kappos L, Montalban X, Craveiro L, Chognot C, Hughes R, et al. Safety of ocrelizumab in patients with relapsing and primary progressive multiple sclerosis. Neurology. 2021;97(16):e1546–59.
Casertano S, Signoriello E, Rossi F, Di Pietro A, Tuccillo F, Bonavita S, et al. Ocrelizumab in a case of refractory chronic inflammatory demyelinating polyneuropathy with anti-rituximab antibodies. Eur J Neurol. 2020;27(12):2673–5.
Hauser SL, Bar-Or A, Cohen JA, Comi G, Correale J, Coyle PK, et al. Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med. 2020;383(6):546–57.
Bar-Or A, Grove RA, Austin DJ, Tolson JM, VanMeter SA, Lewis EW, et al. Subcutaneous ofatumumab in patients with relapsing-remitting multiple sclerosis: the MIRROR study. Neurology. 2018;90(20):e1805–14.
Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1829–39.
Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1819–28.
Coles AJ, Compston DA, Selmaj KW, Lake SL, Moran S, Margolin DH, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008;359(17):1786–801.
Riechmann L, Clark M, Waldmann H, Winter G. Reshaping human antibodies for therapy. Nature. 1988;332(6162):323–7.
Baker D, Herrod SS, Alvarez-Gonzalez C, Giovannoni G, Schmierer K. Interpreting lymphocyte reconstitution data from the pivotal phase 3 trials of alemtuzumab. JAMA Neurol. 2017;74(8):961–9.
Baker D, Ali L, Saxena G, Pryce G, Jones M, Schmierer K, et al. The irony of humanization: alemtuzumab, the first, but one of the most immunogenic, humanized monoclonal antibodies. Front Immunol. 2020;11:124.
Dubuisson N, Baker D, Kang AS, Pryce G, Marta M, Visser LH, et al. Alemtuzumab depletion failure can occur in multiple sclerosis. Immunology. 2018;154(2):253–60.
Saxena G, Moore JM, Jones M, Pryce G, Ali L, Leisegang GR, et al. Detecting and predicting neutralization of alemtuzumab responses in MS. Neurol Neuroimmunol Neuroinflamm. 2020;7(4):e767.
Holgate RG, Weldon R, Jones TD, Baker MP. Characterisation of a novel anti-CD52 antibody with improved efficacy and reduced immunogenicity. PLoS ONE. 2015;10(9): e0138123.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received.
Conflict of interest
Per Soelberg Sorensen has received personal compensation for serving on scientific advisory boards, steering committees, and independent data monitoring committees and has received speaker honoraria from Biogen, Merck, Novartis, TEVA, and Celgene/BMS.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All references are indexed in PubMed.
Code availability
Not applicable.
Author contributions
The author was the sole contributor to this review article and agrees to be accountable for the work.
Additional information
The original online version of this article was revised: Ofatumumab is a fully human anti-CD20 monoclonal antibody and the B-cell-depleting drug most recently approved for the treatment of RRMS. It is administered subcutaneously at a dose of 20 mg every 4 weeks.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sorensen, P.S. Antidrug Antibodies Against Biological Treatments for Multiple Sclerosis. CNS Drugs 36, 569–589 (2022). https://doi.org/10.1007/s40263-022-00920-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-022-00920-6