Abstract
The pathophysiology of sepsis involves activation of acid sphingomyelinase (SMPD1) with subsequent generation of the bioactive mediator ceramide. We herein evaluate the hypothesis that the enzyme exerts biological effects in endothelial stress response. Plasma-secreted sphingomyelinase activity, ceramide generation and lipid raft formation were measured in human microcirculatory endothelial cells (HMEC-1) stimulated with serum obtained from sepsis patients. Clustering of receptors relevant for signal transduction was studied by immunostaining. The role of SMPD1 for macrodomain formation was tested by pharmacological inhibition. To confirm the involvement of the stress enzyme, direct inhibitors (amino bisphosphonates) and specific downregulation of the gene was tested with respect to ADAMTS13 expression and cytotoxicity. Plasma activity and amount of SMPD1 were increased in septic patients dependent on clinical severity. Increased breakdown of sphingomyelin to ceramide in HMECs was observed following stimulation with serum from sepsis patients in vitro. Hydrolysis of sphingomyelin, clustering of receptor complexes, such as the CD95L/Fas-receptor, as well as formation of ceramide enriched macrodomains were abrogated using functional inhibitors (desipramine and NB6). Strikingly, the stimulation of HMECs with serum obtained from sepsis patients or mixture of proinflammatory cytokines resulted in cytotoxicity and ADAMTS13 downregulation which was abrogated using desipramine, amino bisphosphonates and genetic inhibitors. SMPD1 is involved in the dysregulation of ceramide metabolism in endothelial cells leading to macrodomain formation, cytotoxicity and downregulation of ADAMTS13 expression. Functional inhibitors, such as desipramine, are capable of improving endothelial stress response during sepsis and might be considered as a pharmacological treatment strategy to obtain a favorable outcome.
Similar content being viewed by others
Introduction
Sepsis is characterized by an overwhelming host response and development of remote organ failure (1). Despite the development and clinical testing of numerous novel therapeutic compounds, sepsis/septic shock is still accompanied by high mortality rates, reaching up to 50% of patients in intensive care units worldwide (2,3). Through a wide variety of causes, generalized activation of the endothelium leads to a shift to a proinflammatory phenotype. Once an inflammatory response has been instigated by endogenous mediators, such as cytokines, or components of the bacterial cell wall (4,5), a large number of host-derived mediators are capable to further activate endothelial cells. The proinflammatory phenotype results in promotion of endothelial dysfunction, leukocyte trafficking and a prothrombotic state which potentially may lead to thrombotic microangiopathy (TMA), remote organ dysfunction and death (6–8).
SMPD1 activation and rapid ceramide generation have been demonstrated in response to various stressors (9,10) including ligation of death receptors, viral, bacterial and parasitic pathogens and proinflammatory cytokines (11–14). Subsequent to receptor-mediated activation, translocation of SMPD1 to the cell membrane conduces the enzyme in proximity to its substrate onto the anticytosolic leaflet. Since the product, the lipid mediator ceramide consisting of a sphingosine base linked to a fatty acid of different length, exhibits the tendency for spontaneous self-association, the formation of ceramide-enriched macrodomains serves the reorganization and clustering of receptor molecules, such as CD95 (15) and Toll-like receptor 4 (16), all of which are involved in cellular stress response. It has been demonstrated recently that oxidized phospholipids cause plasma membrane nanoplatform disintegration under stress conditions, an effect apparently dependent on acid sphingomyelinase mediated ceramide generation (17). Furthermore, studies reveal that short chain ceramides are capable of functioning as second messengers and modify signal transduction pathways (18). An altered biological function, including sphingomyelin-breakdown and ceramidegeneration, may play an important role in systemic inflammatory response syndrome (SIRS) and sepsis. In sepsis, both enhanced monocytic ceramide content and threshold levels of SMPD1 activity help to predict outcome (19–21). Further evidence suggests a beneficial role of using functional inhibitors of acid sphingomyelinase (FIASMA) in systemic inflammation, which reduced inflammatory response and improved outcome in an endotoxemia mice model (20,22). FIASMA are a group of compounds including Food and Drug Administration (FDA) approved and heavily prescribed antidepressant drugs, such as desipramine, which due to their weak basic properties accumulate in the lysosomes and lead to the degradation of acid sphingomyelinase (23). Pharmacological inhibition by desipramine was proven beneficial to the outcome in Staphylococcus aureus infection (24), however, the role and mechanisms of SMPD1 activation during endothelial stress response due to sepsis remain unclear. Amino bisphosphonates are promising candidates for direct inhibition (25).
In the present study, we used an in vitro model of endothelial stress response to evaluate whether serum from septic patients is capable of modulating ceramide-generation as well as whether this phenomenon is sensitive to desipramine, an FDA approved drug belonging to the group of FIASMA (23). Furthermore, we studied ceramide-enriched macrodomain formation and clustering of receptor complexes. The significance of SMPD1 on regulation of a typical stress regulated transcript, ADAMTS13, that is, A Disintegrin-like And Metalloprotease with ThromboSpondin-type-1-Motif 13 (26), was evaluated using pharmacological inhibition as well as a genetic knockdown model.
Materials and Methods
SMPD1-Assay
Activity of plasma secreted sphingomyelinase was determined by hydrolysis of Nitrobenzoxadiazolylamino (NBD)-labeled sphingomyelin as exogenous substrate in citrated plasma to yield a fluorescently active ceramide derivate for densitometric analysis, as reported previously (20,27,28).
SMPD1-immunoprecipitation and Western blotting of the enzyme were precipitated from diluted plasma with polyclonal anti-SMPD1 antibodies against the N-terminal epitope (H-181) conjugated to Agarose-beads (sc-11352, Santa Cruz Biotechnology Inc., N-17, sc-9816) (29). After washing and electrophoretic separation, proteins were transferred to polyvinylidene difluoride (PVDF) membranes. After blocking (4% skimmed milk) and incubation with primary antibody against a C-terminus epitope (A-19, sc-9815), membranes were washed, incubated with HRP-conjugated secondary antibody and luminescence was visualized.
Cell Culture Experiments
Human microcirculatory endothelial cells (HMEC-1) were cultured under standard conditions in Dulbecco’s Modified Eagle Medium (DMEM) media, 5% CO2, 10% heat inactivates fetal calf serum and 1% penicillin/streptomycin. Stimulation of HMECs were performed with 5% pooled serum of at least six patients with sepsis, proinflammatory cytokines (TNFα, IFNγ and IL-1β [10 ng/mL] each combined with 100 ng/mL of endotoxin, as described previously) (30) or isolated human placenta sphingomyelinase (Sigma Aldrich). For SMPD1 inhibition strategy, HMECs were co-incubated with desipramine (Sigma Aldrich), amino bisphosphonate (Cayman Chemical), NB6 (31) or with siRNA (5nM) targeting SMPD1 performed using Lipofectamin-RNAiMAX reagent according to the manufacturer’s instructions (Life Technologies). siRNA_SMPD1 sense 5′- GGUUA CAUCG CAUAG UGCCd TdT-3′; antisense 5′-GGCAC UAUGC GAUGU AACCd TdG- 3′
Intracellular SM-turnover
Semi-confluent HMECs were washed and labeled with 1.5 mL NBD-SM/BSA complex (4 nmol C6-NBD-Sphingomyelin (Invitrogen) in MeOH) prepared with defatted bovine serum albumin (32). Monolayers were washed, pre-incubated with inhibitors as indicated and exposed to sera from healthy individuals or patients. After washing with phosphate buffered saline solution (PBS), cellular lipids were extracted (33), the organic phase evaporated in vacuo, dissolved in MeOH, separated by thin layer chromatography and quantitated as described previously (20).
Quantitation of Ceramide
Following lipid extraction with MeOH containing C8-ceramide as internal standard, total phosphate content was determined according to Ames and Dubin (34) and data are given as molcer/molPh. For quantification of ceramide isoforms, polar phospholipids were removed by adsorption chromatography. After evaporation and hydrolysis of diacylglycerol, ceramides were labeled with 7-(Diethylamino)-coumarin-3-carbonylazide. Ceramides were separated in LiChrospher-100 RP-18 HPLC-column with stepwise gradient elution and detected at Ex/Em 385/475 nm in a Shimadzu RF-535 monitor. Identification was performed using corresponding standards (35).
Real-time Polymerase Chain Reaction (PCR)
Stimulated HMECs were homogenized in 600 µL lysis buffer (RLT lysis puffer, supplemented with 1% β-mercaptoethanol). RNA was isolated using RNeasy Mini Kit according to the manufacturer’s instructions (Qiagen). RNA concentration and integrity were analyzed using Nanodrop spectrophotometer and QIAxcel microcapillar electrophoresis apparatus and was followed by reverse transcription of 1.0 µg RNA using standard conditions (Thermo Scientific) (36). The expression profile of selected mRNA was measured using the Rotor-Gene Q 2plex system. The following primer sequences were used: GAPDH forw. 5′ CTCTG CTCCT CCTGT TCGAC 3′; rev. 5′ CAATA CGACC AAATC CGTTG AC 3′; ADAMTS13 forw. 5′ CCCAA CCTGA CCAGT GTCTA CA 3′; rev. 5′ CTTCC CAGCC ACGAC ATAGC 3′
Confocal Microscopy
A total number of 5 × 105 cells in a 2-cm poly-L-lysine-coated confocal plate were treated and fixed. After blocking, primary antibodies were incubated and visualized with labeled secondary antibody. Images were acquired using a Zeiss LSM 510meta. For quantitative analysis of ceramide-generation we determined intensity profiles of ceramide-immuno-reactive epitopes using the Zeiss LSM image examiner software. The assay conditions were validated by the detection of both SMPD1 (unconjugated anti-human SMPD1 polyclonal primary antibody, H-181, sc-11352 (29)), and ceramide (unconjugated anti-ceramide monoclonal primary antibody, MID 15B4, Alexis Corp.) (37) immune reactivity after stimulation of endothelial cells with CD95/TNFSF6 (human, recombinant, R&D Systems) (5 ng/mL, 20 min (15)) using an identical experimental setup, resulting in an increase of SMPD1 signals (densitometric analysis: median 15.8 AU [15.1/23.7] versus 13.3 AU [12.3/14.3] in resting cells; p < 0.05) as well as ceramide signals (4.1 [3.5/5.4] versus 1.9 [1.6/2.6] in resting cells; p < 0.01).
Patient Characteristics
After ethical approval and written informed consent by patients or healthcare proxies, healthy individuals and three groups of ICU-patients were investigated: (1) twenty-three patients were enrolled prospectively who underwent elective cardiac surgery with low risk to develop post-operative organ dysfunction. Twelve of these patients underwent off-pump surgery, eleven patients underwent on-pump surgery; both groups met two SIRS criteria in the absence of organ dysfunction. (2) Twenty-two patients following non-elective on-pump cardiac surgery with increased risk of developing organ dysfunction, were enrolled after onset of organ dysfunction. (3) The third group reflected twelve patients meeting ACCP/SCCM Consensus Conference criteria for severe sepsis/septic shock (38). Demographic and clinical data of patients enrolled in the study are summarized in Table 1.
Lactate Dehydrogenase Measurements
Lactate dehydrogenase (LDH) assay were performed by a commercially available kit (Roche) according to the manufacturer’s instructions.
Statistical Analysis
A Kruskal-Wallis-test was performed for comparisons of variance between patient groups. A Mann-Whitney-U test (MWU-test) or t test was performed between control and treated states and/or between patients and healthy controls. A p value of < 0.05 was considered statistically significant. With respect to analysis of expression profile values log2 fold variations at least ± 1 were considered to be of biological significance.
Results
Activity and Amount of Plasma Secreted Sphingomyelinase Are Increased Dependent on Severity of the Inflammatory Response
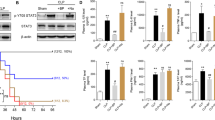
As shown in Figure 1A, an increase of pSMPD1 activity (median 172.7 pmol/(mL*h); interquartile range (IQR) 108.8 pmol/(mL*h), n = 23) was observed in uncomplicated major surgery controls following invasive or non-invasive cardiac surgery fulfilling SIRS-criteria. A more pronounced rise was observed in patients fulfilling SIRS criteria complicated by organ dysfunction on day 1 (291.7 pmol/(mL*h); 75.2 pmol/(mL*h), n = 14). Highest values were found in patients with severe sepsis/septic shock (379.2 pmol/(mL*h); 194.3 pmol/(mL*h), n = 12). The median value of all patients groups exceeded the median value of age matched, healthy controls (118.4 pmol/(mL*h); 56.5 pmol/(mL*h), n = 12). The increase in SMPD1 activity in inflammatory host response of different origin was found increased in patients with systemic inflammation (SIRS without organ dysfunction (OD)), with further increasing values in those with OD or severe sepsis/septic shock compared with intensive care unit (ICU) controls (Kruskal-Wallis-test, p ≤ 0.001). Next, we determined whether there was an increase in enzymatic activity or protein amount in plasma. Immunoprecipitation revealed nearly absent pSMPD1 immune complexes in healthy individuals, whereas a stepwise appearance and severity dependent increase of protein was observed corresponding to the observed activity pattern (Figure 1B).
Activity of pSMPDl during the course of inflammatory response. (A) Activity was determined by densitometric quantification of generated ceramide from a fluorescent dye-labeled sphingomyelin substrate in serum from patients with various degrees of inflammatory response with postoperative SIRS after cardiac surgery without organ dysfunction (SIRS without OD), with SIRS with organ dysfunction (SIRS with OD) and severe sepsis/septic shock (severe sepsis/septic shock). Boxes indicate interquartile range, divided by the median (central horizontal line). The median of an age-matched healthy control cohort is indicated by a dotted horizontal line. Levels of significance between patients groups are indicated by asterisks (*** P ≤ 0.001; Kruskal-Wallis-test) and between the patients groups with OD versus SIRS without OD by a rhombus (### P ≤ 0.001; MWU-test). (B) Plasma appearance of SMPD1 determined by immuno-precipitation and immunological visualization. Results shown are representative of those obtained in three independent experiments.
Instantaneous Endothelial Ceramide Generation During Sepsis and its Abrogation by Desipramine
To investigate a functional role of SMPD1 in stress response, we metabolically labeled human endothelial cells with a fluorescent sphingomyelin derivative according to the method of Pagano (39) and exposed them to freshly prepared serum obtained from patients with sepsis. Densitometric analysis of chromatographically separated reaction products revealed elevated amounts of sphingomyelin breakdown upon stimulation with serum obtained from septic patients as compared with healthy controls (median 18.2; IQR 8.8 versus 12.6; IQR 6.3 pmol, Figure 2A). Increasing concentrations of desipramine abrogated generation of both ceramide and Glc-Cer. In a separate set of experiments, similar results were obtained following preincubation with NB6 (Figure 2B). The stimulation with serum from septic patients resulted in a sharp and rapid increase (15 min) of ceramide generation (>3 pmol/106 cells), whereupon generated ceramide was instantaneously metabolized to a glycosylated derivative (Figure 2D). A similar effect was observed following 30 min of stimulation and minor intensity following 60 and 120 min which reflects a time-dependent decrease of sphingomyelin bound to endothelial membranes. As shown in a representative illustration (Figure 2E), preincubation of endothelial monolayers with increasing concentrations of desipramine (upper panel) or NB6 (lower panel) reduced the generation of both, ceramide and its derivative in endothelial cells.
Inhibition of ceramide generation by (A) desipramine and (B) NB6. Semi-confluent HMECs were metabolically labeled with C6-NBD-sphingomyelin and stimulated with serum (5%) obtained from healthy individuals (n = 12) or patients with severe sepsis for 20 min (n = 12). Monolayers were pretreated with desipramine or NB6 for 30 min (10),20,40 µmolar). Boxes indicate the interquartile range, divided by the median (central horizontal line) as analyzed in twelve independent experiments per group. Significant differences in comparison to monolayers without desipramine or NB6 pretreatment were calculated by Friedmann-Wilcoxon test with adjustment according to Bonferoni-Holm results are indicated by asterisks (*p < 0.05, **p < 0.02). (C) Ceramide generation in proinflammatory stimulated endothelial cells. Semi-confluent HMECs were pretreated with desipramine or NB6 for 60 min (20 µmol each), and stimulated with TNF-α (10 ng/mL) or endotoxin (100 ng/mL) for 240 min in the presence of 5% human serum from healthy individuals. Subsequent to lipid extraction, purification and labeling, ceramides were separated by HPLC. Shown is the total amount of ceramides, since the boxes indicate the interquartile range, divided by the median (central horizontal line) as analyzed in six independent experiments. Significant differences to basal values are indicated by an asterisk (*p < 0.05). (D) Time course of sphingomyelin breakdown in stimulated endothelial cells. Semi-confluent HMECs were metabolically labeled with C6-NBD-sphingomyelin and stimulated with serum (5%) obtained from patients with severe sepsis. Fluorescent sphingolipids such as ceramide as well as GlcCer were separated by TLC quantitated by densitometry. Representative results from three independent experiments are shown. (E) Representative sphingolipid pattern of stimulated endothelial cells used for densitometric analyses. Endothelial monolayers were pretreated with desipramine (upper lines) and NB6 (lower lines) for 30 min (10),20,40 µmolar). Fluorescent sphingolipids such as ceramide (rf 0.8) as well as GlcCer (rf 0.6) were separated by TLC and quantitated by densitometry. Representative results from 24 independent experiments (12 each per group and inhibitor, providing data for A and B) are shown. M, Marker.
Furthermore, we detected variations in the endogenous sphingomyelin pool in an in vitro setting ceramide generation following the stimulation with TNF-α (10 ng/mL−1) and endotoxin (100 ng/mL−1). Basal ceramide levels were ranging around 0.288 molcer/molPh. Figure 2C demonstrates that ceramide generation was highly upregulated in response to either TNF-α or endotoxin for 240 min (0.524 and 0.371 molcer/molPh, respectively). No significant increase was observed following 15 or 60 min of incubation (data not shown). Pre-administration of the inhibitor desipramine or NB6 (60 min, 20 µmol each) completely prevented ceramide-generation.
Pharmacological inhibition abrogates early formation of ceramide-enriched macrodomains upon treatment with serum from patients with sepsis. Here, we investigated the direct effect of serum from septic patients with respect to the formation of ceramide enriched macrodomains in endothelial monolayers using confocal scanning microscopy. Immune staining revealed a brusque formation of ceramide enriched macrodomains following to the exposure to septic serum (Figure 3A, upper panel). Under resting conditions, we found that serum from healthy individuals failed to trigger formation of ceramide-enriched lipid rafts as indicated by weak, diffuse red (Cy5) fluorescence in a random punctuate staining pattern (control; lower panel). Interestingly, following exposure to serum from septic patients we observed green fluorescence patches (FITC) indicating an increase in SMPD1 immunoreactivity in endothelial membranes which was detected in a more diffuse pattern under resting conditions (left panel). To examine whether SMPD1 is colocalizing with ceramide enriched macrodomains, we merged the fluorescence staining. As shown in the right panel of Figure 3A, following the exposure to serum from patients with sepsis, SMPD1 aggregates in the ceramide enriched macrodomains, as demonstrated by patches of yellow fluorescence compared with unstimulated cells. Figures 3B/C summarize the effects of serum from patients and healthy individuals with and without pre-administration of functional inhibitors on membrane SMPD1 immunoreactivity and ceramide enriched macrodomain formation by counting resulting fluorescence intensity in endothelial membranes. Under resting conditions (healthy controls), median SMPD1 immunoreactivity (Figure 3B) amounted to 13.3 AU [first quartile: 12.3/third quartile: 14.3] which was elevated following stimulation with serum from patients with sepsis (19.1 AU [14.7/24.7]; p < 0.02). Pre-administration with desipramine was unable to diminish SMPD1 immunoreactivity following stimulation, median values ranged between 19.2 AU [15.9/21.9] for desipramine and 18.8 AU [16.8/21.2] for NB6 (40 µM each) without reaching significance. As shown in Figure 3C, in cells incubated with serum from healthy controls, fluorescence intensity for membrane associated ceramide amounted to values of 1.9 AU [1.6/2.6] which were doubled after stimulation with serum obtained from septic patients (4.1 AU [3.0/5.7]; p < 0.02). However, NB6 attenuated the extent of ceramide generation significantly (3.0 AU [2.4/3.7]; p < 0.05), while upon treatment with desipramine nearly basal levels of ceramide were reached (2.5 AU [1.9/2.9]; p < 0.02).
(A) Ceramide enriched macrodomain formation and its association with SMPD1 in stimulated endothelial cells. Semi-confluent HMECs were stimulated for 20 min with serum (5%) from healthy individuals (lower panel) or from patients with sepsis (upper panel). Subsequent to fixation and blockade of unspecific binding, immuno reactivity was tested with polyclonal anti-SMPD1 antibody and monoclonal anti-ceramide antibody using identical experimental settings in all conditions. Representative images from at least six independent experiments are shown. (B) Inhibition of SMPD1 membrane immuno reactivity and (C) ceramide enriched macrodomain formation by desipramine and NB6. Semi-confluent HMECs were pretreated for 30min with SMPD1 functional inhibitors desipramine or NB6, stimulated for 20 min with serum (5%) from healthy individuals or from patients with sepsis. Subsequent to fixation and blockade of unspecific binding, immuno reactivity was proved with (B) polyclonal anti-SMPD1 antibody and (C) monoclonal anti-ceramide antibody using identical experimental settings in all conditions. At 200 × magnification, fluorescence intensity was analyzed densitometrically using Carl Zeiss Image Examiner software. The boxes indicate the interquartile range, divided by the median (central horizontal line) as analyzed in at least 12 independent experiments. Significant differences between the patient groups w and w/o inhibitors compared with these cells treated with serum from healthy controls were calculated using t-test for paired samples (**p < 0.02), the effectiveness of inhibition (desipramine or NB6) is indicated by rhombus symbol (# p < 0.05, ## p < 0.02, compared with vehicle treated sepsis control).
Ceramide Promotes Receptor Clustering and GM1 Formation upon Stimulation with Serum from Septic Patients
Next, we analyzed the presence and clustering of the CD95 receptor protein, known as the binding protein for Fasligand. We found CD95 levels significantly increased in response to stimulation with serum from patients with sepsis (Figure 4A, left panel). To examine whether CD95 might be capable to directly interfere with ceramide-enriched macrodomains, double staining revealed clustering of CD95 protein in ceramide-enriched macrodomains of endothelial membranes following stimulation (right panel).
(A) Receptor clustering in ceramide-enriched macrodomains in stimulated endothelial cells. (B) GM1-membrane sorting and ceramide-enriched macrodomain formation in stimulated endothelial cells. Semi-confluent HMECs were stimulated for 20 min with serum (5%) from healthy individuals (lower panel) or from patients with sepsis (upper panel). Subsequent to fixation and blockade of unspecific binding, immunoreactivity was assessed with polyclonal anti-CD95, GM1 antibody and monoclonal anti-ceramide antibody using identical experimental settings in all conditions. Representative images from at least six independent experiments are shown.
The ganglioside GM1 is a common component of membrane raft microdomains which is demonstrated to contain antiapoptotic and antiinflammatory properties (40). We found in resting cells a strong signal diffusely distributed in the cytoplasm (Figure 4B, lower panel). Subsequent to incubation with serum obtained from patients with sepsis, this signal for GM1 translocated to the cell membrane, suggesting an indirect contribution to the formation of raft clusters, since staining with ceramide-reactive antibodies displayed intense formation of large ceramide enriched lipid rafts (Figure 4B, upper panel). We also observed that both lipid mediators (ceramide, GM1), although they were simultaneously upregulated upon stimulation, were within the membrane spatially separated as shown by double immune staining (right panel).
Pharmacological and Genetic Inhibition of SMPD1 Abrogates ADAMTS13 Downregulation Following the Stimulation with Serum of Patients with Sepsis as well as with Mixture of Cytokines
Furthermore, we were interested in whether the rapid generation of ceramide by SMPD1 upon stimulation is directly related to expression changes of the newly discovered marker of endothelial stress response, ADAMTS13. Treatment of HMECs with serum obtained from patients with sepsis significantly downregulated the expression of ADAMTS13 transcripts. To clarify our result, we targeted SMPD1 with specific siRNA and a random control siRNA. Genetic knock-down of SMPD1 blocked the downregulation of ADAMTS13 following the stimulation with serum from septic patients, whereas random siRNA revealed similar results as compared with positive control (Figure 5A).
Detrimental role of SMPD1 in sepsis-induced endothelial stress response Semi-confluent HMECs (1 × 104) were incubated for 24 h with serum (5%) obtained from healthy individuals, from patients with sepsis (n = 6) or proinflammatory cytokines. Co-incubation with (A) SMPD1 specific siRNA (5 nmol/L, preincubation for 48 h) or (B) SMPD1 direct inhibitor amino bisphosphonate (0.1 µmol/L) prevented ADAMTS13 down-regulation as normalized to reference transcript GAPDH. Stimulation of HMECs with (C) isolated human acid sphingomyelinase resulted in significant LDH release into the supernatant, whereas (D) amino bisphophonates abrogated LDH release following cytokine stimulation. The boxes indicate the interquartile range, divided by the median (central horizontal line) as analyzed in at least four independent experiments.
Similar results were obtained following stimulation with a mixture of proinflammatory cytokines, where the ADAMTS13 downregulation was completely abolished by co-incubation with specific inhibition of SMPD1 activity, a geminal amino bisphosphonate (Figure 5B). The specificity of the proposed mechanism is also shown by release of LDH into the supernatant, either by exposure to isolated enzyme (Figure 5C) or the breakdown of autocrine destruction triggered by cytokine and secreted SMPD1 following exposure to amino bisphosphonate (Figure 5D).
Desipramine Abrogates ADAMTS13 Downregulation and Reveals Cytoprotective Effects in HMECs During Sepsis
Cytoprotection was achieved by incubation of HMECs with desipramine, a functional inhibitor of SMPD1, following exposure to serum obtained from septic patients (Figure 6A) or stimulation with proinflammatory cytokines (Figure 6B). Treatment with desipramine was also able to diminish the amount of LDH in the supernatants in presence of serum obtained from septic patients (Figure 6C). Strikingly, the functional inhibitor also normalized LDH release from cytokine stimulated cells (Figure 6D).
Desipramine improves endothelial stress response during sepsis. Semi-confluent HMECs (1 × 104) were incubated for 24 h with serum (5%) obtained from healthy individuals, from patients with sepsis (n = 6) or proinflammatory cytokines (CM). (A, B) Co-incubation with desipramine (20 µmolL, preincubation for 24 h) abrogated ADAMTS13 expression as normalized to reference transcript GAPDH. Desipramine treatment of HMECs diminished LDH release into the supernatant following the stimulation with serum obtained from sepsis patients (C) or proinflammatory cytokines (D). The boxes indicate the interquartile range, divided by the median (central horizontal line) as analyzed in at least four independent experiments. OD optical density.
Discussion
Many functions of the endothelium have been described in sepsis, for example, the regulation of hemostasis, inflammation and the development of organ dysfunction (6,41). In this context, the endothelium undergoes structural and functional alterations which may result in remote organ failure that is central to the pathophysiological derangements of multiple organ dysfunction syndrome (5,42). Despite numerous reports describing an increased activity of SMPD1 in several clinical conditions (43,44), there is a lack of information regarding the action of SMPD1 in particular on endothelial cells mediated by a plethora of proinflammatory serum constituents. Therefore, we used a human in vitro setting (HMEC-1) to study various pathophysiological processes as these cells display most of the morphological and functional characteristics of endothelial cells (45). It is well known that properties of endothelial cells are dependent on persistent environmental signals triggered by origin, nurture as well as the status of confluence (46,47), whereas the latter critically influences the cellular response with respect to stress induced signaling cascades (46). For prevention of a potential bias, we used microvascular endothelial cells in an almost semiconfluent condition as a fundamental prerequisite of our study design.
This is the first report describing the kinetics and dynamics of a severity-dependent gradual increase comprising both the amount of protein and the biological activity affecting endothelial ceramide generation as well as lipid raft formation in critical care (Figures 1A, B). The lipid mediator ceramide, generated by the pace-making activity of SMPD1, controls cellular functions ranging from proliferation and differentiation to growth arrest and apoptosis (48,49). The isoform investigated in the present study (SMPD1) is the only one known to be responsible for rapid and transient induction of ceramide generation (50). Beyond the increase of the major reaction product ceramide, we also observed glycosylated ceramide (51) as a secondary reaction product of sphingolipid signaling in endothelial cells stimulated with serum obtained from septic patients (Figures 2C, D). Cellular ceramide content has been measured by derivatization procedures of ceramide followed by chromatographic separation (35). Compared with serum, treatment with both TNF-α and endotoxin induced a delayed but robust elevation in total endogenous ceramide as early as 2 h and remained elevated for the next 2 h of treatment (Figure 2C). Previous studies demonstrated that the use of desipramine was capable of decreasing inflammatory response and increasing the outcome in an endotoxemia mice model (20,22). Hence, we pharmacologically inhibited SMPD1 with weak basic compounds such as desipramine and NB6 which were identified as functional inhibitors of SMPD1 (FIASMA) with high potency (23) and were capable of abrogating ceramide generation (Figures 2A, B, C). This group of compounds gained our attention due to the fact that FIASMAs include FDA approved and heavily prescribed drugs in daily clinical routine which might enable a novel pharmacological treatment strategy in systemic inflammation and sepsis.
Data obtained in this study and presented from other groups suggested that TNF-α may amplify the injury of endothelial cells. We propose that the injury-amplifying effects are mediated by ceramide generation and the formation of lipid rafts with subsequent clustering of receptors relevant for initiation of apoptosis (15). The formation of the ceramide-enriched macrodomains seems to be mediated by the tendency of ceramide molecules to associate with each other (52). We demonstrated that the stimulation of HMECs with serum obtained from sepsis patients resulted in the formation of ceramide enriched macrodomains (Figures 3, 4). These domains serve the reorganization of other cellular “signalosomes” and, in particular, the clustering of receptor molecules. SMPD1 mediated clustering was shown in both in vivo and in vitro experimentation for CD95 (15), amplifying signal intensity which is required for initiation of apoptosis. Previously, it was shown in an in vitro model for tissue fixed macrophages that exposure of cultured THP-1 cells to endotoxin resulted in PKCζ mediated activation of SMPD1, ceramide generation as well as assembly of TLR4 receptors within lipid rafts which was attenuated by functional inhibition with imipramine (16,53). A clearly protective role of inhibition of macrodomain formation was recently shown in irradiation-induced mortality (54). Otherwise, non-lysosomal sphingomyelinase can protect against cytotoxic agents (55).
In accordance with results obtained from model membrane bilayers, we found the gangliosides GM1 enriched in lipid rafts (56), supporting the hypothesis that lipid rafts may act as protein/lipid platforms sorting specific membrane components and clustering them stably together. These results are consistent with previous studies, suggesting that translocation and intra-membranous accumulation play a critical role in stress induced signaling in endothelial cells, for example, for maintenance of vascular barrier integrity and cell-cell communication (57). In membranes, an association of ceramide/GM1 reorganization with endothelial dysfunction may represent a novel mechanism contributing to altered signal transduction, increased endothelial permeability and edema formation.
Rapid and transient ceramide generation is triggered by either an endolysosomal or an extracellularly secreted isoform of the protein, but the latter one might also act in an autocrine manner following proinflammatory stimulation (58). The tricyclic antidepressant desipramine modulates ceramide generation and lipid raft formation by limited proteolysis of the lysosomal SMPD1 (Figures 3, 4). Besides, it is known that this group of drugs might inhibit other enzymes essentially involved in ceramide metabolism such as ceramidases (59), thus a delayed inactivation of ceramide might also trigger a preconditioning effect as shown in myocardial ischemia (60). To further clarify our results we pharmacologically as well as genetically inhibited SMPD1 and measured a newly established marker of endothelial stress response during sepsis, ADAMTS13 (26). This protease is responsible for hydrolyzing unprocessed ultralarge von-Willebrand factor (ulVWF) multimers to restrict their function and thereby abrogating a prothrombotic state. ADAMTS13 was found to be deficient in patients with sepsis correlating to the intense of inflammatory response (8). Targeting SMPD1 with specific siRNA demonstrate a novel molecular mechanism involved in downregulating the expression of ADAMTS13 in endothelial cells during sepsis which was demonstrated in a clinical (Figure 5A, exposure to serum) as well as rather experimental setting (Figure 5B, exposure to cytokines). Independent from molecular measures, treatment with desipramine abolished the release of LDH as a global marker of cellular damage (Figures 6C, D). In addition, we provide a pharmacological treatment strategy with desipramine resulting in an abrogation of the effects.
Differing on that, amino bisphosphonates are known as effective and direct inhibitors of secreted SMPD1 due to complexation of essential Zn++-ions trapped by the protein (25). The down-regulation of ADAMTS13 in our experimental setting was normalized following inhibition of secreted SMPD1 (Figure 5B). Also the abrogation of LDH release clearly supports the concept that during sepsis and inflammatory response, SMPD1 release is a critical effector of cellular damage control (Figure 5D) including autocrine signaling. In our study, the inhibition of both effector proteins, the endolysosomal as well as the secreted one, results in protection of the endothelial cells either from the detrimental constituents in serum obtained from septic patients and an artificial mixture of proinflammatory cytokines.
Conclusion
In summary, these data provide first evidence that both isoforms of SMPD1 affect regulation of endothelial ceramide generation during systemic inflammation. Results from experiments using the set of pharmacological and genetic inhibitors support the concept that activation of SMPD1 in critically ill patients is responsible for endothelial stress response and might contribute to remote organ failure, which can be improved using functional inhibitors, such as desipramine. The amplifying function of ceramide generation and macrodomain formation results in endothelial stress response and ADAMTS13 downregulation. This concept and the availability of FDA-approved functional inhibitors might offer an additional approach to affect and to limit development of organ dysfunction, for example, in patients with sepsis at risk of multiple organ failure.
Disclosure
The authors declare they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
References
Angus, DC, Pereira, CA and Silva, E (2006) Epidemiology of severe sepsis around the world. Endocr Metab Immune Disord Drug Targets 6(2): 207–12.
Deutschman, CS, Tracey KJ (2014) Sepsis: current dogma and new perspectives. Immunity 40(4): 463–75.
Opal, SM et al. (2014) The next generation of sepsis clinical trial designs: what is next after the demise of recombinant human activated protein C? Crit. Care Med. 42(7): 1714–21.
Henneke, P Golenbock, DT (2002) Innate immune recognition of lipopolysaccharide by endothelial cells. Crit. Care Med. 30(5 Suppl): S207–13.
Schouten, M., et al. (2008) Inflammation, endothelium, and coagulation in sepsis. J. Leukoc. Biol. 83(3):536–45.
Aird, WC (2007) Endothelium as a therapeutic target in sepsis. Curr Drug Targets 8(4):501–7.
Kinasewitz, GT et al. (2005) Prognostic value of a simple evolving disseminated intravascular coagulation score in patients with severe sepsis. Crit. Care Med. 33(10): 2214–21.
Bockmeyer, CL et al. (2008) Inflammation-associated ADAMTS13 deficiency promotes formation of ultra-large von Willebrand factor. Haematologica 93(1): 137–140.
Gulbins, E et al. (2004) Ceramide, membrane rafts and infections. J Mol. Med. 82(6):357–63.
Claus, RA et al. (2010) Approaching clinical reality: markers for monitoring systemic inflammation and sepsis. Curr Mol. Med. 10(2): 227–35.
Marchesini, N and Hannun, YA (2004) Acid and neutral sphingomyelinases: roles and mechanisms of regulation. Biochem Cell Biol 82(1):27–44.
Gulbins, E. and Li, PL (2006) Physiological and pathophysiological aspects of ceramide. Am. J. Physiol. 290(1):R11–26.
Jenkins, RW et al. (2010) Regulated secretion of acid sphingomyelinase: implications for selectivity of ceramide formation. J Biol. Chem. 285(46) 35706–18.
Miller, ME et al. (2012) Ebolavirus requires acid sphingomyelinase activity and plasma membrane sphingomyelin for infection. J Virol 86(14): 7473–83.
Grassme, H, Schwarz H and Gulbins E (2001) Molecular mechanisms of ceramide-mediated CD95 clustering. Biochem. Biophys. Res. Commun. 284(4):1016–30.
Cuschieri, J et al. (2007) Acid sphingomyelinase is required for lipid Raft TLR4 complex formation. Surg Infect. (Larchmt) 8(1):91–106.
Brameshuber, M et al. (2016) Oxidized Phospholipids Inhibit the Formation of Cholesterol-Dependent Plasma Membrane Nanoplatforms. Biophys J 110(1): 205–13.
van Blitterswijk, WJ et al. (2003) Ceramide: second messenger or modulator of membrane structure and dynamics? Biochem. J. 369(Pt 2): p. 199–211.
Delogu, G et al., (1999) Ceramide concentrations in septic patients: a possible marker of multiple organ dysfunction syndrome. Crit. Care Med. 27(11): 2413–7.
Claus, RA, et al. (2005) Plasma platelet-activating factor acetylhydrolase activity in critically ill patients. Crit. Care Med. 33(6): 1416–9.
Kott, M et al. (2014) Acid sphingomyelinase serum activity predicts mortality in intensive care unit patients after systemic inflammation: a prospective cohort study. PLoS One 9(11): e112323.
Roumestan, C et al. (2007) Anti-inflammatory properties of desipramine and fluoxetine. Respir. Res. 8: 35.
Kornhuber, J et al. (2010) Functional Inhibitors of Acid Sphingomyelinase (FIASMAs): a novel pharmacological group of drugs with broad clinical applications. Cell Physiol Biochem 26(1): 9–20.
Peng, H et al. (2015) Acid sphingomyelinase inhibition protects mice from lung edema and lethal Staphylococcus aureus sepsis. J Mol. Med. 93(6): 675–89.
Arenz, C (2010) Small molecule inhibitors of acid sphingomyelinase. Cell Physiol Biochem 26(1): 1–8.
Ekaney, ML et al. (2015) Preserved Expression of mRNA Coding von Willebrand Factor-Cleaving Protease ADAMTS13 by Selenite and Activated Protein C. Mol. Med. 21: 355–63.
Loidl, A et al. (2002) High-precision fluorescence assay for sphingomyelinase activity of isolated enzymes and cell lysates. J Lipid Res 43(5): 815–23.
Loidl, A et al. (2004) Role of ceramide in activation of stress-associated MAP kinases by minimally modified LDL in vascular smooth muscle cells. Biochim Biophys Acta 1690(2): 150–8.
Osawa, Y et al. (2011) Acid sphingomyelinase regulates glucose and lipid metabolism in hepatocytes through AKT activation and AMP-activated protein kinase suppression. FASEB J. 25(4): 1133–44.
Ceppi, ED, Smith, FS and Itheradge MA (1996) Effect of multiple cytokines plus bacterial endotoxin on glucose and nitric oxide production by cultured hepatocytes. Biochem. J. 317 (Pt 2): 503–7.
Deigner, HP et al. (2001) Ceramide induces aSMase expression: implications for oxLDL-induced apoptosis. Faseb J 15(3): 807–14.
Martin, OC and Pagano, RE (1994) Internalization and sorting of a fluorescent analogue of glucosylceramide to the Golgi apparatus of human skin fibroblasts: utilization of endocytic and nonendocytic transport mechanisms. J. Cell Biol. 125(4): 769–81.
Bligh, EG and Dyer, WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8): 911–7.
Ames, BN and Dubin, DT The role of polyamines in the neutralization of bacteriophage deoxyribo-nucleic acid. J Biol. Chem. 235: 769–75.
Blaess, M, Claus, RA and Deigner, HP (2015) HPLC separation and ultrasensitive optical quantification of ceramide species applying 7-(diethylamino)coumarin-3-carbonyl azide derivatisation. J Chromatogr B Analyt Technol Biomed Life Sci. 986–987: 123–8.
Sieber, MW et al. (2010) Substantial performance discrepancies among commercially available kits for reverse transcription quantitative polymerase chain reaction: a systematic comparative investigator-driven approach. Anal. Biochem. 401(2): 303–11.
Gassert, E et al. (2009) Induction of membrane ceramides: a novel strategy to interfere with T lymphocyte cytoskeletal reorganisation in viral immunosuppression. PLoS Pathog. 5(10): e1000623.
American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit. Care Med., 1992. 20(6): 864–74.
Koval, M and Pagano RE, (1991) Intracellular transport and metabolism of sphingomyelin. Biochim Biophys Acta. 1082(2): 113–25.
Nikolaeva, S et al. (2014) GM1 and GD1a gangliosides modulate toxic and inflammatory effects of E. coli lipopolysaccharide by preventing TLR4 translocation into lipid rafts. Biochim Biophys Acta.
Opal, SM and van der Poll, T (2015) Endothelial barrier dysfunction in septic shock. J. Intern. Med. 277(3): 277–93.
Abraham, E and Singer, M (2007) Mechanisms of sepsis-induced organ dysfunction. Crit. Care Med. 35(10): 2408–16.
Fernandez, A et al. (2013) ASMase is required for chronic alcohol induced hepatic endoplasmic reticulum stress and mitochondrial cholesterol loading. J Hepatol 59(4): 805–13.
Garcia-Ruiz, C et al. (2015) Acid sphingomyelinase-ceramide system in steatohepatitis: a novel target regulating multiple pathways. J Hepatol. 62(1): 219–33.
Bouis, D et al. (2001) Endothelium in vitro: a review of human vascular endothelial cell lines for blood vessel-related research. Angiogenesis 4(2): 91–102.
Vinals, F and Pouyssegur, J (1999) Confluence of vascular endothelial cells induces cell cycle exit by inhibiting p42/p44 mitogen-activated protein kinase activity. Mol. Cell. Biol. 19(4): 2763–72.
Regan, ER and Aird, WC (2012) Dynamical systems approach to endothelial heterogeneity. Circ. Res. 111(1): 110–30.
Smith, EL and Schuchman, EH (2008) The unexpected role of acid sphingomyelinase in cell death and the pathophysiology of common diseases. FASEB J. 22(10): 3419–31.
Moles, A et al. (2010) Acidic sphingomyelinase controls hepatic stellate cell activation and in vivo liver fibrogenesis. Am. J. Pathol. 177(3): 1214–24.
Hannun, YA and Obeid, LM (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol. Cell. Biol. 9(2): 139–50.
Kok, JW et al. (1995) Fluorescent, short-chain C6-NBD-sphingomyelin, but not C6-NBD-glucosylceramide, is subject to extensive degradation in the plasma membrane: implications for signal transduction related to cell differentiation. Biochem. J. 309 (Pt 3): 905–12.
Kolesnick, RN, Goni, FM and Alonso, A (2000) Compartmentalization of ceramide signaling: physical foundations and biological effects. J Cell Physiol. 184(3): 285–300.
Olsson, S and Sundler, R (2006) The role of lipid rafts in LPS-induced signaling in a macrophage cell line. Mol. Immunol. 43(6): 607–12.
Rotolo, J et al. (2012) Anti-ceramide antibody prevents the radiation gastrointestinal syndrome in mice. J. Clin. Invest. 122(5): 1786–90.
Petersen, NH et al. (2013) Transformation-associated changes in sphingolipid metabolism sensitize cells to lysosomal cell death induced by inhibitors of acid sphingomyelinase. Cancer Cell 24(3): 379–93.
Chiantia, S et al. (2008) Role of ceramide in membrane protein organization investigated by combined AFM and FCS. Biochim Biophys Acta 1778(5): 1356–64.
Zhao, J et al. (2009) Phosphotyrosine protein dynamics in cell membrane rafts of sphingosine-1-phosphate-stimulated human endothelium: Role in barrier enhancement. Cell Signal.
Castro-Gomes, T et al. (2016) Plasma Membrane Repair Is Regulated Extracellularly by Proteases Released from Lysosomes. PLoS One 11(3): e0152583.
Elojeimy, S et al. (2006) New insights on the use of desipramine as an inhibitor for acid ceramidase. FEBS Lett. 580(19): 4751–6.
Der, P, Cui, J and Das, DK (2006) Role of lipid rafts in ceramide and nitric oxide signaling in the ischemic and preconditioned hearts. J Mol Cell Cardiol. 40(2): 313–20.
Acknowledgments
The authors would like to thank Edith Walther, Brigitte Specht, Jacqueline Fischer and Markus Zacharias for technical assistance and André Scherag for statistical advice. Special thanks to Silke Keiner and Otto Witte (Department for Neurology, Jena University Hospital) for assistance during preparation of microphotographs.
The study was supported by the Center of Sepsis Control and Care (Integriertes Forschungs- und Behandlungszentrum Sepsis und Sepsisfolgen — Center for Sepsis Control and Care, sponsored by German Federal Ministry of Education and Research, BMBF FKZ 01EO1002, to H.Y.C., G.P.O., and R.A.C.), in part by a grant from the Thuringian Ministry of Science and Arts (TMWFK, project B-309-00014) and by the German Research Council, DFG (SPP 1267; CL 173/3-1, 3-2). CLB, MJD and DCH have received financial support from the IZKF/‘Förderverein’ of the Jena University Hospital (Loder-Grant for young investigators).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, and provide a link to the Creative Commons license. You do not have permission under this license to share adapted material derived from this article or parts of it.
The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this license, visit (http://creativecommons.org/licenses/by-nc-nd/4.0/)
About this article
Cite this article
Chung, HY., Hupe, D.C., Otto, G.P. et al. Acid Sphingomyelinase Promotes Endothelial Stress Response in Systemic Inflammation and Sepsis. Mol Med 22, 412–423 (2016). https://doi.org/10.2119/molmed.2016.00140
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2119/molmed.2016.00140