Abstract
Myocarditis is a major cause of heart failure and sudden cardiac death in young adults and adolescents. Many cases of myocarditis are associated with autoimmune processes in which cardiac myosin is a major autoantigen. Conventional immunosuppressive therapies often provide unsatisfactory results and are associated with adverse toxicities during the treatment of autoimmune myocarditis. Cannabidiol (CBD) is a nonpsychoactive constituent of marijuana that exerts antiinflammatory effects independent of classical cannabinoid receptors. Recently, 80 clinical trials have investigated the effects of CBD in various diseases from inflammatory bowel disease to graft versus host disease. CBD-based formulations are used for the management of multiple sclerosis in numerous countries, and CBD also received U.S. Food and Drug Administration approval for the treatment of refractory childhood epilepsy and glioblastoma multiforme. Herein, using a well-established mouse model of experimental autoimmune myocarditis (EAM) induced by immunization with cardiac myosin emmulsified in adjuvant resulting in T cell-mediated inflammation, cardiomyocyte cell death, fibrosis and myocardial dysfunction, we studied the potential beneficial effects of CBD. EAM was characterized by marked myocardial T-cell infiltration, profound inflammatory response and fibrosis (measured by quantitative real-time polymerase chain reaction, histology and immunohistochemistry analyses) accompanied by marked attenuation of both systolic and diastolic cardiac functions measured with a pressure-volume conductance catheter technique. Chronic treatment with CBD largely attenuated the CD3+ and CD4+ T cell-mediated inflammatory response and injury, myocardial fibrosis and cardiac dysfunction in mice. In conclusion, CBD may represent a promising novel treatment for managing autoimmune myocarditis and possibly other autoimmune disorders and organ transplantation.
Similar content being viewed by others
Introduction
The report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of Cardiomyopathies (1) defined myocarditis as an inflammatory heart disease associated with cardiac dysfunction. Myocarditis is associated with several serious cardiac complications, ranging from sudden cardiac death (2) to chronic heart failure. Myocarditis often results in the development of dilated cardiomyopathy, which is one of the most important indications for cardiac transplantation (3,4). Different etiologies can lead to the development of myocarditis in humans, such as infectious diseases, autoimmune diseases, hypersensitivity or toxic reactions to drugs (3). The course of myocarditis can be separated into acute, subacute and chronic phases (5). The prognosis and outcome of myocarditis vary from fully recovering to death or chronic heart failure, depending on the clinical phase, signs and parameters and on the patient’s response to the applied medical treatment (3). In the pathogenesis of chronic myocarditis, autoimmunity plays a crucial role. Lv et al. (6) showed that α myosin heavy chain (MyHCα) is not expressed in the thymus of mice and humans; thus, the CD4+ T-cell population does not undergo negative selection regarding to MyHCα specificity (6). In different pathologies where the heart is injured (microbial infections, toxins, ischemic infarction and so on), the dendritic cells residing in the cardiac draining lymph nodes become activated and start to present MyHCα to naive T cells specific for MyHCα. In the pathogenesis of autoimmune myocarditis (in mice or humans), these T lymphocytes play a leading role either by their direct cytotoxic effects or by inducing B cells to produce pathogenic antibodies (7), leading to necrosis, oxidative stress, fibrotic remodeling and myocardial dysfunction (8–10).
Although, a wide range of specific and nonspecific drugs could be used to treat myocarditis on the basis of etiology, the conventional immunosuppressive and heart failure therapies often provide partial and/or unsatisfactory results. Because of the high tendency of toxicity and adverse effects of immunosuppressive therapy and the lack of clinical trials focusing on the treatment of myocarditis, the medical treatment regimen is still limited and new treatment options are needed (3).
Cannabidiol (CBD) is a nonpsychoactive ingredient of marijuana (Cannabis sativa). Although CBD is considered a biologically inactive molecule (11,12), it was reported to exert cytoprotective effects in various preclinical models and was shown to be safe in patients (13). Several preclinical studies described the protective effect of CBD in diseases associated with increased oxidative stress, inflammation and cell death such as in colitis (14), diabetic complications (15), drug-induced nephrotoxicity (16), alcohol-induced steatohepatosis (17) or hypoxia-ischemia induced brain injury (18). Because of the success of the preclinical studies, CBD has become a widely investigated drug in different clinical settings. To date, 80 clinical trials investigating the effects of CBD in various autoimmune and neurological disorders, and in graft versus host disease, have been cited on www.clinicaltrials.gov. CBD is approved in 27 countries as an oromucosal spray (Sativex) to treat spasticity in multiple sclerosis and was granted Orphan Drug Designation by the U.S. Food and Drug Administration (FDA) (Epidiolex) for the treatment of Dravet and Lennox-Gastaut syndromes.
In this study, we investigated the effects of CBD on myocardial inflammation, remodeling and dysfunction in an animal model of experimental autoimmune myocarditis (EAM).
Materials and Methods
Animals
The investigation was performed according to the Guide for the Care and Use of Laboratory Animals (National Research Council of the National Academies, 2011, 8th edition, Washington, DC: National Academies Press) and was reviewed and approved by the Institutional Animal Care and Use Committee. Forty male A/J mice (The Jackson Laboratory) weighing 18–22 g were housed in the animal facility of NIAAA and received standard laboratory diet and water ad libitum.
Induction of EAM and Experimental Groups
The myocarditogenic MyHCα334–352 (amino acid sequence: DSAF DVLS FTAE EKAG VYK) (19) (Genscript) was used to induce EAM in our animals, as described previously (20). Briefly, on d 0 and 7, mice were treated subcutaneously (axillary region) with 100 µg MyHCα334–352 emulsified in Freund’s complete adjuvant (CFA) (Sigma-Aldrich) supplemented with heat-killed Mycobacterium tuberculosis H37Ra. On d 0, mice were given 500 ng pertussis toxin intraperitoneally (List Biologicals). Animals were divided into four experimental groups, namely control (CTL) (vehicle treated; n = 11), CFA treated (CFA treated with vehicle; n = 10), experimental autoimmune myocarditis (EAM treated with vehicle; n = 16) and CBD-treated EAM (EAM + CBD; n = 12).
Drug Treatment
CBD was extracted as previously described (21) and was dissolved in physiological saline vehicle solution containing Tween-80 and dimethyl sulfoxide in a ratio of 1:1:18. Animals were treated daily either with vehicle or CBD (10 mg/kg) intraperitoneally. Drug treatment was initiated from d 1 to 46. Body weight of the animals were recorded daily, and CBD doses were adjusted accordingly.
Hemodynamic Measurements
On d 46, left ventricular (LV) performance was assessed under 1–2% isoflurane by using a pressure-conductance microcatheter system (MPVS-Ultra, Millar Instruments) coupled with PVR-1045 P-V microcatheter (Millar Instruments) as described previously (22). Ejection fraction, maximal slope of systolic pressure increment (dP/dtmax) and cardiac output were assessed as systolic and left ventricular end-diastolic pressure (LVEDP), and time constant of LV relaxation (Tau; according to Weiss method) was calculated as diastolic parameters. The slope of the LV end-systolic pressure-volume relationship (ESPVR) and the preload recruitable stroke work (PRSW) were used as load-independent LV contractility indices, and the slope of the LV end-diastolic pressure-volume relationship (EDPVR) was determined as an index of LV diastolic stiffness.
Gene Expression Analysis
After the hemodynamic measurements, heart was excised and snap-frozen in liquid nitrogen. Total RNA was isolated from homogenated LV myocardium using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. To remove genomic DNA contamination, RNase-free DNase (Ambion, Thermo Fisher Scientific) was applied, and total RNA was reverse-transcribed by using a high-capacity cDNA Reverse Transcription Kit (Applied Biosystems). Real-time polymerase chain reaction (PCR) for target genes (Tables 1, 2) was performed by using Syber Green Master Mix (Applied Biosystems) and an HT7900 (Applied Biosystems) PCR system as described previously (23). Relative gene expression quantification was calculated by the comparative CT method. Data were normalized to the housekeeping gene β-actin.
Determination of Myocardial 3-Nitrotyrosine and 4-Hydroxynonenal Contents
3-Nitrotyrosine (3-NT) was measured by the Nitrotyrosine ELISA kit (Hycult Biotechnology) according to the manufacturer’s instruction. 4-Hydroxynonenal (4-HNE) content was measured by the OxiSelect™ HNE Adduct Competitive ELISA Kit (Cell Biolabs) as described in the manufacturer’s instruction. 3-NT and 4-HNE values are presented as fold changes compared with CTL.
Histology and Immunohistochemistry
Hearts were excised and fixed in 4% phosphate-buffered formalin and embedded in paraffin. LV myocardial sections (5 µm) were stained with hematoxylin and eosin (H&E) to examine histological characteristics and inflammation. Myocarditis severity was evaluated as described previously (24) by two independent investigators blinded to the study groups. Masson trichrome and Sirius red staining were performed on LV sections (5 µm) to assess fibrotic remodeling. Fibrotic area was quantified using ImageJ software (NIH). 3-NT immunohistochemistry was performed as described previously (15). Briefly, 5-µm thin sections were stained with 3-NT (1:100, Nitrotyrosine Monoclonal Antibody, Cayman Chemical) overnight at 4°C. The sections were incubated with anti-mouse horseradish peroxidase reagent (Mouse on Mouse [M.O.M.™] Elite Peroxidase Kit, Vector Laboratories) for 1 h at room temperature and developed with a peroxide-based substrate Vectastain DAB kit (Vector Laboratories). Sections were counterstained with Nuclear Fast Red for 3 min, dehydrated in ethanol and cleared in xylene and mounted.
Statistics
Data are expressed as means ± standard error of the mean. Normal distribution was examined by Shapiro-Wilk test. Statistical significance among study groups was tested by ANOVA with Tukey post hoc test or Kruskal-Wallis ANOVA with Dunn post hoc test appropriately using GraphPad Prism 6 software. P <0.05 was considered statistically significant.
Results
CBD Attenuates Inflammation in EAM
First, we studied the effect of EAM and CBD treatment on the histological structure of the myocardium in our animals. Experimental autoimmune myocarditis induced by application of MyHCα334–352 was associated with inflammation, necrosis and mononuclear infiltration of the LV myocardium evidenced by H&E staining (myocarditis score: CTL: 0.16 ± 0.06 versus EAM: 3.6 ± 0.4, P < 0.05) (Figures 1A, B). CBD treatment largely decreased the inflammatory cell invasion and necrosis in EAM myocardium on d 46 on the H&E-stained sections (myocarditis score: EAM + CBD: 1.4 ± 0.2, P < 0.05) (Figures 1A, B). Second, we investigated the gene expression of different inflammatory cell markers and proinflammatory chemokines/cytokines in the LV. We observed increased gene expression levels of T-cell markers CD3a, CD3g, CD4, CD8a, monocyte and dendritic cell marker Itgax (complement component 3 receptor) and macrophage marker EMR f4/80 in the LV of EAM mice in comparison with the CTL animals (Figure 2) that were markedly reduced by the CBD treatment (Figure 2). EAM was associated with elevated mRNA expression values of several proinflammatory markers, including cytokines and chemokines (interleukin [IL]-6, interferon [IFN]-γ, IL1β and monocyte chemoattractant protein 1 [MCP-1]) (Figure 3). CBD treatment significantly attenuated mRNA expression of the proinflammatory IL-6, IL1β and IFN-γ (Figure 3) and had the tendency to reduce the mRNA levels of MCP1 (Figure 3).
CBD Attenuates Inflammation-Associated Oxidative Stress Markers in EAM
Secondary, as a consequence of inflammation, we also investigated the presence of oxidative stress in the myocardium of EAM animals. We observed significant upregulation of NADPH oxidase isoform p47phox in EAM compared with the CTL group (Figure 4A). CBD treatment reduced the mRNA expression of p47phox (Figure 4A). We also found significant downregulation of sarco/endoplasmic reticulum ATPase2a2 (SERCA) (Figure 4A), which was reverted by the application of CBD (Figure 4A). 3-NT and 4-HNE content of the LV was significantly increased in the LV of EAM animals (Figures 4B, C), which was markedly reduced by CBD treatment (Figures 4B, C).
Effects of CBD on oxidative stress and inflammation in EAM. (A) Cardiac mRNA expression of oxidative stress markers (p47phox and SERCA). (B) Myocardial 3-NT and 4-HNE content. (C) Representative images of cardiac 3-NT immunohistochemistry. Magnification: 200×. *P < 0.05 versus CTL; #P < 0.05 versus EAM.
CBD Protects against Fibrotic Remodeling of the Myocardium in EAM
The excessive inflammation in EAM was associated with the extreme fibrotic remodeling of the LV myocardium on d 46, shown by Masson trichrome staining (Figure 5A) and Sirius red staining (Figure 5C). Quantification of the Sirius red-stained sections (Figure 5D) (CTL: 1.4 ± 0.2 versus EAM: 7.6 ± 0.8, percent of fibrotic area) and upregulation of the myocardial Col1a (Figure 5B) confirmed the significant fibrotic remodeling in EAM compared controls. CBD treatment dramatically protected against fibrotic remodeling of the heart (percent of fibrotic area in EAM + CBD: 2.9 ± 1.4) (Figure 5).
Effects of CBD on myocardial fibrotic remodeling induced by EAM. (A) Representative images of Masson trichrome-stained LV myocardial sections. Magnification: 100×. (B) LV mRNA expression of collagen 1α (Col1a). (C) Representative images of Sirius red-stained LV myocardium sections. Magnification: 100×. (D) Quantification of Sirius red positive area in LV myocardium. *P < 0.05 versus CTL; #P < 0.05 versus EAM.
Impact of CBD on Myocardial Dysfunction and Body Weight in EAM
In comparison with the CTL group, we observed a significant decrease in ejection fraction, cardiac output and dP/dtmax suggesting impaired systolic function (Figure 6A). The load-independent cardiac parameters such as ESPVR and PRSW showed a significantly impaired contractility in myocarditis (Figures 6A, B). In addition to impaired systolic function, we found markedly increased Tau and LVEDP as the sign of diastolic dysfunction and markedly elevated slope of EDPVR as evidence of increased diastolic stiffness (Figures 6A, B). CBD treatment significantly improved systolic function and LV myocardium contractility and reverted EAM-associated diastolic dysfunction and myocardial stiffness (Figures 6A, B). After the induction of autoimmune myocarditis, we observed loss of body weight in our animals (Figure 6C). Loss of body weight reached its peak during the acute inflammatory phase, which was mostly recovered by the time of sacrifice (Figure 6C).
Effects of CBD on EAM-associated myocardial dysfunction and loss of body weight. (A) Classic indices of left ventricular systolic (ejection fraction, cardiac output, dP/dtmax) and diastolic (left ventricular end-diastolic pressure [LVEDP], TauW) and load-independent contractility (ESPVR), PRSW and diastolic stiffness (slope of EDPVR) parameters. (B) Representative pressure-volume loops of CFA, EAM and EAM + CBD groups. (C) Graph of body weight changes during the study course. *P < 0.05 versus CTL; #P < 0.05 versus EAM.
Discussion
Myocarditis is a major cause of dilated cardiomyopathy and subsequent chronic heart failure leading to arrhythmias, sudden cardiac death and cardiac transplantation (3). Among several factors, autoimmunity plays an important role in the pathophysiology of myocarditis. As a result of different cardiac injuries (including microbial infection, ischemic and toxic injury), MyHCα can be released and become an antigen for the immune system (25), causing a complex autoimmune response that leads to cardiac dysfunction and remodeling (26). Despite the growing number of studies investigating the pathology of autoimmune myocarditis, the therapeutic options are limited, and new possible treatment options are needed (3). The nonpsychoactive CBD, a constituent of Cannabis sativa, has been reported to be antiinflammatory, antioxidant and cytoprotective independently of cannabinoid 1 and 2 receptors (15,27,28).
In the present study, we describe that CBD (a) inhibits T cell-mediated myocardial inflammation and consequent myocardial remodeling/fibrosis and (b) improves myocardial dysfunction in the cardiac myosin-induced experimental autoimmune myocarditis model.
Consistent with previous investigations (8,10,20,25), we found that immunization with the MyHCα334–352 of mice resulted in severe inflammatory cell infiltration and necrosis on H&E-stained myocardial sections. Autoimmune myocarditis is considered to be a disease primarily driven by T-cell activation induced by cardiac myosin release (29). Consistent with this assumption, we detected significantly increased mRNA expression levels of CD3e, CD3g, CD4 and CD8a, reflecting the accumulation of the helper and cytotoxic T cells. A complex interplay between T cells and various proinflammatory mediators (for example, TNF-α and IL12 [30]) leads to a complex immunological response including activation of macrophages (31,32) and dendritic cells (30). In agreement with these, we observed significantly increased gene expression of Itgax, EmR f4/80, MCP1 and IL1β markers, suggesting activation, chemotaxis and infiltration of macrophages, monocytes and dendritic cells in the myocardium. Although T-cell activation has a critical role in the development of autoimmune myocarditis, other crucial processes have been identified during the past decades. Kaya et al. (33) showed that the activation of the complement system acting via complement receptors 1 and 2 is critical for the induction of experimental autoimmune myocarditis in mice (33). Eriksson et al. (34) showed that IL-6 is necessary for the development of EAM by using IL-6−/− mice. They found that IL-6 is required for the upregulation of the C3 complement factor during the immunization. The research group of Čiháková showed that IL-6 is upregulated both at 14 and 21 d after immunization and that it could be a possible mechanism contributing to the development of dilated cardiomyopathy in EAM (26). Consistent with these findings, we showed that IL-6 is upregulated in our animals with the plausible upregulation of the complement system.
CBD was shown to protect in various diseases associated with inflammation. Rajesh et al. (35) demonstrated that CBD attenuated high glucose-induced monocyte transendothelial migration and adhesion to endothelium and restored endothelial barrier function in vitro (35). CBD also attenuated neutrophil infiltration in a hepatic ischemia-reperfusion injury model in vivo and TNF-α secretion by hepatic Kupffer cells in vitro (28). CBD treatment protected against cisplatin-induced kidney injury in a mouse model, in which the complement system activation plays an important role in mediating inflammation and tissue injury (16,36). CBD has also been shown to attenuate inflammatory cell infiltration in models of colitis, hepatitis and neuroinflammation, among others (12). In agreement with these previous findings, we observed tremendous inhibition of inflammatory cell infiltration on H&E-stained myocardium sections in the CBD-treated animals reflected by decreased mRNA expression levels of different T-cell markers (namely CD3e, CD3g, CD4 and CD8), likewise markers of macrophage, monocyte and dendritic cell activation (Itgax, EmR f4/80, MCP1 and IL1β). CBD also reduced the elevated IL-6 mRNA levels. It was reported that CBD might inhibit specific immune response (B cells and CD4+ as well as CD8+ T cell-associated responses) and the high risk of autoimmune response (37). In agreement with this, we propose that CBD treatment may affect both helper and cytotoxic T-cell infiltration, thus preventing EAM-related inflammation.
It is well known that inflammation is associated with excessive oxidative/nitrative stress. When the macrophages and monocytes become activated, they begin to produce various cytokines and reactive oxygen species (ROS) and reactive nitrogen species (RNS) (38), which contributes to the detrimental effects of the overactivation of the immune response and leads to a vicious cycle of oxidative stress and inflammation. ROS/RNS rapidly modulates the expression or activity of various key proteins involved in Ca2+ handling (for example, SERCA) (39). One of the major sources of ROS in macrophages, cardiomyocytes, fibroblasts and endothelial or smooth muscle cells is the NADPH oxidase system (40,41), which has been implicated in the pathophysiology of EAM (42). We found that CBD attenuated excessive oxidative/nitrative stress (evidenced by decreased myocardial 3-NT and 4-HNE levels in treated mice with myocarditis) and the increased expression of NADPH oxidase isoform p47phox associated with the autoimmune inflammation, as well as improved the downregulation of SERCA mRNA.
Fibrotic remodeling of the myocardium is a well-known consequence of EAM (8,10). Consistent with previous studies, we observed marked fibrotic remodeling in the heart of EAM mice. The activation of profibrotic signaling and subsequent fibrosis was shown to be associated with IL-6 overexpression (43) and overactivation of IL1 signaling (44), with the transition of CD133+ progenitor cells into myofibroblasts (45) and with the subsequent excessive oxidative stress. We found that CBD treatment had a significant antifibrotic effect in EAM, at least in part, by decreasing the profibrotic IL-6 and IL1 signaling and limiting the number of macrophages able to transform into myofibroblasts.
It is a well-known phenomenon that EAM is associated with systolic and diastolic cardiac dysfunction due to the ongoing inflammation, necrosis, cardiac cell death and fibrosis (46,47). In agreement with previous results, we found markedly decreased global (systolic and diastolic) cardiac function in our EAM model. The inflammation-driven necrosis, loss of functional myocytes and oxidative/nitrative stress-induced contractile protein damage can play a crucial role in the development of systolic dysfunction (48,49). We observed significant deterioration of the systolic performance derived from classic load-dependent (ejection fraction, cardiac output and dP/dtmax) and load-independent (ESPVR, PRSW) functional parameters. The excessive inflammatory response, coupled with oxidative/nitrative stress, can play a role by inactivating key proteins participating in diastole (for example, sarco-/endoplasmic reticulum Ca2+ pump SERCA) (38) and in promoting fibrotic remodeling. With diastolic dysfunction, we consistently found that the relaxation marker Tau and the slope of EDPVR and LVEDP, indices of diastolic stiffness, were significantly increased in EAM. CBD treatment markedly improved both systolic and diastolic dysfunction in mice with EAM.
Conclusion
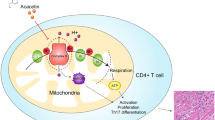
Collectively, our study demonstrates that CBD treatment markedly attenuates autoimmune myocarditis and improves myocardial dysfunction and heart failure primarily by its antiinflammatory and antifibrotic effects. The proposed mechanisms by which CBD might exert its beneficial cardioprotective effects are summarized on Figure 7. These results, coupled with the proven safety of CBD in human clinical trials and its current orphan drug approval by the FDA for different neurological disorders, suggest that it has tremendous therapeutic potential in the therapy of myocarditis with different etiologies and various autoimmune disorders. The latter is also supported by beneficial effects of CBD in preventing graft versus host disease after allogeneic hematopoietic cell transplantation in a recent phase II human study (50), as well as in mice with arthritis (51). Attenuation of the T cell-mediated injury by CBD also suggests that it may have therapeutic utility in management of organ transplantation/rejection.
Disclosure
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
References
Richardson P, et al. (1996) Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of Cardiomyopathies. Circulation. 93:841–2.
Fabre A, Sheppard MN. (2006) Sudden adult death syndrome and other non-ischaemic causes of sudden cardiac death. Heart. 92:316–20.
Kindermann I, et al. (2012) Update on myocarditis. J. Am. Coll. Cardiol. 59:779–92.
Stehlik J, et al. (2010) The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult heart transplant report—2010. J. Heart Lung Transplant. 29:1089–103.
Kawai C. (1999) From myocarditis to cardiomyopathy: mechanisms of inflammation and cell death: learning from the past for the future. Circulation. 99:1091–100.
Lv H, et al. (2011) Impaired thymic tolerance to alpha-myosin directs autoimmunity to the heart in mice and humans. J. Clin. Invest. 121:1561–73.
Lichtman AH. (2013) The heart of the matter: protection of the myocardium from T cells. J. Autoimmun. 45:90–6.
Hirakawa H, et al. (2015) A DPP-4 inhibitor suppresses fibrosis and inflammation on experimental autoimmune myocarditis in mice. PLoS One. 10:e0119360.
Sukumaran V, et al. (2011) Telmisartan ameliorates experimental autoimmune myocarditis associated with inhibition of inflammation and oxidative stress. Eur. J. Pharmacol. 652:126–35.
Ong S, et al. (2015) Natural killer cells limit cardiac inflammation and fibrosis by halting eosinophil infiltration. Am. J. Pathol. 185:847–61.
Pacher P, Batkai S, Kunos G. (2006) The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 58:389–462.
Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. (2009) Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 30:515–27.
Cunha JM, et al. (1980) Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology. 21:175–85.
Borrelli F, et al. (2009) Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J. Mol. Med. (Berl). 87:1111–21.
Rajesh M, et al. (2010) Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J. Am. Coll. Cardiol. 56:2115–25.
Pan H, et al. (2009) Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J. Pharmacol. Exp. Ther. 328:708–14.
Yang L, et al. (2014) Cannabidiol protects liver from binge alcohol-induced steatosis by mechanisms including inhibition of oxidative stress and increase in autophagy. Free Radic. Biol. Med. 68:260–7.
Pazos MR, et al. (2012) Cannabidiol administration after hypoxia-ischemia to newborn rats reduces long-term brain injury and restores neurobehavioral function. Neuropharmacology. 63:776–83.
Donermeyer DL, Beisel KW, Allen PM, Smith SC. (1995) Myocarditis-inducing epitope of myosin binds constitutively and stably to I-Ak on antigen-presenting cells in the heart. J. Exp. Med. 182:1291–300.
Cihakova D, Sharma RB, Fairweather D, Afanasyeva M, Rose NR. (2004) Animal models for autoimmune myocarditis and autoimmune thyroiditis. Methods Mol. Med. 102:175–93.
Gaoni Y, Mechoulam R. (1971) The isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish. J. Am. Chem. Soc. 93:217–24.
Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA. (2008) Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat. Protoc. 3:1422–34.
Hao E, et al. (2015) Cannabidiol protects against doxorubicin-induced cardiomyopathy by modulating mitochondrial function and biogenesis. Mol. Med. 21:38–45.
Barin JG, et al. (2013) Fatal eosinophilic myocarditis develops in the absence of IFN-gamma and IL-17A. J. Immunol. 191:4038–47.
Fairweather D, Kaya Z, Shellam GR, Lawson CM, Rose NR. (2001) From infection to autoimmunity. J Autoimmun. 16:175–86.
Baldeviano GC, et al. (2010) Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circ. Res. 106:1646–55.
Hampson AJ, Grimaldi M, Axelrod J, Wink D. (1998) Cannabidiol and (−)delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc. Natl. Acad. Sci. U. S. A. 95:8268–73.
Mukhopadhyay P, et al. (2011) Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free Radic. Biol. Med. 50:1368–81.
Smith SC, Allen PM. (1991) Myosin-induced acute myocarditis is a T cell-mediated disease. J. Immunol. 147:2141–7.
Eriksson U, et al. (2003) Activation of dendritic cells through the interleukin 1 receptor 1 is critical for the induction of autoimmune myocarditis. J. Exp. Med. 197:323–31.
Martinez FO, Sica A, Mantovani A, Locati M. (2008) Macrophage activation and polarization. Front Biosci. 13:453–61.
Cihakova D, et al. (2008) Interleukin-13 protects against experimental autoimmune myocarditis by regulating macrophage differentiation. Am. J. Pathol. 172:1195–208.
Kaya Z, et al. (2001) Contribution of the innate immune system to autoimmune myocarditis: a role for complement. Nat. Immunol. 2:739–45.
Eriksson U, et al. (2003) Interleukin-6-defirient mice resist development of autoimmune myocarditis associated with impaired upregulation of complement C3. Circulation. 107:320–5.
Rajesh M, et al. (2007) Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am. J. Physiol. Heart Circ. Physiol. 293:H610–9.
Pan H, et al. (2009) Anaphylatoxin C5a contributes to the pathogenesis of cisplatin-induced nephrotoxicity. Am. J. Physiol. Renal Physiol. 296:F496–504.
Ignatowska-Jankowska B, Jankowski M, Glac W, Swiergel AH. (2009) Cannabidiol-induced lymphopenia does not involve NKT and NK cells. J. Physiol. Pharmacol. 60 Suppl 3:99–103.
Pacher P, Beckman JS, Liaudet L. (2007) Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 87:315–424.
Lokuta AJ, et al. (2005) Increased nitration of sarcoplasmic reticulum Ca2+-ATPase in human heart failure. Circulation. 111:988–95.
Lee CF, Qiao M, Schroder K, Zhao Q, Asmis R. (2010) Nox4 is a novel inducible source of reactive oxygen species in monocytes and macrophages and mediates oxidized low density lipoprotein-induced macrophage death. Circ. Res. 106:1489–97.
Bedard K, Krause KH. (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 87:245–313.
Sukumaran V, et al. (2012) Olmesartan attenuates the development of heart failure after experimental autoimmune myocarditis in rats through the modulation of ANG 1–7 mas receptor. Mol. Cell. Endocrinol. 351:208–19.
Diaz JA, et al. (2009) Critical role for IL-6 in hypertrophy and fibrosis in chronic cardiac allograft rejection. Am. J. Transplant. 9:1773–83.
Blyszczuk P, et al. (2009) Myeloid differentiation factor-88/interleukin-1 signaling controls cardiac fibrosis and heart failure progression in inflammatory dilated cardiomyopathy. Circ. Res. 105:912–20.
Blyszczuk P, et al. (2013) Nitric oxide synthase 2 is required for conversion of pro-fibrogenic inflammatory CD133(+) progenitors into F4/80(+) macrophages in experimental autoimmune myocarditis. Cardiovasc. Res. 97:219–29.
Liu X, Zhu X, Wang A, Fan H, Yuan H. (2009) Effects of angiotensin-II receptor blockers on experimental autoimmune myocarditis. Int. J. Cardiol. 137:282–8.
Nakagawa P, et al. (2012) Treatment with N-acetyl-seryl-aspartyl-lysyl-proline prevents experimental autoimmune myocarditis in rats. Am. J. Physiol. Heart Circ. Physiol. 303:H1114–27.
Canton M, et al. (2011) Oxidation of myofibrillar proteins in human heart failure. J. Am. Coll. Cardiol. 57:300–9.
Varga ZV, et al. (2013) MicroRNA-25-dependent up-regulation of NADPH oxidase 4 (NOX4) mediates hypercholesterolemia-induced oxidative/nitrative stress and subsequent dysfunction in the heart. J. Mol. Cell. Cardiol. 62:111–21.
Yeshurun M, et al. (2015) Cannabidiol for the prevention of graft-versus-host-disease after allogeneic hematopoietic cell transplantation: results of a phase II study. Biol. Blood Marrow Transplant. 21:1770–5.
Malfait AM, et al. (2000) The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. U. S. A. 97:9561–6.
Acknowledgments
This work was supported by the Intramural Research Program of NIAAA/NIH to P Pacher and by the scholarship of the Hungarian-American Enterprise Scholarship Fund/Council on International Educational Exchange to C Matyas. ZV Varga was supported by the Rosztoczy Foundation. The authors are grateful to George Kunos, the Scientific Director of NIAAA, for continuous support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, and provide a link to the Creative Commons license. You do not have permission under this license to share adapted material derived from this article or parts of it.
The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this license, visit (http://creativecommons.org/licenses/by-nc-nd/4.0/)
About this article
Cite this article
Lee, WS., Erdelyi, K., Matyas, C. et al. Cannabidiol Limits T Cell-Mediated Chronic Autoimmune Myocarditis: Implications to Autoimmune Disorders and Organ Transplantation. Mol Med 22, 136–146 (2016). https://doi.org/10.2119/molmed.2016.00007
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2119/molmed.2016.00007