Abstract
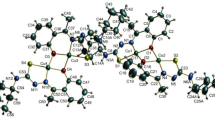
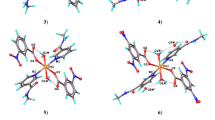
Synthesis and characterization of eight new complexes of various structural types are reported. With 5-nitro-2-furancarboxylic acid (5-NO2-2-fucH), two monomeric complexes, [Cu(5-NO2-2-fuc)2(H2O)2] (II) and [Cu(5-NO2-2-fuc)2(H2O)4] (III), as well as a dimeric complex with ethylnicotinate (Etnic), [Cu(5-NO2-2-fuc)2(Etnic)2]2 (V), were prepared. With other acids: 2,5-dimethyl-3-furancarboxylic acid (2,5-Me2-3-fucH), 2-thiophencarboxylic acid (2-tpcH), 3-methyl-2-thiophencarboxylic acid (3-Me-2-tpcH) or 5-methyl-2-thiophencarboxylic acid (5-Me-2-tpcH), only dimeric complexes [Cu(2,5-Me2-3-fuc)2(H2O)]2 (I), [Cu(2,5-Me2-3-fuc)2(Etnic)]2 (IV), [Cu(2-tpc)2(Etnic)]2 (VI), [Cu(3-Me-2-tpc)2(Etnic)]2 (VII) and Cu(5-Me-2-tpc)2(Etnic)]2 (VIII) have been synthesised. Characterizations of the complexes were based on elemental analysis and infrared, electronic, EPR and magnetic measurements. Moreover, complexes III, V, VII and VIII were also studied by X-ray structural analysis. Two structural types of dimeric complexes were observed differing in the number of carboxylate bridges. Most of the dinuclear complexes exhibit the common “paddle-wheel” structural motif while the molecular structure of V contains two pentacoordinated copper(II) ions bridged by two carboxylate groups of two 5-nitro-2-furancarboxylate ligands resulting in the intramolecular copper-copper distance of 4.4960(8) Å. Magnetic properties (monomeric EPR signal and isotropic exhchange constant (J of approximately 0 cm−1) of V confirmed a very weak magnetic interaction between copper centres.
Similar content being viewed by others
References
Bencini, A., & Gatteschi, D. (1990). EPR of exchange-coupled systems. Berlin, Germany: Springer.
Betteridge, P. W., Carruthers, J. R., Copper, R. I., Prout, K., & Watkin, D. J. (2003). CRYSTALS version 12: software for guided crystal structure analysis. Journal of Applied Crystallography, 36, 1487. DOI: 10.1107/s0021889803021800.
Bourhis, L. J., Dolomanov, O. V., Gildea, R. J., Howard, J. A. K., & Puschmann, H. (2015). The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment — Olex2 dissected. Acta Crystallographica, Section A: Foundation and Advances, 71, 59–75. DOI: 10.1107/s2053273314022207.
Bruker (2001). SADABS [computer software]. Madison, WI, USA: Bruker.
Burla, M. C., Caliandro, R., Carrozzini, B., Cascarano, G. L., Cuocci, C., Giacovazzo, C., Mallamo, M., Mazzone, A., & Polidori, G. (2015). Crystal structure determination and refinement via SIR2014. Journal of Applied Crystallography, 48, 306–309. DOI: 10.1107/s1600576715001132.
Buvaylo, E. A., Kokozay, V. N., Vassilyeva, O. Y., Skelton, B. W., Jezierska, J., & Ozarowski, A. (2011). A new Cu/Zn carboxylato-bridged 1D polymer: Direct synthesis, X-ray structure and magnetic properties. Inorganica Chimica Acta, 373, 27–31. DOI: 10.1016/j.ica.2011.03.040.
Catterick, J., & Thornton, P. (1977). Structures and physical properties of polynuclear carboxylates. Advances in Inorganic Chemistry and Radiochemistry, 20, 291–362. DOI: 10.1016/s0065-2792(08)60041-2.
Chen, C. L., Zou, Y., Qiu, P., Wen, Y. H., Li, J. Y., Hong, Z. H., Lin, X. M., Xu, A. X., & Cai, Y. P. (2009). Synthesis and characterization of two temperature-dependent copper(II) complexes based on 2,6-dimethylpyridine-3,5-dicarboxylate. Journal of Coordination Chemistry, 62, 2480–2489. DOI: 10.1080/00958970902862636.
Davies, H. O., Gillard, R. D., Hursthouse, M. B., & Karaulov, A. (1995). Natural glycine unidentate on copper(II): a novel mode of bonding. Journal of the Chemical Society, Dalton Transactions, 1995, 2333–2336. DOI: 10.1039/dt9950002333.
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., & Puschmann, H. (2009). OLEX2: a complete structure solution, refinement and analysis program. Journal of Applied Crystallography, 42, 339–341. DOI: 10.1107/s0021889808042726.
Etaiw, S. E. H., Sultan, A. S., & El-bendary, M. M. (2011). In vitro and in vivo activity of novel 3D-organotin supramolecular coordination polymers based on CuCN and pyridine bases. Journal of Organometallic Chemistry, 696, 1668–1676. DOI: 10.1016/j.jorganchem.2011.02.003.
Groom, C. R., & Allen, F. H. (2014). The Cambridge structural database in retrospect and prospect. Angewandte Chemie International Edition, 53, 662–671. DOI: 10.1002/anie.201306438.
Guo, J. Y., Zhang, T. L., Zhang, J. G., Liu, Y. H., & Yu, K. B. (2006). Direct synthesis, crystal structures and thermal analyses of the two-dimensional Cu(ntp)2(H2O)4 crystal (ntp = 2-nitroterephthalate. Chinese Journal of Inorganic Chemistry, 22, 995–999.
Huang, J., Li, Y. Z., Sun, G. C., Dai, R. B., Li, Q. X., Wang, L. F., & Xia, C. G. (2000). Tetraaqua(5-fluorouracil-1-acetato-O)copper(II) tetrahydrate. Acta Crystallographica, Section C: Crystal Structure Communications, 56, e489–e490. DOI: 10.1107/s0108270100013044.
In, Y., Hayashi, C., & Ishida, T. (1997). Metal dependent coordination of biomolecular ligand: X-ray crystal structures of copper, cobalt and calcium complexes of (3-hydroxy-5-(hydroxymethyl)-2-methylisonicotinic acid) 5-phosphate, an oxidized pyridoxal 5-phosphate. Inorganica Chimica Acta, 260, 111–118. DOI: 10.1016/s0020-1693(96)05538-7.
Karpova, E. V., Boltalin, A. I., Zakharov, M. A., Sorokina, N. I., Korenev, Y. M., & Troyanov, S. I. (1998). Synthesis and crystal structure of copper(II) trifluoroacetates, Cu2(CF3COO)4 · 2CH3CN and Cu(CF3COO)2(H2O)4. Zeitschrift für Anorganische und Allgemeine Chemie, 624, 741–744. DOI: 10.1002/SICI)1521-3749(199804)624:4<741∷AID-ZAAC741>3.0.CO;2-4.
Kato, M., & Muto, Y. (1988). Factors affecting the magnetic properties of dimeric copper(II) complexes. Coordination Chemistry Reviews, 92, 45–83. DOI: 10.1016/0010-8545(88)85005-7.
Kennard, C. H. L., Stewart, S. W., O’Reilly, E. J., Smith, G., & White, A. H. (1985). Metal-phenoxyalkanoic acid interactions-XVI. Molecular structures of the divalent cobalt, copper, manganese, nickel and zinc complexes with (2-nitrophe. Polyhedron, 4, 697–705. DOI: 10.1016/s0277-5387(00)86685-1.
König, E. (1966). Magnetic properties of coordination and organometalic transition metal compounds. Exeter, UK: Springer-Verlag.
Kuchtanin, V., Moncoľ, J., Mroziński, J., Kalińska, B., Padělková, Z., Švorec, J., Segľa, P., & Melník, M. (2013). Study of copper(II) thiophenecarboxylate complexes with N-methylnicotinamide. Polyhedron, 50, 546–555. DOI: 10.1016/j.poly.2012.11.041.
McGowan, P. C. (2005). Metal complexes as pharmaceuticals. Annual Reports Section “A” (Inorganic Chemistry), 101, 631–648. DOI: 10.1039/b413633k.
Melník, M. (1981). Mono-, bi-, tetra- and polynuclear copper(II) halogenocarboxylates. Coordination Chemistry Reviews, 36, 1–44. DOI: 10.1016/s0010-8545(00)80504-4.
Melník, M. (1982). Study of the relation between the structural data and magnetic interaction in oxo-bridged binuclear copper(II) compounds. Coordination Chemistry Reviews, 42, 259–293. DOI: 10.1016/s0010-8545(00)80537-8.
Melník, M., Kabešová, M., Koman, M., Macášková, L., Garaj, J., Holloway, C. E., & Valent, A. (1998). Copper(II) coordination compounds: Classification and analysis of crystallographic and structural data III. Dimeric compounds. Journal of Coordination Chemistry, 45, 147–359. DOI: 10.1080/00958979808027144.
Moncol J., Mudra, M., Lönnecke, P., Hewitt, M., Valko, M., Morris, H., Svorec, J., Melnik, M., Mazur, M., & Koman, M. (2007). Crystal structures and spectroscopic behavior of monomeric, dimeric and polymeric copper(II) chloroacetate adducts with isonicotinamide, N-methylnicotinamide and N, N-diethylnicotinamide. Inorganica Chimica Acta, 360, 3213–3225. DOI: 10.1016/j.ica.2007.03.27.
Moncoľ, J., Kuchtanin, V., Polakovičová, P., Mroziński, J., Kalińska, B., Koman, M., Padělková, Z., Segľa, P., & Melník, M. (2012). Study of copper(II) thiophenecarboxylate complexes with nicotinamide. Polyhedron, 45, 94–102. DOI: 10.1016/j.poly.2012.07.069.
Nakamoto, K. (1997). Infrared and Raman spectra of inorganic and coordination compounds. New York, NY, USA: Wiley.
Ozarowski, A. (2008). The zero-field-splitting parameter D in binuclear copper(II) carboxylates is negative. Inorganic Chemistry, 47, 9760–9762. DOI: 10.1021/ic801560e.
Ozarowski, A., Szymańska, I. B., Muzioł, T., & Jezierska, J. (2009). High-field EPR and magnetic susceptibility studies on binuclear and tetraniclear copper trifluoroacetate complexes. X-ray structure determination of three tetranuclear quinoline adducts of copper(II) trifluoroacetate. Journal of the American Chemical Society, 131, 10279–10292. DOI: 10.1021/ja902695y.
Palatinus, L., & Chapuis, G. (2007). SUPERFLIP — a computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. Journal of Applied Crystallography, 40, 786–790. DOI: 10.1107/s0021889807029238.
Paluchowska, B., Maurin, J. K., & Leciejewicz, J. (1998). Dinuclear copper(II) complexes with 3-furancarboxylic acid and 2-thiophenecarboxylic acid. Journal of Coordination Chemistry, 44, 183–192. DOI: 10.1080/00958979808022892.
Perec, M., & Baggio, R. (2010). Di-μ-acetato-bis[(acetato)-κ2O, O′)bis(isonicotinamide-κN)copper(II)]. Acta Crystallographica, Section E: Structure Reports Online, 66, m275–m276. DOI: 10.1107/s1600536810004393.
Segľa, P., Palicová, M., Mikloš, D., Koman, M., Melník, M., Korábik, M., Mroziński, J., Głowiak, T., Sundberg, M. R., & Lönnecke, P. (2004). Synthesis, spectral and magnetic properties, and crystal structures of copper(II) pyridinecarboxylate adducts with N-heterocyclic ligands. Zeitschrift für Anorganische und Allgemeine Chemie, 630, 470–478. DOI: 10.1002/zaac.200300365.
Sertçelik, M., Cåaylak Delibas, N., Necefoglu, H., & Hökelek, T. (2013). Bis(μ-4-formylbenzoato-κ2O:O′)bis[(4-formylbenzoato-κ2O,O′)bis(isonicotinamide-κN1)copper(II)]. Acta Crystallographica, Section E: Structure Reports, 69, m290–m291. DOI: 10.1107/s1600536813010908.
Sheldrick, G. M. (2015a). SHELXT — Integrated space-group and crystal structure determination. Acta Crystallographica, Section A: Foundation and Advances, 71, 3–8. DOI: 10.1107/s2053273314026370.
Sheldrick, G. M. (2015b). Crystal structure refinement with SHELXL. Acta Crystallographica, Section C: Structural Chemistry, 71, 3–8. DOI: 10.1107/s2053229614024218.
Siddiqi, Z. A., Khalid, M., Kumar, S., Shahid, M., & Noor, S. (2010). Antimicrobial and SOD activities of novel transition metal complexes of pyridine-2,6-dicarboxylic acid containing 4-picoline as auxilliary ligand. European Journal of Medicinal Chemistry, 45, 264–269. DOI: 10.1016/j.ejmech.2009.10.005.
Siemens (1994). XEMP, Siemens analytical X-ray instruments. Madison, WI, USA: Siemens.
Smith, G., & Wermuth, U. D. (2010). The structure of the copper(II) complex with 4,5-dichlorophthalic acid: The 1:1 complex ‘adduct’ salt trans-tetraaqua(2-carboxy-4,5-dichlorobenzoato)(4,5-dichlorobenzene-1,2-dicarb oxylic acid) copper(II) 2-carboxy-4,5-dichlorobenzoate. Journal of Chemical Crystallography, 40, 151–155. DOI: 10.1007/s10870-009-9623-z.
Stomberg, R., & Lundquist, K. (1989). The crystal structure of tetraaquabis[(2-methoxyphenoxy)-acetato]copper(II), [Cu(C9H9O4)2(H2O)4]. Acta Chemica Scandinavica, 43, 160–163. DOI: 10.3891/acta.chem.scand.43-0160.
Švorec, J., Valko, M., Moncoľ, J., Mazúr, M., Melník, M., & Telser, J. (2009). Determination of intermolecular copper-copper distances from the EPR half-field transitions and their comparison with distances from X-ray structures: applications to copper(II) complexes with biologically important ligands. Transition Metal Chemistry, 34, 129–134. DOI: 10.1007/s11243-008-9168-6.
Švorec, J., Polakovičová, P., Moncoľ, J., Kuchtanin, V., Breza, M., Šoralová, S., Padělková, Z., Mrozinski, J., Lis, T., & Segľa, P. (2014). Structural, magnetic and quantum-chemical study of dinuclear copper(II) thiophenecarboxylate and furancarboxylate complexes. Polyhedron, 81, 216–226. DOI: 10.1016/j.poly.2014.05.071.
Swaminathan, S., & Alangaden, G. J. (2010). Treatment of resistant enterococcal urinary tract infections. Current Infectious Disease Reports, 12, 455–464. DOI: 10.1007/s11908-010-0138-8.
Telser, J., Krzystek, J., & Ozarowski, A. (2014). High-frequency and high-field electron paramagnetic resonance (HFEPR): a new spectroscopic tool for bioinorganic chemistry. JBIC Journal of Biological Inorganic Chemistry, 19, 297–318. DOI: 10.1007/s00775-013-1084-3.
Weder, J. E., Dillon, C. T., Hambley, T. W., Kennedy, B. J., Lay, P. A., Biffin, J. R., Regtop, H. L., & Davies, N. M. (2002). Copper complexes of non-steroidal anti-inflammatory drugs: an opportunity yet to be realized. Coordination Chemistry Reviews, 232, 95–126. DOI: 10.1016/s0010-8545(02)00086-3.
Yang, E. C., Feng, W., Wang, J. Y., & Zhao, X. J. (2010). Crystal structure, thermal stability and theoretical investigation on four 1,3-bis(1,2,4-triazol-1-yl)propane-based copper(II) complexes. Inorganica Chimica Acta, 363, 308–316. DOI: 10.1016/j.ica.2009.10.014.
Yenikaya, C., Poyraz, M., Sarı, M., Demirci, F., Ýlkimen, H., & Büyükgüngör, O. (2009). Synthesis, characterization and biological evaluation of a novel Cu(II) complex with the mixed ligands 2,6-pyridinedicarboxylic acid and 2-aminopyridine. Polyhedron, 28, 3526–3532. DOI: 10.1016/j.poly.2009.05.079.
Yeşilela, O. Y., Ölmez, H., Uçar, I., Bulut, A., & Kazak, C. (2005). Synthesis, spectrothermal behaviour and molecular structure of aquaorotatotriethanolaminenickel(II) monohydrate. Zeitschrift für Anorganische und Allgemeine Chemie, 631, 3100–3103. DOI: 10.1002/zaac.200500297.
Yuan, S., & Li, X. D. (2008). Crystal structure of tetraaqua-cis-bis(4-(sulfonylglycinato)benzoato)-copper(II), Cu(H2O)4 (C9H8NO6S)2. Zeitschrift für Kristallographie — New Crystal Structures, 223, 267–268. DOI: 10.1524/ncrs.2008.0113.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bertová, P., Kuchtanin, V., Růžičková, Z. et al. Different structural types of copper(II) furan- and thiophencarboxylates: X-ray structural, EPR, spectral and magnetic analyses. Chem. Pap. 70, 114–125 (2016). https://doi.org/10.1515/chempap-2015-0204
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/chempap-2015-0204