ABSTRACT
Background
Matrix metalloproteinase 11 (MMP-11) is a matrix degrading enzyme known to be involved in the remodeling of extracellular matrix proteins. This enzyme recently has been reported to play a key role in tumor progression and results in poor clinical outcomes for several different types of tumors.
Methods
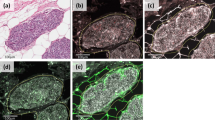
A total of 192 patients diagnosed with invasive ductal carcinoma between 2000 and 2005 were included in this study. MMP-11 expression in tumors and stromal fibroblast-like cells was analyzed by immunohistochemical staining on a tissue microarray. Subsequently, evaluation of the associations between MMP-11 expression and clinicopathological characteristics was performed.
Results
MMP-11 expression of stromal fibroblast-like cells was correlated with prognostic factors, including tumor size, metastasis, histological grade, central tumor fibrosis, p53 expression, and luminal A subtype and was linked to therapeutic markers, such as ER and HER2 (all p < 0.05). There was a significant relationship between worse overall survival and MMP-11 expression in both tumors and stromal fibroblast-like cells (all p < 0.05). In multivariate analysis, MMP-11 expression of stromal fibroblast-like cells was still significantly associated with poor prognosis (p = 0.043).
Conclusions
MMP-11 expression was significantly related to clinicopathological parameters, which may be essential to the prediction of disease outcome in patients with invasive ductal carcinoma of the breast.



Similar content being viewed by others
REFERENCES
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Dixon JM, Page DL, Anderson TJ, et al. Long-term survivors after breast cancer. Br J Surg. 1985;72:445–8.
Jezierska A, Motyl T. Matrix metalloproteinase-2 involvement in breast cancer progression: a mini-review. Med Sci Monit. 2009;15:RA32–40.
Peppercorn J, Perou CM, Carey LA. Molecular subtypes in breast cancer evaluation and management: divide and conquer. Cancer Invest. 2008;26:1–10.
Yurchenco PD, Schittny JC. Molecular architecture of basement membranes. FASEB J. 1990;4:1577–90.
Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000;18:1135–49.
Wolf C, Rouyer N, Lutz Y, et al. Stromelysin 3 belongs to a subgroup of proteinases expressed in breast carcinoma fibroblastic cells and possibly implicated in tumor progression. Proc Nati Acad Sci. 1993;90:1843–7.
Engel G, Heselmeyer K, Auer G, Backdahl M, Eriksson E, Linder S. Correlation between stromelysin-3 mRNA level and outcome of human breast cancer. Int J Cancer. 1994;58:830–5.
Westermarck J, Kahari VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–92.
Pei D, Majmudar G, Weiss SJ. Hydrolytic inactivation of a breast carcinoma cell-derived serpin by human stromelysin-3. J Biol Chem. 1994;269:25849–55.
Kahari VM, Saarialho-Kere U. Matrix metalloproteinases in skin. Exp Dermatol. 1997;6:199–213.
Basset P, Bellocq JP, Wolf C, et al. A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature. 1990;348:699–704.
Okada A, Bellocq JP, Rouyer N, et al. Membrane-type matrix metalloproteinase (MT-MMP) gene is expressed in stromal cells of human colon, breast, and head and neck carcinomas. Proc Nati Acad Sci. 1995;92:2730–4.
Takeuchi T, Adachi Y, Nagayama T, Furihata M. Matrix metalloproteinase-11 overexpressed in lobular carcinoma cells of the breast promotes anoikis resistance. Virchows Archiv. 2011;459:291–7.
Nakopoulou L, Panayotopoulou EG, Giannopoulou I, et al. Stromelysin-3 protein expression in invasive breast cancer: relation to proliferation, cell survival and patients’ outcome. Mod Pathol. 2002;15:1154–61.
Cheng CW, Yu JC, Wang HW, et al. The clinical implications of MMP-11 and CK-20 expression in human breast cancer. Clin Chim Acta. 2010;411:234–41.
Asch PH, Basset P, Roos M, Grosshans E, Bellocq JP, Cribier B. Expression of stromelysin 3 in keratoarcanthoma and squamous cell carcinoma. Am J Dermatopathol. 1999;21:146–50.
Anderson IC, Sugarbaker DJ, Ganju RK, et al. Stromelysin-3 is overexpressed by stromal elements in primary non-small cell lung cancers and regulated by retinoic acid in pulmonary fibroblasts. Cancer Res. 1995;55:4120–6.
Munck-Wikland E, Heselmeyer K, Lindholm J, Kuylenstierna R, Auer G, Engel G. Stromelysin-3 mRNA expression in dysplasias and invasive epithelial cancer of the larynx. Int J Oncol. 1998;12:859–64.
Masson R, Lefebvre O, Noel A, et al. In vivo evidence that the stromelysin-3 metalloproteinase contributes in a paracrine manner to epithelial cell malignancy. J Cell Biol. 1998;140:1535–41.
Peruzzi D, Mori F, Conforti A, et al. MMP11: a novel target antigen for cancer immunotherapy. Clin Cancer Res. 2009;15:4104–13.
Genestie C, Zafrani B, Asselain B, et al. Comparison of the prognostic value of Scarff-Bloom-Richardson and Nottingham histological grades in a series of 825 cases of breast cancer: major importance of the mitotic count as a component of both grading systems. Anticancer Res. 1998;18:571–6.
Robbins P, Pinder S, de Klerk N, et al. Histological grading of breast carcinomas: a study of interobserver agreement. Hum Pathol. 1995;26:873–9.
Sousa B, Paredes J, Milanezi F, et al. P-cadherin, vimentin and CK14 for identification of basal-like phenotype in breast carcinomas: an immunohistochemical study. Histol Histopathol. 2010;25:963–74.
Del Casar JM, Gonzalez LO, Alvarez E, et al. Comparative analysis and clinical value of the expression of metalloproteases and their inhibitors by intratumor stromal fibroblasts and those at the invasive front of breast carcinomas. Breast Cancer Res Treat. 2009;116:39–52.
Remmele W, Stegner HE. [Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue]. Pathologe. 1987;8:138–40.
Gonzalez LO, Pidal I, Junquera S, et al. Overexpression of matrix metalloproteinases and their inhibitors in mononuclear inflammatory cells in breast cancer correlates with metastasis-relapse. Br J Cancer. 2007;97:957–63.
Gonzalez LO, Corte MD, Junquera S, et al. Expression and prognostic significance of metalloproteases and their inhibitors in luminal A and basal-like phenotypes of breast carcinoma. Hum Pathol. 2009;40:1224–33.
Gonzalez LO, Gonzalez-Reyes S, Marin L, et al. Comparative analysis and clinical value of the expression of metalloproteases and their inhibitors by intratumour stromal mononuclear inflammatory cells and those at the invasive front of breast carcinomas. Histopathology. 2010;57:862–76.
Garcia MF, Gonzalez-Reyes S, Gonzalez LO, et al. Comparative study of the expression of metalloproteases and their inhibitors in different localizations within primary tumours and in metastatic lymph nodes of breast cancer. Int J Exp Pathol. 2010;91:324–34.
Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth–bystanders turning into key players. Curr Opin Genet Dev. 2009;19:67–73.
Behrens P, Rothe M, Wellmann A, Krischler J, Wernert N. The Ets-1 transcription factor is up-regulated together with MMP 1 and MMP 9 in the stroma of pre-invasive breast cancer. J Pathol. 2001;194:43–50.
Sato T, Sakai T, Noguchi Y, Takita M, Hirakawa S, Ito A. Tumor-stromal cell contact promotes invasion of human uterine cervical carcinoma cells by augmenting the expression and activation of stromal matrix metalloproteinases. Gynecol Oncol. 2004;92:47–56.
Vizoso FJ, Gonzalez LO, Corte MD, et al. Study of matrix metalloproteinases and their inhibitors in breast cancer. Br J Cancer. 2007;96:903–11.
Drac P, Klein J, Tichy T, Kolek V, Skarda J. [Prognostic impact of matrix metalloproteinases 2,9, and 11 in stromal cells stage I non-small cell lung cancer]. Cas Lek Cesk. 2007;146:45–7.
Perigny M, Bairati I, Harvey I, et al. Role of immunohistochemical overexpression of matrix metalloproteinases MMP-2 and MMP-11 in the prognosis of death by ovarian cancer. Am J Clin Pathol. 2008;129:226–31.
Thewes M, Worret WI, Engst R, Ring J. Stromelysin-3 (ST-3): immunohistochemical characterization of the matrix metalloproteinase (MMP)-11 in benign and malignant skin tumours and other skin disorders. Clin Exp Dermatol. 1999;24:122–6.
Ito Y, Yoshida H, Kakudo K, Nakamura Y, Kuma K, Miyauchi A. Inverse relationships between the expression of MMP-7 and MMP-11 and predictors of poor prognosis of papillary thyroid carcinoma. Pathology. 2006;38:421–5.
Ahmad A, Hanby A, Dublin E, et al. Stromelysin 3: an independent prognostic factor for relapse-free survival in node-positive breast cancer and demonstration of novel breast carcinoma cell expression. Am J Pathol. 1998;152:721–8.
Selvey S, Haupt LM, Thompson EW, Matthaei KI, Irving MG, Griffiths LR. Stimulation of MMP-11 (stromelysin-3) expression in mouse fibroblasts by cytokines, collagen and co-culture with human breast cancer cell lines. BMC Cancer. 2004;4:40.
Tetu B, Brisson J, Wang CS, Lapointe H, Beaudry G, Blanchette C. Expression of cathepsin D, stromelysin-3, and urokinase by reactive stromal cells on breast carcinoma prognosis. Cancer. 2001;92:2957–64.
Chenard MP, O’Siorain L, Shering S, et al. High levels of stromelysin-3 correlate with poor prognosis in patients with breast carcinoma. Int J Cancer. 1996;69:448–51.
Hahnel E, Harvey JM, Joyce R, Robbins PD, Sterrett GF, Hahnel R. Stromelysin-3 expression in breast cancer biopsies: clinico-pathological correlations. Int J Cancer. 1993;55:771–4.
Hahnel R. Prospective-study of stromelysin-3 expression in breast-cancer biopsies and disease-free survival. Int J Oncol. 1995;7:1315–8.
Kwon YJ, Hurst DR, Steg AD, et al. Gli1 enhances migration and invasion via up-regulation of MMP-11 and promotes metastasis in ERalpha negative breast cancer cell lines. Clin Exp Metastasis. 2011;28:437–49.
Decock J, Hendrickx W, Drijkoningen M, et al. Matrix metalloproteinase expression patterns in luminal A type breast carcinomas. Dis Markers. 2007;23:189–96.
Kasper G, Reule M, Tschirschmann M, et al. Stromelysin-3 over-expression enhances tumourigenesis in MCF-7 and MDA-MB-231 breast cancer cell lines: involvement of the IGF-1 signalling pathway. BMC Cancer. 2007;7:12.
Jia L, Cao J, Wei W, Wang S, Zuo Y, Zhang J. CD147 depletion down-regulates matrix metalloproteinase-11, vascular endothelial growth factor-A expression and the lymphatic metastasis potential of murine hepatocarcinoma Hca-F cells. Int J Biochem Cell Biol. 2007;39:2135–42.
Acknowledgment
The authors are grateful to Dr. Min Kim at the Department of Ophthalmology, Yonsei Medical Center, Dr. Hack Lyoung Kim at the Department of Internal Medicine, Seoul National University Hospital, and English teacher Joung Woo Hong for editing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Min, KW., Kim, DH., Do, SI. et al. Diagnostic and Prognostic Relevance of MMP-11 Expression in the Stromal Fibroblast-Like Cells Adjacent to Invasive Ductal Carcinoma of the Breast. Ann Surg Oncol 20 (Suppl 3), 433–442 (2013). https://doi.org/10.1245/s10434-012-2734-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-012-2734-3