Abstract
Background
Vascular endothelial growth factor (VEGF) is a proangiogenic molecule produced by melanoma cells. We hypothesized that administration of bevacizumab (Bev), a monoclonal antibody that neutralizes VEGF, with low-dose interferon alfa-2b (IFN-α2b), an inhibitor of basic fibroblast growth factor (FGF), would lead to the regression of metastatic melanoma.
Methods
Patients with metastatic melanoma were randomized to receive Bev (15 mg/kg intravenously every 2 weeks) with or without low-dose IFN-α2b (1 MU/m2 subcutaneously daily). Patients exhibiting a clinical response or stable disease after 12 weeks were treated until disease progression.
Results
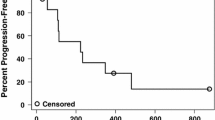
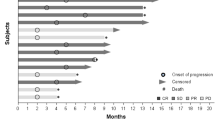
Thirty-two patients (16 per arm) were accrued (18 male, 14 female; mean age 57.5 years). Both regimens were well tolerated. Six patients developed easily managed exacerbations of preexisting hypertension. Two patients developed grade 3 proteinuria that resolved after a treatment break. IFN-α2b therapy was associated with grade 1 to 2 constitutional symptoms. Arterial thromboembolic complications were observed in three patients (two mild myocardial infarctions, one transient ischemic attack), all of whom had risk factors. One patient (Bev plus IFN-α2b arm) had locally recurrent scalp disease that partially responded to therapy. Eight patients (five Bev, three Bev plus IFN-α2b) had prolonged disease stabilization (24 to 146 weeks). Plasma levels of VEGF and FGF did not correlate with any clinical parameter. The patient with the longest period of stable disease had the highest baseline VEGF and FGF.
Conclusions
Bev was well tolerated at this dose and prolonged disease stabilization was achieved in one-quarter of metastatic melanoma patients. Low-dose IFN-α2b did not augment the activity of Bev.


Similar content being viewed by others
References
Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med 1999;15:1359–64
Parangi S, O’Reilly M, Christofori G, et al. Antiangiogenic therapy of transgenic mice impairs de novo tumor growth. Proc Natl Acad Sci U S A 1996;93:2002–7
Investigator’s brochure: recombinant humanized anti-VEGF antibody (rhuMAb VEGF; bevacizumab). August 14, 2000
Mustonen T, Alitalo K. Endothelial receptor tyrosine kinases involved in angiogenesis. J Cell Biol 1995;129:895–8
Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003;9:669–76
Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005;69(Suppl 3):4–10
Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–42
Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non–small cell cancer. J Clin Oncol 2004;22:2184–91
Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 2005;23:792–9
Dinney CP, Bilenberg DR, Perrotte P, et al. Inhibition of basic fibroblast growth factor expression, angiogenesis, and growth of human bladder carcinoma in mice by systemic interferon-alpha administration. Cancer Res 1998;58:808–14
Sangfelt O, Erickson S, Castro J, et al. Molecular mechanisms underlying interferon-alpha–induced G0/G1 arrest: CKI-mediated regulation of G1 Cdk-complexes and activation of pocket proteins. Oncogene 1999;18:2798–810
Ezekowitz RA, Mulliken JB, Folkman J. Interferon alpha-2a therapy for life-threatening hemangiomas of infancy. N Engl J Med 1992;326:1456–63
Simon R. Optimal two-stage designs for phase II clinical trials. Controlled Clin Trials 1989;10:1–10
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–16
Kim CJ, Reintgen DS, Balch CM. The new melanoma staging system. Cancer Control 2002;9:9–15
Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 2003;21:60–5
Gordon MS, Cunningham D. Managing patients treated with bevacizumab combination therapy. Oncology 2005;69(Suppl 3):25–33
Crane CH, Ellis LM, Abbruzzese JL, et al. Phase I trial evaluating the safety of bevacizumab with concurrent radiotherapy and capecitabine in locally advanced pancreatic cancer. J Clin Oncol 2006;24:1145–51
Kabbinavar FF, Jambleton J, Mass RD, et al. Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol 2005;23:3706–12
Herbst RS, Johnson DH, Mininberg E, et al. Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1 epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non–small-cell lung cancer. J Clin Oncol 2005;23:2544–5
Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti- vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 2003;349:427–34
Scappaticci FA, Fehrenbacher L, Cartwright T, et al. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J Surg Oncol 2005;91:173–80
Hurwitz H, Kavvinavar F. Bevacizumab combined with standard fluoropyrimidine- based chemotherapy regimens to treat colorectal cancer. Oncology 2005;69(suppl 3):17–24
Bottasso B, Mari D, Coppola R, et al. Hypercoagulability and hyperfibrinolysis in patients with melanoma. Thromb Res 1996;81:345–52
Blackwell K, Hurwitz H, Lieberman G, et al. Circulating d-dimer levels are better predictors of overall survival and disease progression than carcinoembryonic antigen levels in patients with metastatic colorectal carcinoma. Cancer 2004;101:77–82
Marcoval J, Moreno A, Graells J, et al. Angiogenesis and malignant melanoma. Angiogenesis is related to the development of vertical (tumorigenic) growth phase. J Cutan Pathol 1997;24:212–8
Salven P, Heikkila P, Joensuu H. Enhanced expression of vascular endothelial growth factor in metastatic melanoma. Br J Cancer 1997;76:930–4
Gorski DH, Leal AD, Goydos JS. Differential expression of vascular endothelial growth factor-A isoforms at different stages of melanoma progression. J Am Coll Surg 2003;197:408–18
Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005;69(Suppl 3):4–10
Lacal PM, Failla CM, Pagani E, et al. Human melanoma cells secrete and respond to placenta growth factor and vascular endothelial growth factor. J Invest Dermatol 2000;115:1000–7
Straume O, Akslen LA. Expression of vascular endothelial growth factor, its receptors (FLT-1, KDR) and TSP-1 relate to microvessel density and patient outcome in vertical growth phase melanomas. Am J Pathol 2001;159:223–35
Ugurel S, Rappl G, Tilgen W, et al. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol 2001;19:577–83
Oku T, Tjuvajev JG, Miyagawa T, et al. Tumor growth modulation by sense and antisense vascular endothelial growth factor gene expression: effects on angiogenesis, vascular permeability, blood volume, blood flow, fluorodeoxyglucose uptake, and proliferation of human melanoma xenografts. Cancer Res 1998;58:4185–92
Rofstad EK, Halsor EF. Vascular endothelial growth factor, interleukin 8, platelet- derived endothelial cell growth factor, and basic fibroblast growth factor promote angiogenesis and metastasis in human melanoma xenografts. Cancer Res 2000;20:4932–8
Li Y, Wang MN, Li H, et al. Active immunization against the vascular endothelial growth factor flk 1 inhibits tumor angiogenesis and metastasis. J Exp Med 2002;195:1575–84. Erratum in: J Exp Med 2002; 196:557
Niethammer AG, Xiang R, Becker JC, et al. A DNA vaccine against VEGF receptor 2 prevents effective angiogenesis and inhibits tumor growth. Nat Med 2002;8:1369–75
Wedge SR, Ogilvie DJ, Dukes M, et al. ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res 2002;62:4645–55
Tao J, Tu YT, Huag CZ, et al. Inhibiting the growth of malignant melanoma by blocking the expression of vascular endothelial growth factor using an RNA interference approach. Br J Dermatol 2005;153:715–24
Sun J, Blaskovich MA, Jain RK, et al. Blocking angiogenesis and tumorigenesis with GFA-116, a synthetic molecule that inhibits binding of vascular endothelial growth factor to its receptor. Cancer Res 2004;64:3586–92
Traxler P, Allegrini PR, Brandt R, et al. AEE788: a dual family epidermal growth factor/ErbB2 and vascular endothelial growth factor receptor tyrosine kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res 2004;64:4931–41
Giantonio BJ, Catalano PJ, O’Dwyer PJ, Meropol NJ, Benson AB. Impact of bevacizumab dose reduction on clinical outcomes for patients treated on the Eastern Cooperative Oncology Group’s Study E3200. J Clin Oncol 2006; 24(18 Suppl):3538
Eggermont AM, Kirkwood JM. Re-evaluating the role of dacarbazine in metastatic melanoma: what have we learned in 30 years? Eur J Cancer 2004;40:1825–36
Chapman PB, Einhorn LH, Meyers ML, et al. Phase III randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol 1999;17:2745–51
Jansen B, Wacheck V, Heere-Rees, et al. Clinical, pharmacologic, and pharmacodynamic study of genasense (G3139, Bcl-2 antisense oligonucleotide) and dacarbazine (DTIC) in patients with metastatic melanoma. J Clin Oncol 2001; 20:1426
Acknowledgments
Supported by National Institutes of Health grants CA95426, P30 CA16058-28, P01 CA95426, K24 CA93670 (W.E.C.), and U01 CA-076576-06. K.A.V. is an NRSA T32 fellow (T32 CA09338-27).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Varker, K.A., Biber, J.E., Kefauver, C. et al. A Randomized Phase 2 Trial of Bevacizumab with or without Daily Low-Dose Interferon Alfa-2b in Metastatic Malignant Melanoma. Ann Surg Oncol 14, 2367–2376 (2007). https://doi.org/10.1245/s10434-007-9389-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-007-9389-5