Abstract
Purpose
Melanomas are vascular tumors with a high incidence of BRAF mutations driving tumor proliferation. Complete inhibition of vascular endothelial growth factor (VEGF) signaling has potential for enhanced antitumor efficacy.
Methods
Patients with advanced melanoma and adequate organ function were eligible. Sorafenib was given orally at 200 mg BiD for 5 days every week; bevacizumab was administered 5 mg/kg intravenously every 14 days. The primary objective was to determine clinical biological activity. The secondary objectives were safety, tolerability, and time to progression (TTP). Pharmacodynamic analysis included serum VEGF and soluble VEGF receptor-1 and VEGF receptor-2 performed at baseline, C1D15 and C2D1. The study was terminated during the first stage of a Simon two-stage design, after 14 of planned 21 subjects were enrolled.
Results
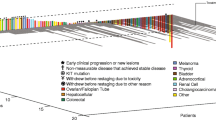
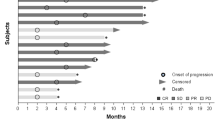
Of the 14 patients who received treatment, no objective tumor responses were observed. Stable disease (SD) ≥16 weeks was observed in 57 % patients, including three patients with SD lasting ≥1 year. Median TTP was 32 weeks. The most frequently reported drug-related adverse events (AEs) were hand–foot syndrome (57.1 %), fatigue (57.1 %), hypertension (64.3 %), and proteinuria (35.7). Grade 3/4 drug-related AEs were hypertension (14.2 %), hand–foot syndrome, proteinuria, and thrombocytopenia (7 % each). Patients with low VEGF (<300 pg/ml) experienced longer TTP than those with high VEGF [median 50 vs. 15 weeks, p = 0.02). A similar pattern was seen for VEGFR1 and VEGFR2, although it did not reach statistical significance.
Conclusions
Combined VEGF/VEGFR blockade using bevacizumab with sorafenib shows clinical activity. The linkage between VEGF levels and time to tumor progression needs further exploration.


Similar content being viewed by others
References
Jemal A, Saraiya M, Patel P, Cherala S, Barnholtz-Sloan J, Kim J, Wiggins C, Wingo P (2011) Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992–2006. J Am Acad Dermatol 65(5 Suppl 1):S17–25 e11–13. doi:10.1016/j.jaad.2011.04.032
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63(1):11–30. doi:10.3322/caac.21166
Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC Jr, Morton DL, Ross MI, Sober AJ, Sondak VK (2009) Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 27(36):6199–6206. doi:10.1200/jco.2009.23.4799
Manola J, Atkins M, Ibrahim J, Kirkwood J (2000) Prognostic factors in metastatic melanoma: a pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol 18(22):3782–3793
Pisacane AM, Risio M (2005) VEGF and VEGFR-2 immunohistochemistry in human melanocytic naevi and cutaneous melanomas. Melanoma Res 15(1):39–43
Hendrix MJ, Seftor EA, Hess AR, Seftor RE (2003) Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer 3(6):411–421. doi:10.1038/nrc1092
Folberg R, Hendrix MJ, Maniotis AJ (2000) Vasculogenic mimicry and tumor angiogenesis. Am J Pathol 156(2):361–381. doi:10.1016/s0002-9440(10)64739-6
Warso MA, Maniotis AJ, Chen X, Majumdar D, Patel MK, Shilkaitis A, Gupta TK, Folberg R (2001) Prognostic significance of periodic acid-Schiff-positive patterns in primary cutaneous melanoma. Clin Cancer Res 7(3):473–477
Ugurel S, Rappl G, Tilgen W, Reinhold U (2001) Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol 19(2):577–583
Presta LG, Chen H, O’Connor SJ, Chisholm V, Meng YG, Krummen L, Winkler M, Ferrara N (1997) Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res 57(20):4593–4599
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356(2):125–134. doi:10.1056/NEJMoa060655
Azad NS, Posadas EM, Kwitkowski VE, Steinberg SM, Jain L, Annunziata CM, Minasian L, Sarosy G, Kotz HL, Premkumar A, Cao L, McNally D, Chow C, Chen HX, Wright JJ, Figg WD, Kohn EC (2008) Combination targeted therapy with sorafenib and bevacizumab results in enhanced toxicity and antitumor activity. J Clin Oncol 26(22):3709–3714. doi:10.1200/jco.2007.10.8332
Lee JM, Sarosy GA, Annunziata CM, Azad N, Minasian L, Kotz H, Squires J, Houston N, Kohn EC (2010) Combination therapy: intermittent sorafenib with bevacizumab yields activity and decreased toxicity. Br J Cancer 102(3):495–499. doi:10.1038/sj.bjc.6605514
Sosman J, Flaherty K, Atkins M, Puzanov I, McDermott D, Vermeulen W, Harlacker K, Hsu A, Rothenberg M (2006) A phase I/II trial of Sorafenib with Bevacizumab in metastatic renal cell cancer patients. J Clin Oncol 24:(Abstr 3031)
Galanis E, Jaeckle KA, Anderson S, Kaufmann T, Uhm JH, Giannini C, Kumar S, Northfelt D, Flynn P, Buckner J (2010) NCCTG phase II trial of Bevacizumab in combination with Sorafenib in recurrent GBM. J Clin Oncol 28:(abstr 2018)
Grothey A, Lafky J, Morlan B, Stella P, Dakhil S, Steen P, Loui W, Bot B, Alberts S, Reynolds J (2010) Dual VEGF inhibition with Sorafenib and Bevacizumab as salvage therapy in metastatic colorectal cancer: results of the phase II North Central Cancer Treatment Group study N054C. J Clin Oncol 28:(abstr 3549)
Byrne GJ, Ghellal A, Iddon J, Blann AD, Venizelos V, Kumar S, Howell A, Bundred NJ (2000) Serum soluble vascular cell adhesion molecule-1: role as a surrogate marker of angiogenesis. J Natl Cancer Inst 92(16):1329–1336
Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, Ginsberg MS, Kim ST, Baum CM, DePrimo SE, Li JZ, Bello CL, Theuer CP, George DJ, Rini BI (2006) Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol 24(1):16–24. doi:10.1200/jco.2005.02.2574
Lane HA, Wood JM, McSheehy PM, Allegrini PR, Boulay A, Brueggen J, Littlewood-Evans A, Maira SM, Martiny-Baron G, Schnell CR, Sini P, O’Reilly T (2009) mTOR inhibitor RAD001 (everolimus) has antiangiogenic/vascular properties distinct from a VEGFR tyrosine kinase inhibitor. Clin Cancer Res 15(5):1612–1622. doi:10.1158/1078-0432.ccr-08-2057
Longo R, Gasparini G (2007) Challenges for patient selection with VEGF inhibitors. Cancer Chemother Pharmacol 60(2):151–170. doi:10.1007/s00280-006-0403-6
Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O’Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur GA (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364(26):2507–2516. doi:10.1056/NEJMoa1103782
Kim KB, Sosman JA, Fruehauf JP, Linette GP, Markovic SN, McDermott DF, Weber JS, Nguyen H, Cheverton P, Chen D, Peterson AC, Carson WE III, O’Day SJ (2012) BEAM: a randomized phase II study evaluating the activity of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced melanoma. J Clin Oncol 30(1):34–41. doi:10.1200/jco.2011.34.6270
Varker KA, Biber JE, Kefauver C, Jensen R, Lehman A, Young D, Wu H, Lesinski GB, Kendra K, Chen HX, Walker MJ, Carson WE III (2007) A randomized phase 2 trial of bevacizumab with or without daily low-dose interferon alfa-2b in metastatic malignant melanoma. Ann Surg Oncol 14(8):2367–2376. doi:10.1245/s10434-007-9389-5
Denduluri N, Yang SX, Berman AW, Nguyen D, Liewehr DJ, Steinberg SM, Swain SM (2008) Circulating biomarkers of bevacizumab activity in patients with breast cancer. Cancer Biol Ther 7(1):15–20
Leighl N, Reck M, Haas S, Evers S, Delmar P, Manegold C, Scherer S (2009) 9172 Analysis of biomarkers (BMs) in the AVAiL phase III randomised study of first-line Bevacizumab (Bv) with cisplatin-gemcitabine (CG) in patients (pts) with non-small cell lung cancer (NSCLC). Eur J Cancer 7(2):558
Hegde PS, Jubb AM, Chen D, Li NF, Meng YG, Bernaards C, Elliott R, Scherer SJ, Chen DS (2013) Predictive impact of circulating vascular endothelial growth factor in four phase III trials evaluating bevacizumab. Clin Cancer Res 19(4):929–937. doi:10.1158/1078-0432.ccr-12-2535
Si L, Han M, Chi Z, Cui C, Sheng X, Li S, Kong Y, Guo J (2010) Durable response of the triple combination of temozolomide, sorafenib, and bevacizumab to treat refractory stage IV acral melanoma. J Clin Oncol 28(15s):(abstr 8564)
Acknowledgments
This study was funded by National Cancer Institute.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahalingam, D., Malik, L., Beeram, M. et al. Phase II study evaluating the efficacy, safety, and pharmacodynamic correlative study of dual antiangiogenic inhibition using bevacizumab in combination with sorafenib in patients with advanced malignant melanoma. Cancer Chemother Pharmacol 74, 77–84 (2014). https://doi.org/10.1007/s00280-014-2479-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2479-8