Abstract
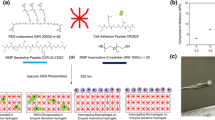
Herein, we report on continued efforts to understand an implantable poly(ethylene glycol) diacrylate (PEGDA) hydrogel drug delivery system that responds to extracellular enzymes, in particular matrix metalloproteinase-2 (MMP-2) to provide controlled drug delivery. By attaching peptide as pendant groups on the hydrogel backbone, drug release occurs at an accelerated rate in the presence of active protease. We investigated MMP-2 entry and optimized parameters of the drug delivery system. Mesh size for different PEGDA molecular weight macromers was measured with PEGDA 3,400 hydrogels having a mesh size smaller than the dimensions of MMP-2 and PEGDA 10,000 and PEGDA 20,000 hydrogels having mesh sizes larger than MMP-2. Purified MMP-2 increased release of peptide fragment compared to buffer at several loading concentrations. Cell-stimulated release was demonstrated using U-87 MG cells embedded in collagen. GM6001, an MMP inhibitor, diminished release and altered the identity of the released peptide fragment. The increase in ratio of release from PEGDA 10,000 and PEGDA 20,000 hydrogels compared to PEGDA 3,400 hydrogels suggests MMP-2 enters the hydrogel. PEGDA molecular weight of 10,000 and 15 % (w/V) were the optimal conditions for release and handling. The use of protease-triggered drug delivery has great advantage particularly with the control of protease penetration as a parameter for controlling rate of release.






Similar content being viewed by others
REFERENCES
Central Brain Tumor Registry of the United States. Statistical report: primary brain tumors in the United States, 1992–1997, 2001. 2001.
Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011. CA Cancer J Clin. 2011;61(4):212–36.
Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507.
Central Brain Tumor Registry of the United States. Primary Brain Tumors in the United States. Chicago, IL. 2007–2008.
Tauro JR, Gemeinhart RA. Extracellular protease activation of chemotherapeutics from hydrogel matrices: a new paradigm for local chemotherapy. Mol Pharm. 2005;2(5):435–8.
Tauro JR, Gemeinhart RA. Matrix metalloprotease triggered local delivery of cancer chemotherapeutics. Bioconjug Chem. 2005;16(5):1133–9.
Tauro JR, Lee BS, Lateef SS, Gemeinhart RA. Matrix metalloprotease selective peptide substrates cleavage within hydrogel matrices for cancer chemotherapy activation. Peptides. 2008;29(11):1965–73.
Anseth KS, Bowman CN, Brannon-Peppas L. Mechanical properties of hydrogels and their experimental determination. Biomaterials. 1996;17(17):1647–57.
Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50(1):27–46.
Kim SW, Bae YH, Okano T. Hydrogels: swelling, drug loading, and release. Pharm Res. 1992;9(3):283–90.
Levi E, Fridman R, Miao HQ, Ma YS, Yayon A, Vlodavsky I. Matrix metalloproteinase 2 releases active soluble ectodomain of fibroblast growth factor receptor 1. Proc Natl Acad Sci USA. 1996;93(14):7069–74.
Overall CM, Kleifeld O. Validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6(3):227–39.
Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2(9):657–72.
Vartak D, Gemeinhart RA. Matrix metalloproteases: underutilized targets for drug delivery. J Drug Target. 2007;15(1):1–21.
Fridman R. Surface association of secreted matrix metalloproteinases. Cell Surf Proteases. 2003;54:75–100.
Foda HD, Zucker S. Matrix metalloproteinases in cancer invasion, metastasis and angiogenesis. Drug Discov Today. 2001;6(9):478–82.
Haas TL, Madri JA. Extracellular matrix-driven matrix metalloproteinase production in endothelial cells: implications for angiogenesis. Trends Cardiovasc Med. 1999;9(3–4):70–7.
Lu MK, Chen PH, Shih YW, Chang YT, Huang ET, Liu CR, et al. alpha-Chaconine inhibits angiogenesis in vitro by reducing matrix metalloproteinase-2. Biol Pharm Bull. 2010;33(4):622–30.
Rao JS, Yamamoto M, Mohaman S, Gokaslan ZL, Fuller GN, Stetler-Stevenson WG, et al. Expression and localization of 92 kDa type IV collagenase/gelatinase B (MMP-9) in human gliomas. Clin Exp Metastasis. 1996;14(1):12–8.
Sawaya RE, Yamamoto M, Gokaslan ZL, Wang SW, Mohanam S, Fuller GN, et al. Expression and localization of 72 kDa type IV collagenase (MMP-2) in human malignant gliomas in vivo. Clin Exp Metastasis. 1996;14(1):35–42.
Chau Y, Tan FE, Langer R. Synthesis and characterization of dextran-peptide-methotrexate conjugates for tumor targeting via mediation by matrix metalloproteinase II and matrix metalloproteinase IX. Bioconjug Chem. 2004;15(4):931–41.
Chau Y, Langer RS. Important factors in designing targeted delivery of cancer therapeutics via MMP-2 mediation. J Control Release. 2003;91(1–2):239–40.
Albright CF, Graciani N, Han W, Yue E, Stein R, Lai ZH, et al. Matrix metalloproteinase-activated doxorubicin prodrugs inhibit HT1080 xenograft growth doxorubicin with less toxicity. Mol Cancer Ther. 2005;4(5):751–60.
Lim SH, Jeong YI, Moon KS, Ryu HH, Jin YH, Jin SG, et al. Anticancer activity of PEGylated matrix metalloproteinase cleavable peptide-conjugated adriamycin against malignant glioma cells. Int J Pharm. 2010;387(1–2):209–14.
Bae M, Cho S, Song J, Lee GY, Kim K, Yang J, et al. Metalloprotease-specific poly(ethylene glycol) methyl ether-peptide-doxorubicin conjugate for targeting anticancer drug delivery based on angiogenesis. Drugs Exptl Clin Res. 2003;29(1):15–23.
Lee GY, Park K, Kim SY, Byun Y. MMPs-specific PEGylated peptide-DOX conjugate micelles that can contain free doxorubicin. Eur J Pharm Biopharm. 2007;67(3):646–54.
Terada T, Iwai M, Kawakami S, Yamashita F, Hashida M. Novel PEG-matrix metalloproteinase-2 cleavable peptide-lipid containing galactosylated liposomes for hepatocellular carcinoma-selective targeting. J Control Release. 2006;111(3):333–42.
Tokatlian T, Shrum CT, Kadoya WM, Segura T. Protease degradable tethers for controlled and cell-mediated release of nanoparticles in 2- and 3-dimensions. Biomaterials. 2010;31(31):8072–80.
Laromaine A, Koh LL, Murugesan M, Ulijn RV, Stevens MM. Protease-triggered dispersion of nanoparticle assemblies. J Am Chem Soc. 2007;129(14):4156–7.
Rawsterne RE, Gough JE, Rutten FJM, Pham NT, Poon WCK, Flitsch SL, et al. Controlling protein retention on enzyme-responsive surfaces. Surf Interface Anal. 2006;38(11):1505–11.
Thornton PD, McConnell G, Ulijn RV. Enzyme responsive polymer hydrogel beads. Chem Commun. 2005;47:5913–5.
Ulijn RV. Enzyme-responsive materials: a new class of smart biomaterials. J Mater Chem. 2006;16(23):2217–25.
West JL, Hubbell JA. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules. 1999;32(1):241–4.
Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23(1):47–55.
Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc Natl Acad Sci USA. 2003;100(9):5413–8.
Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, Davies N, et al. Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB J. 2003;17(15):2260–2.
Gojgini S, Tokatlian T, Segura T. Utilizing cell-matrix interactions to modulate gene transfer to stem cells inside hyaluronic acid hydrogels. Mol Pharm. 2011;8(5):1582–91.
Peppas NA, Keys KB, Torres-Lugo M, Lowman AM. Poly(ethylene glycol)-containing hydrogels in drug delivery. J Control Release. 1999;62(1–2):81–7.
Kim J, Hefferan TE, Yaszemski MJ, Lu L. Potential of hydrogels based on poly(ethylene glycol) and sebacic acid as orthopedic tissue engineering scaffolds. Tissue Eng, Part A. 2009;15(8):2299–307.
Bryant SJ, Lynn AD, Kyriakides TR. Characterization of the in vitro macrophage response and in vivo host response to poly(ethylene glycol)-based hydrogels. J Biomed Mater Res A. 2010;93A(3):941–53.
Salinas CN, Anseth KS. Mixed mode thiol-acrylate photopolymerizations for the synthesis of PEG-peptide hydrogels. Macromolecules. 2008;41(16):6019–26.
Hughes SW. Archimedes revisited: a faster, better, cheaper method of accurately measuring the volume of small objects. Phys Educ. 2005;40(5):468–74.
Peppas NA. Hydrogels in medicine and pharmacy. Boca Raton: CRC Press; 1986.
Flory PJ. Principles of polymer chemistry. Ithaca: Cornell University Press; 1953.
Vartak DG. Integrins and matrix metalloprotease-2 as dual targets in angiogenesis. Ph.D. Dissertation, University of Illinois at Chicago, Chicago; 2009.
Vartak DG, Gemeinhart RA. In vitro evaluation of functional interaction of integrin αvβ3 and matrix metalloprotease-2. Mol Pharm. 2009;6(6):1856–67.
Azzam HS, Arand G, Lippman ME, Thompson EW. Association of MMP-2 activation potential with metastatic progression in human breast cancer cell lines independent of MMP-2 production. J Natl Cancer Inst. 1993;85(21):1758–64.
Lustig S, Peppas NA. Solute diffusion in swollen membranes. IX. Scaling laws for solute diffusion in gels. J Appl Polym Sci. 1988;36(4):735–47.
Morgunova E, Tuuttila A, Bergmann U, Isupov M, Lindqvist Y, Schneider G, et al. Structure of human pro-matrix metalloproteinase-2: activation mechanism revealed. Science. 1999;284(5420):1667–70.
Cowie JMG, Arrighi V. Polymers: chemistry and physics of modern materials. 3rd ed. Boca Raton: CRC Press; 2008.
Brannon-Peppas L. Preparation and characterization of crosslinked hydrophilic networks. In: Brannon-Peppas L, Harland RS, editors. Absorbent Polymer Technology. Amesterdam: Elsevier; 1990. p. 45–65.
Hansen S. Translational friction coefficients for cylinders of arbitrary axial ratios estimated by Monte Carlo simulation. J Chem Phys. 2004;121(18):9111–5.
Tanaka T, Fillmore DJ. Kinetics of swelling gels. J Chem Phys. 1970;70(3):1214–8.
Snoek-van Beurden PAM, Von den Hoff JW. Zymographic techniques for the analysis of matrix metalloproteinases and their inhibitors. Biotechniques. 2005;38(1):73–83.
Rome C, Arsaut J, Taris C, Couillaud F, Loiseau H. MMP-7 (matrilysin) expression in human brain tumors. Mol Carcinog. 2007;46(6):446–52.
Woessner Jr JF, Taplin CJ. Purification and properties of a small latent matrix metalloproteinase of the rat uterus. J Biol Chem. 1988;263(32):16918–25.
Peppas NA, Huang Y, Torres-Lugo M, Ward JH, Zhang J. Physicochemical, foundations and structural design of hydrogels in medicine and biology. Annu Rev Biomed Eng. 2000;2:9–29.
Cussler EL. Diffusion: mass transfer in fluid systems. 3rd ed. Cambridge: Cambridge University Press; 2009.
ACKNOWLEDGMENT
The authors would like to thank Dr. William Beck for use of equipment. We thank Dr. J.H. “Robert” Chang for insightful discussion. This work was supported by NIH R01 NS055095 (RAG). This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR15482 from the National Center for Research Resources, NIH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ross, A.E., Tang, M.Y. & Gemeinhart, R.A. Effects of Molecular Weight and Loading on Matrix Metalloproteinase-2 Mediated Release from Poly(Ethylene Glycol) Diacrylate Hydrogels. AAPS J 14, 482–490 (2012). https://doi.org/10.1208/s12248-012-9356-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-012-9356-3