Abstract
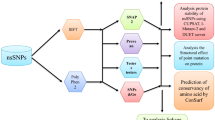
UDP glucuronosyltransferases (UGTs) are an important class of Phase II enzymes involved in the metabolism and detoxification of numerous xenobiotics including therapeutic drugs and endogenous compounds (e.g. bilirubin). To date, there are 21 human UGT genes identified, and most of them contain single-nucleotide polymorphisms (SNPs). Non-synonymous SNPs (nsSNPs) of the human UGT genes may cause absent or reduced enzyme activity and polymorphisms of UGT have been found to be closely related to altered drug clearance and/or drug response, hyperbilirubinemia, Gilbert’s syndrome, and Crigler-Najjar syndrome. However, it is unlikely to study the functional impact of all identified nsSNPs in humans using laboratory approach due to its giant number. We have investigated the potential for bioinformatics approach for the prediction of phenotype based on known nsSNPs. We have identified a total of 248 nsSNPs from human UGT genes. The two algorithms tools, sorting intolerant from tolerant (SIFT) and polymorphism phenotyping (PolyPhen), were used to predict the impact of these nsSNPs on protein function. SIFT classified 35.5% of the UGT nsSNPs as “deleterious”; while PolyPhen identified 46.0% of the UGT nsSNPs as “potentially damaging” and “damaging”. The results from the two algorithms were highly associated. Among 63 functionally characterized nsSNPs in the UGTs, 24 showed altered enzyme expression/activities and 45 were associated with disease susceptibility. SIFT and Polyphen had a correct prediction rate of 57.1% and 66.7%, respectively. These findings demonstrate the potential use of bioinformatics techniques to predict genotype–phenotype relationships which may constitute the basis for future functional studies.

Similar content being viewed by others
Abbreviations
- UGTs:
-
UDP glucuronosyltransferase
- nsSNP:
-
non-synonymous single-nucleotide polymorphism
References
Guillemette C. Pharmacogenomics of human UDP-glucuronosyltransferase enzymes. Pharmacogenomics J. 2003;3:136–58.
Evans WE, Relling MV. Pharmacogenomics: translating functional genomics into rational therapeutics. Science. 1999;286:487–91.
Mackenzie PI, Owens IS, Burchell B, et al. The UDP glycosyltransferase gene superfamily: recommended nomenclature update based on evolutionary divergence. Pharmacogenetics. 1997;7:255–69.
King CD, Rios GR, Green MD, Tephly TR. UDP-glucuronosyltransferases. Curr Drug Metab. 2000;1:143–61.
Mackenzie PI, Bock KW, Burchell B, et al. Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenet Genomics. 2005;15:677–85.
Harding D, Jeremiah SJ, Povey S, Burchell B. Chromosomal mapping of a human phenol UDP-glucuronosyltransferase, GNT1. Ann Hum Genet. 1990;54:17–21.
Turgeon D, Carrier JS, Levesque E, Hum DW, Belanger A. Relative enzymatic activity, protein stability, and tissue distribution of human steroid-metabolizing UGT2B subfamily members. Endocrinology. 2001;142:778–87.
Ramensky V, Bork P, Sunyaev S. Human non-synonymous SNPs: server and survey. Nucleic Acids Res. 2002;30:3894–900.
Ng PC, Henikoff S. Accounting for human polymorphisms predicted to affect protein function. Genome Res. 2002;12:436–46.
Ng PC, Henikoff S. Predicting the effects of amino acid substitutions on protein function. Annu Rev Genomics Hum Genet. 2006;7:61–80.
Herrgard S, Cammer SA, Hoffman BT, et al. Prediction of deleterious functional effects of amino acid mutations using a library of structure-based function descriptors. Proteins. 2003;53:806–16.
Dobson RJ, Munroe PB, Caulfield MJ, Saqi MA. Predicting deleterious nsSNPs: an analysis of sequence and structural attributes. BMC Bioinformatics. 2006;7:217.
Ehmer U, Vogel A, Schutte JK, Krone B, Manns MP, Strassburg CP. Variation of hepatic glucuronidation: novel functional polymorphisms of the UDP-glucuronosyltransferase UGT1A4. Hepatology. 2004;39:970–7.
Guillemette C, Millikan RC, Newman B, Housman DE. Genetic polymorphisms in uridine diphospho-glucuronosyltransferase 1A1 and association with breast cancer among African Americans. Cancer Res. 2000;60:950–6.
Crigler JF Jr, Najjar VA. Congenital familial nonhemolytic jaundice with kernicterus. Pediatrics. 1952;10:169–80.
Black M, Billing BH. Hepatic bilirubin udp-glucuronyl transferase activity in liver disease and Gilbert’s syndrome. N Engl J Med. 1969;280:1266–71.
Moghrabi N, Clarke DJ, Boxer M, Burchell B. Identification of an A-to-G missense mutation in exon 2 of the UGT1 gene complex that causes Crigler-Najjar syndrome type 2. Genomics. 1993;18:171–3.
Bosma PJ, Chowdhury JR, Huang TJ, et al. Mechanisms of inherited deficiencies of multiple UDP-glucuronosyltransferase isoforms in two patients with Crigler-Najjar syndrome, type I. Faseb J. 1992;6:2859–63.
Iyer L, Das S, Janisch L, et al. UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity. Pharmacogenomics J. 2002;2:43–7.
Ando Y, Saka H, Ando M, et al. Polymorphisms of UDP-glucuronosyltransferase gene and irinotecan toxicity: a pharmacogenetic analysis. Cancer Res. 2000;60:6921–6.
Wasserman E, Myara A, Lokiec F, et al. Severe CPT-11 toxicity in patients with Gilbert’s syndrome: two case reports. Ann Oncol. 1997;8:1049–51.
Kaniwa N, Kurose K, Jinno H, et al. Racial variability in haplotype frequencies of UGT1A1 and glucuronidation activity of a novel single nucleotide polymorphism 686C> T (P229L) found in an African-American. Drug Metab Dispos. 2005;33:458–65.
Danoff TM, Campbell DA, McCarthy LC, et al. A Gilbert’s syndrome UGT1A1 variant confers susceptibility to tranilast-induced hyperbilirubinemia. Pharmacogenomics J. 2004;4:49–53.
Strassburg CP, Vogel A, Kneip S, Tukey RH, Manns MP. Polymorphisms of the human UDP-glucuronosyltransferase (UGT) 1A7 gene in colorectal cancer. Gut. 2002;50:851–6.
Zheng Z, Park JY, Guillemette C, Schantz SP, Lazarus P. Tobacco carcinogen-detoxifying enzyme UGT1A7 and its association with orolaryngeal cancer risk. J Natl Cancer Inst. 2001;93:1411–8.
Vogel A, Ockenga J, Ehmer U, et al. Polymorphisms of the carcinogen detoxifying UDP-glucuronosyltransferase UGT1A7 in proximal digestive tract cancer. Z Gastroenterol. 2002;40:497–502.
Vogel A, Kneip S, Barut A, et al. Genetic link of hepatocellular carcinoma with polymorphisms of the UDP-glucuronosyltransferase UGT1A7 gene. Gastroenterology. 2001;121:1136–44.
Darbari DS, van Schaik RH, Capparelli EV, Rana S, McCarter R, van den Anker J. UGT2B7 promoter variant -840G>A contributes to the variability in hepatic clearance of morphine in patients with sickle cell disease. Am J Hematol. 2008;83:200–2.
Holthe M, Rakvag TN, Klepstad P, et al. Sequence variations in the UDP-glucuronosyltransferase 2B7 (UGT2B7) gene: identification of 10 novel single nucleotide polymorphisms (SNPs) and analysis of their relevance to morphine glucuronidation in cancer patients. Pharmacogenomics J. 2003;3:17–26.
MacLeod SL, Nowell S, Plaxco J, Lang NP. An allele-specific polymerase chain reaction method for the determination of the D85Y polymorphism in the human UDP-glucuronosyltransferase 2B15 gene in a case-control study of prostate cancer. Ann Surg Oncol. 2000;7:777–82.
Ng PC, Henikoff S. Predicting deleterious amino acid substitutions. Genome Res. 2001;11:863–74.
Ng PC, Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31:3812–4.
Sunyaev S, Ramensky V, Koch I, Lathe W 3rd, Kondrashov AS, Bork P. Prediction of deleterious human alleles. Hum Mol Genet. 2001;10:591–7.
Ng PC, Henikoff JG, Henikoff S. PHAT: a transmembrane-specific substitution matrix. Predicted hydrophobic and transmembrane. Bioinformatics. 2000;16:760–6.
Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–80.
Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–4.
Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6.
Xi T, Jones IM, Mohrenweiser HW. Many amino acid substitution variants identified in DNA repair genes during human population screenings are predicted to impact protein function. Genomics. 2004;83:970–9.
Aono S, Adachi Y, Uyama E, et al. Analysis of genes for bilirubin UDP-glucuronosyltransferase in Gilbert’s syndrome. Lancet. 1995;345:958–9.
Ciotti M, Chen F, Rubaltelli FF, Owens IS. Coding defect and a TATA box mutation at the bilirubin UDP-glucuronosyltransferase gene cause Crigler-Najjar type I disease. Biochim Biophys Acta. 1998;1407:40–50.
Ciotti M, Obaray R, Martin MG, Owens IS. Genetic defects at the UGT1 locus associated with Crigler-Najjar type I disease, including a prenatal diagnosis. Am J Med Genet. 1997;68:173–8.
Ciotti M, Werlin SL, Owens IS. Delayed response to phenobarbital treatment of a Crigler-Najjar type II patient with partially inactivating missense mutations in the bilirubin UDP-glucuronosyltransferase gene. J Pediatr Gastroenterol Nutr. 1999;28:210–3.
Erps LT, Ritter JK, Hersh JH, Blossom D, Martin NC, Owens IS. Identification of two single base substitutions in the UGT1 gene locus which abolish bilirubin uridine diphosphate glucuronosyltransferase activity in vitro. J Clin Invest. 1994;93:564–70.
Labrune P, Myara A, Hadchouel M, et al. Genetic heterogeneity of Crigler-Najjar syndrome type I: a study of 14 cases. Hum Genet. 1994;94:693–7.
Maruo Y, Nishizawa K, Sato H, Sawa H, Shimada M. Prolonged unconjugated hyperbilirubinemia associated with breast milk and mutations of the bilirubin uridine diphosphate-glucuronosyltransferase gene. Pediatrics. 2000;106:E59.
Seppen J, Bosma PJ, Goldhoorn BG, et al. Discrimination between Crigler-Najjar type I and II by expression of mutant bilirubin uridine diphosphate-glucuronosyltransferase. J Clin Invest. 1994;94:2385–91.
Seppen J, Steenken E, Lindhout D, Bosma PJ, Elferink RP. A mutation which disrupts the hydrophobic core of the signal peptide of bilirubin UDP-glucuronosyltransferase, an endoplasmic reticulum membrane protein, causes Crigler-Najjar type II. FEBS Lett. 1996;390:294–8.
Chalasani N, Chowdhury NR, Chowdhury JR, Boyer TD. Kernicterus in an adult who is heterozygous for Crigler-Najjar syndrome and homozygous for Gilbert-type genetic defect. Gastroenterology. 1997;112:2099–103.
Farheen S, Sengupta S, Santra A, et al. Gilbert’s syndrome: high frequency of the (TA)7 TAA allele in India and its interaction with a novel CAT insertion in promoter of the gene for bilirubin UDP-glucuronosyltransferase 1 gene. World J Gastroenterol. 2006;12:2269–75.
Huang CS, Luo GA, Huang ML, Yu SC, Yang SS. Variations of the bilirubin uridine-diphosphoglucuronosyl transferase 1A1 gene in healthy Taiwanese. Pharmacogenetics. 2000;10:539–44.
Kadakol A, Ghosh SS, Sappal BS, Sharma G, Chowdhury JR, Chowdhury NR. Genetic lesions of bilirubin uridine-diphosphoglucuronate glucuronosyltransferase (UGT1A1) causing Crigler-Najjar and Gilbert syndromes: correlation of genotype to phenotype. Hum Mutat. 2000;16:297–306.
Labrune P, Myara A, Chalas J, Le Bihan B, Capel L, Francoual J. Association of a homozygous (TA)8 promoter polymorphism and a N400D mutation of UGT1A1 in a child with Crigler-Najjar type II syndrome. Hum Mutat. 2002;20:399–401.
Maruo Y, Serdaroglu E, Iwai M, et al. A novel missense mutation of the bilirubin UDP-glucuronosyltransferase gene in a Turkish patient with Crigler-Najjar syndrome type 1. J Pediatr Gastroenterol Nutr. 2003;37:627–30.
Petit F, Gajdos V, Capel L, et al. Crigler-Najjar type II syndrome may result from several types and combinations of mutations in the UGT1A1 gene. Clin Genet. 2006;69:525–7.
Servedio V, d’apolito M, Maiorano N, et al. Spectrum of UGT1A1 mutations in Crigler-Najjar (CN) syndrome patients: identification of twelve novel alleles and genotype-phenotype correlation. Hum Mutat. 2005;25:325.
Sutomo R, Laosombat V, Sadewa AH, et al. Novel missense mutation of the UGT1A1 gene in Thai siblings with Gilbert’s syndrome. Pediatr Int. 2002;44:427–32.
Aono S, Yamada Y, Keino H, et al. Identification of defect in the genes for bilirubin UDP-glucuronosyl-transferase in a patient with Crigler-Najjar syndrome type II. Biochem Biophys Res Commun. 1993;197:1239–44.
Huang CS, Luo GA, Huang MJ, Chen ES, Young TH, Chao YC. A novel compound heterozygous variation of the uridine-diphosphoglucuronosyl transferase 1A1 gene that causes Crigler-Najjar syndrome type II. Pharmacogenetics. 2001;11:639–42.
Udomuksorn W, Elliot DJ, Lewis BC, Mackenzie PI, Yoovathaworn K, Miners JO. Influence of mutations associated with Gilbert and Crigler-Najjar type II syndromes on the glucuronidation kinetics of bilirubin and other UDP-glucuronosyltransferase 1A substrates. Pharmacogenet Genomics. 2007;17:1017–29.
Chen G, Blevins-Primeau AS, Dellinger RW, Muscat JE, Lazarus P. Glucuronidation of nicotine and cotinine by UGT2B10: loss of function by the UGT2B10 Codon 67 (Asp>Tyr) polymorphism. Cancer Res. 2007;67:9024–9.
Levesque E, Beaulieu M, Green MD, Tephly TR, Belanger A, Hum DW. Isolation and characterization of UGT2B15(Y85): a UDP-glucuronosyltransferase encoded by a polymorphic gene. Pharmacogenetics. 1997;7:317–25.
Tukey RH, Strassburg CP. Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol. 2000;40:581–616.
Gsur A, Preyer M, Haidinger G, et al. A polymorphism in the UDP-Glucuronosyltransferase 2B15 gene (D85Y) is not associated with prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11:497–8.
Li Y, Wang Y, Li Y, Yang L. Prediction of the deleterious nsSNPs in ABCB transporters. FEBS Lett. 2006;580:6800–6.
Evans WE, Johnson JA. Pharmacogenomics: the inherited basis for interindividual differences in drug response. Annu Rev Genomics Hum Genet. 2001;2:9–39.
Ingelman-Sundberg M, Oscarson M, McLellan RA. Polymorphic human cytochrome P450 enzymes: an opportunity for individualized drug treatment. Trends Pharmacol Sci. 1999;20:342–9.
Yamamoto K, Soeda Y, Kamisako T, et al. Analysis of bilirubin uridine 5′-diphosphate (UDP)-glucuronosyltransferase gene mutations in seven patients with Crigler-Najjar syndrome type II. J Hum Genet. 1998;43:111–4.
Zhang EY, Fu DJ, Pak YA, et al. Genetic polymorphisms in human proton-dependent dipeptide transporter PEPT1: implications for the functional role of Pro586. J Pharmacol Exp Ther. 2004;310:437–45.
Wang L. A bioinformatics approach for the phenotype prediction of non-synonymous single nucleotide polymorphisms in human cytochrome P450s. Drug Metab Dispos. 2009;37:977–91.
Bosma PJ, Goldhoorn B, Oude Elferink RP, Sinaasappel M, Oostra BA, Jansen PL. A mutation in bilirubin uridine 5′-diphosphate-glucuronosyltransferase isoform 1 causing Crigler-Najjar syndrome type II. Gastroenterology. 1993;105:216–20.
Yusoff S, Van Rostenberghe H, Yusoff NM, et al. Frequencies of A(TA)7TAA, G71R, and G493R mutations of the UGT1A1 gene in the Malaysian population. Biol Neonate. 2006;89:171–6.
Iwai M, Maruo Y, Ito M, Yamamoto K, Sato H, Takeuchi Y. Six novel UDP-glucuronosyltransferase (UGT1A3) polymorphisms with varying activity. J Hum Genet. 2004;49:123–8.
Caillier B, Lepine J, Tojcic J, et al. A pharmacogenomics study of the human estrogen glucuronosyltransferase UGT1A3. Pharmacogenet Genomics. 2007;17:481–95.
Wiener D, Fang JL, Dossett N, Lazarus P. Correlation between UDP-glucuronosyltransferase genotypes and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone glucuronidation phenotype in human liver microsomes. Cancer Res. 2004;64:1190–6.
Saeki M, Saito Y, Jinno H, et al. Genetic variations and haplotypes of UGT1A4 in a Japanese population. Drug Metab Pharmacokinet. 2005;20:144–51.
Guillemette C, Ritter JK, Auyeung DJ, Kessler FK, Housman DE. Structural heterogeneity at the UDP-glucuronosyltransferase 1 locus: functional consequences of three novel missense mutations in the human UGT1A7 gene. Pharmacogenetics. 2000;10:629–44.
Villeneuve L, Girard H, Fortier LC, Gagne JF, Guillemette C. Novel functional polymorphisms in the UGT1A7 and UGT1A9 glucuronidating enzymes in Caucasian and African-American subjects and their impact on the metabolism of 7-ethyl-10-hydroxycamptothecin and flavopiridol anticancer drugs. J Pharmacol Exp Ther. 2003;307:117–28.
Huang YH, Galijatovic A, Nguyen N, et al. Identification and functional characterization of UDP-glucuronosyltransferases UGT1A8*1, UGT1A8*2 and UGT1A8*3. Pharmacogenetics. 2002;12:287–97.
Jinno H, Saeki M, Saito Y, et al. Functional characterization of human UDP-glucuronosyltransferase 1A9 variant, D256N, found in Japanese cancer patients. J Pharmacol Exp Ther. 2003;306:688–93.
Saeki M, Ozawa S, Saito Y, et al. Three novel single nucleotide polymorphisms in UGT1A10. Drug Metab Pharmacokinet. 2002;17:488–90.
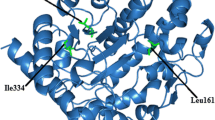
Martineau I, Tchernof A, Belanger A. Amino acid residue ILE211 is essential for the enzymatic activity of human UDP-glucuronosyltransferase 1A10 (UGT1A10). Drug Metab Dispos. 2004;32:455–9.
Acknowledgements
The authors appreciate the technical assistance of Dr Lin-Lin Wang of Institute of Reproductive and Child Health, Peking University, Beijing, China. Ms. Yuan Ming Di is a holder of RMIT University PhD Scholarship. The authors appreciate the support of RMIT Health Innovations Research Institute, RMIT University, Bundoora, Victoria, Australia. We also would like to thank Associate Professor Clifford Da Costa (Department of Mathematics and Statistics, RMIT University, Melbourne, Australia) for his assistance in the statistical analysis of the data in this paper.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(XLS 158 kb)
Rights and permissions
About this article
Cite this article
Di, Y.M., Chan, E., Wei, M.Q. et al. Prediction of Deleterious Non-synonymous Single-Nucleotide Polymorphisms of Human Uridine Diphosphate Glucuronosyltransferase Genes. AAPS J 11, 469–480 (2009). https://doi.org/10.1208/s12248-009-9126-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-009-9126-z