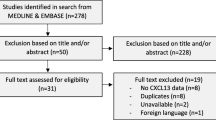
Abstract
Background
Rheumatoid arthritis (RA) affects about 1–3% of the population making it one of the most popular autoimmune diseases. Chemokines through switching on their receptors exert a climacteric role in RA pathogenesis. The purpose of this cross-sectional study was to quantify the serum levels of serum B lymphocyte chemoattractant protein13 (CXCL13) chemokine in recent onset RA patients and to correlate its levels with clinical, laboratory, and musculoskeletal ultrasonographic parameters (MSUS) of disease activity and severity.
Results
The mean serum CXCL13 value showed a significant increase in the RA patients (388.86 ± 283.63 pg/ml) than in the controls (62.94 ± 31.62 pg/ml) (P < 0.001). Highly active RA patients had significantly the highest mean of CXCL13 (mean ± SD 819.13 ± 191.05) compared with the moderately active RA patients (mean ± SD 284.95 ± 137.93) (P < 0.001) and the RA patients with low disease activity (mean ± SD 129.5 ± 21.27) (P < 0.001) and its levels were positively related with clinical disease activity and musculoskeletal ultrasonographic severity parameters.
Conclusion
Serum CXCL13 is correlated with clinical disease activity and MSUS disease severity that encourages its use for monitoring the activity and severity of synovitis in recent onset RA patients. Future studies to detect the effect of disease activity control by medications on CXCL13 levels and the effect of the CXCL13 antagonist on controlling RA disease activity and severity are recommended.
Similar content being viewed by others
Background
Rheumatoid arthritis (RA) affects about 1–3% of the world’s population, and it is considered one of the most popular autoimmune diseases. Hands and feet’s small synovial joints are primarily involved [1]. It is characterized by synovitis extending to the adjacent articular cartilage leading to erosion and further tissue damage. It is not just an articular disease, extra-articular organs could be affected thereby worsening disease prognosis and leading to lifelong disability and discomfort [2].
Etiopathogenesis for RA is obscure, it is a prototypic inflammatory disease that results from the interplay between genetic and environmental factors which alter the immunological homeostasis, wherein immunological stimulation and unwanted inflammation predominate. Inflammatory changes involving the synovium of the inflamed joints leading to pain, warmth, swelling, and stiffness that usually experienced early in the morning or after prolonged inactivity that lasts more than 30 min [3].
Chemotactic cytokines and their receptors exert a climacteric role in the pathogenesis of RA. They synchronize and induce the immune cells migration that direct not just the ordinary turn of events and homeostasis of the immune system but also responsible for diverse immune system intolerance reactions leading to inflammatory and destructive immune responses in many rheumatic disorders [4]. Chemokines super family are grouped according to the gathering of cysteine molecule in their two amino acid terminals. Their names remain on the position of the two cysteine buildups differentiating them into 4 groups: CC, CXC, CX3C, and XC. No other amino acid isolates the two cysteine deposits in the CC chemokines while at least an amino acid isolates the cysteine buildups in the CXC chemokines [5].
Contribution of the CXCL13 in the pathogenesis of synovitis in RA has been recently revealed. At the point when it is created locally, it is related with extra nodal lymphoid aggregates that house ectopic B lymphocyte responses with other cell subsets and intercede development of the immunoglobulin genes. It follows up on various cells having CXCR5 receptors like dendritic cells and bone cells and aids chemotaxis of CD4+ follicular T partner cells. CXCL13 has been thus proposed as a proxy marker of synovitis [1].
Initial outcomes recommended that CXCL13 serum levels and its mRNA expression in the synovium are associated, proposing that the inflamed synovium is a significant wellspring of the circling CXCL13 and clinically, this makes it conceivable and simple to identify and measure this chemokine not just from the synovial membrane, anyway additionally in the synovial liquid and circulation [6].
Early and strict control of the inflammation and the possibility to predict severity of synovial inflammation is vital to set proper algorithm to treat the patients with RA [7].
Remission that is detected clinically does not necessarily mean actual concealment of the inflammation, as remittent patients may proceed to have subclinical active synovitis that can be identified and scored specifically by power doppler (PD) musculoskeletal ultrasound (MSUS) mode. PD recognized signals in the synovium can anticipate joint harm and short-term relapse in treated patients who are considered to be clinically remittent [8, 9]. In this manner, identification of surrogate markers dependent on understanding RA pathogenesis and to correlate with the seriousness of synovitis at the clinical and sub-clinical level, is required [10]. We planned to quantify levels of CXCL13 chemokine in the sera of recent onset RA patients and to associate its levels with clinical, laboratory, and musculoskeletal ultrasonographic findings of disease activity and severity.
Methods
This cross-sectional study included 50 recent onset RA patients who fulfilled the updated American College of Rheumatology/European League Against Rheumatism criteria for the classification of RA [11].
They had disease duration ≤ 12 months attending the outpatients′ clinic and the in-patients’ units of our department. Exclusion criteria included patients with liver or endocrine disorders, metabolic diseases, recent infections, trauma and, malignancy or any other rheumatic disease.
RA patients had intra-articular steroid injection in the examined joints since 3 months of the study were also excluded.
Forty apparently healthy subjects were filled in as a benchmark group. The study was endorsed in concordance to the 1983 Helsinki Declaration Statement. All members gave an oral consent before partaking in the study.
Full history was taken from the patients including the age, sex, disease duration, morning stiffness duration, patient global health assessment based on visual analog scale from 0 to 10 (pVAS) and medical treatments.
Thorough clinical examination was done, disease activity 28-joint (DAS-28) score was used to assess RA disease activity. Remission was considered if DAS-28 is < 2.6, low activity was considered if DAS-28 was ≥ 2.6 and < 3.2, moderate activity was considered if DAS-28 was ≥ 3.2 and < 5.1 and high activity was considered if DAS-28 was ≥ 5. 1[12].
Radiological examination
Plain X-ray of hands and feet were assessed and graded according to Larsen et al. [13]. The Larsen list score was applied to 20 joints reciprocally including metacarpophalangeal joints (MCPs), wrists, the second to the fifth metatarsophalangeal joints (MTPs), each joint was graded from (0-5) with a total score range from 0 to 100.
Musculoskeletal ultrasonography (MSUS)/power doppler assessment (PD):
RA patients were scanned by MSUS that was done by a rheumatologist experienced in MSUS who didn't know the clinical information of the patients, using a GE Logiqe 9 scanner (General Electric Medica Systems, USA) with a linear transducer (8–13 MHZ).
Gray-scale (GS) and power doppler (PD) examination were done in both longitudinal and transverse scan for 6 joints in every RA patient; the wrist (dorsal radiocarpal and midcarpal joints), 2nd MCP and supra patellar recess of the knee joint bilaterally [14]. The gain and depth were adjusted according to examined joint.
Each joint was assessed by GS mode for the presence of synovial hypertrophy (SH) and erosions. According to European League against rheumatism- Outcome Measures in Rheumatology (EULAR-OMERACT), synovitis was diagnosed by the presence of a hypoechoic synovial hypertrophy (SH) regardless of the presence of effusion or any grade of PD signal [15]. US-SH was defined as presence of abnormal hypoechoic synovial tissue within the capsule that was not displaceable and poorly compressible and it may exhibit PD signals. US-SH was graded from 0 to 3; grade 0 (normal): no SH regardless the presence of effusion, grade 1: minimal hypoechoic SH up to the level of the horizontal line joining bone surfaces, grade 2: moderate hypoechoic SH extending out the joint line but with the upper surface concave, grade 3: severe hypoechoic SH with or without effusion extending above the joint line but with the upper surface convex.
US detected erosions were defined as intra- and/or extra-articular discontinuity of bone surface that should be seen in two perpendicular planes [15].US synovitis activity was assessed by PD mode for detecting US-PD signals in the SH of each joint that was graded from 0 to 3; grade 0: no PD signal, grade 1: from one to three single spots or one confluent spot and two single spots or up to two confluent spots, grade 2: > grade 1 but < 50% of doppler signals in the background, grade 3: more than grade 2; > 50% of the total gray-scale back ground [15]. For each patient the total US-SH and US-PD score ranged from 0 to 18. Total musculoskeletal score (0 to 36) is the sum of US-SH and US-PD grades of the 6 joints for the patient [14].
Laboratory investigations
Complete blood cell count (CBC), hemoglobin (HB) concentration (gm/dl), erythrocyte sedimentation rate in the 1st hour (ESR mm/1st hour), C-reactive protein (CRP mg/dl), rheumatoid factor (RF u/ml), and anti-cyclic citrullinated antibody (Anti-CCP Abs u/ml) were measured.
Quantitative detection of serum CXCL13 level
Serum samples from all enrolled subjects were gathered and were put away at − 20°C until investigation. Levels were estimated by enzyme-linked immunosorbent assay (ELISA) (Boster Biological Technology Co.) recorded in pg/ml [16]. Serum CXCL13 levels were evaluated by the manufacturer’s guidelines utilizing a commercially accessible ELISA unit (Quantikine human CXCL13/BCL/BCA-1, #DCX130 R&D systems, USA) that was previously validated [17]. All tests were diluted 1:2 in Calibrator Diluent RD6-41 enhanced with mouse and bovine immunoglobulin G to guarantee preaggregation of heterophilic antibodies. Tests were examined in duplicates and the cutoff limit was determined as two standard deviations of the blanks.
Statistical methods
Data were presented as means ± standard deviation (SD), median and interquartile range (IQR), or numbers and percentage. The significance of difference was tested using Student’s t test to compare between the mean of two groups of parametric data. For continuous non-parametric data, Mann-Whitney U test was used. Chi-square test was used for categorical parameters. Spearman’s relationship coefficient was considered. Regression analysis was used to identify the independent parameters that could predict the US-SH activity being the dependent factor. The receiver operating characteristics (ROC) curve was done to evaluate the diagnostic value of serum CXCL13 levels in recent onset RA. A statistical significance is considered when a P value was < 0.05 and a P value < 0.001 was considered highly significant. All data were tabulated, coded, and analyzed using STATA/SE version 11.2 for Windows.
Results
This study included 50 RA patients; 37 (74%) females and 13 (26%) males with ages extended in the range between 19 and 63 years and a mean of 36.82 ± 13.66 years. They had disease duration ranged between 3 months and 12 months with a mean of 8.9 ± 3.09 months. Forty apparently healthy volunteers; 28 (70%) female and 12 (30%) male with ages ranging between 21 and 63 years with a mean of 39.3 ± 13.07 years were included as a control group. Both patients and controls were age (P = 0.8) and gender matched (P = 0.5). Table 1 and Fig. 1 showed the patients’ clinical, laboratory, and radiographic characteristics.
Comparison between the RA patients and controls regarding the mean serum CXCL13 levels. This figure showed that the mean CXCL13 value was significantly higher in the RA patients (388.86 ± 283.63 pg/ml), than in the controls (62.96 ± 32.5 pg/ml) (P < 0.001). Cut-off level of CXCL13 is 111 pg/ml. CXCL13 serum B lymphocyte chemoattractant protein13, P < 0.001** highly significant
DAS-28 ranged between 2.9 and 6.2 with a mean of 4.25 ± 0.99; 7 patients had low disease activity (13%), 31 (62 %) patients were moderately active, and 12 (24%) patients had high disease activity. Eighteen (36%) of patients had extra-articular manifestations in the form of pleurisy, subcutaneous nodules and Sjogren’ syndrome.
RA patients were on conventional synthetic disease modifying anti-rheumatic drugs (csDMARD) and/or biologic (b) DMARDS ± oral corticosteroid ranged between 5 and 15 mg/day. Twenty-four RA patients were treated with methotrexate (MTX) alone, MTX and Sulfasalazine (3 patients), Leflunomide (5 patients), combination of MTX, Sulfasalazine, and Leflunomide (3 patients). Fifteen RA patients were receiving bDMARDs: 9 patients were receiving Adalimumab and 6 patients were on Etanercept ± MTX.
Periarticular erosions were detected in the hand and feet X-ray in 17 patients (34%) while erosions were detected by MSUS examination in 23 (46%) patients.
The mean CXCL13 value was fundamentally higher in the RA patients (388.86 ± 283.63 pg/ml), than in the controls (62.96 ± 32.5 pg/ml) (P < 0.001) (Fig. 1).
No significant differences in the mean serum CXCL13 levels were found according to the gender (P = 0.26), extra-articular organ involvement (P = 0.75), seropositivity for RF (P = 0.23) or Anti-CCP Abs (P = 0.83) in RA patients’ group but its levels were statistically significantly increased in the erosive RA patients detected by X-ray or MSUS (553.18 ± 291.85) than patients without erosions (201.07 ± 99.84) (P < 0.001) (Table 2).
The mean CXCL13 serum level was significantly the highest in the highly active RA patients’ group (mean ± SD 819.13 ± 191.05) compared with the moderately active RA patients (mean ± SD 284.95 ± 137.93) (P < 0.001) and the RA patients with Low disease activity (mean ± SD 129.5 ± 21.27) (P < 0.001) (Table 2).
Serum levels CXCL13 levels were significantly positively correlated with MS durations (r = 0.68) (P < 0.001), SJCs (r = 0.85) (P < 0.001), TJCs(r = 0.84) (P < 0.001), patient global assessment scores (pVASs) (r = 0.28) (P < 0.001), ESR 1st h values (r = 0.70) (P < 0.001), platelets counts (r = 0.56) (P < 0.003), RF (r = 0.66) (P < 0.001), Anti-CCP Abs (r = 0.64) (P < 0.001), DAS (r = 0.85) (P < 0.001) and significantly negatively correlated with HB concentrations (r = − 0.46) (P < 0.01) while they showed no significant correlations with ages (r = 0.15) (P = 0.26), disease durations (r = 0.16, P = 0.23), or CRP levels (r = 0.23) (P = 0.09) (Table 3).
Serum CXCL13 levels were significantly positively correlated with the total MSUS scores for the patient (r = 0.64) (P < 0.001) and US-SH scores (r = 0.46) (P < 0.001) while there were not correlated with Larsen scores (r = 0.18) (P > 0.05) nor US-PD scores (r = 0.30) (P = 0.11). Figure 2a showed GS dorsal longitudinal MSUS scan of a 2nd MCP joint with SH of grade 2 and an erosion in RA patient with increased serum CXCL13 level of 120 pg/ml, Fig. 2b showed GS dorsal longitudinal midline MSUS scan of the wrist joint (radiocarpal and midcarpal joints) of the same patient with an erosion in the lunate bone and Fig. 2c showed dorsal PDUS longitudinal midline MSUS scan of a wrist joint ( radiocarpal and midcarpal joints) with severe synovitis marked by SH grade 3 and PD activity grade 3 in RA patient with increased serum CXCL13 level of 803 pg/ml. Multivariate regression analysis considering the US-SH the dependent variable showed that serum CXCL13 levels significantly (P = 0.001) associated and predicting US-SH scores among the studied variables including the ESR (P = 0.9), CRP (P = 0.08), RF (P = 0.18), and Anti-CCP abs (P = 0.98).
a–c Dorsal longitudinal MSUS dorsal scan of radiocarpal joint in two different RA patients. a Gray-scale dorsal longitudinal musculoskeletal ultrasonographic scan of a 2nd metacarpophalangeal joint (m: metacarpal bone, p: proximal phalanx) shows synovial hypertrophy (black arrow) grade 2 and an erosion (e) in RA patient with increased serum CXCL13 level of 120 pg/ml. b Grey-scale dorsal longitudinal midline musculoskeletal ultrasonographic scan of the wrist joint (radiocarpal and midcarpal joints) of the same patient shows an erosion (arrow) in the lunate bone (r: radius bone, L:lunate bone, C: capitate bone). c Dorsal PDUS longitudinal midline musculoskeletal ultrasonographic scan of a wrist joint (radiocarpal and midcarpal joints) (r: radius bone, L: lunate bone, C: capitate bone) shows severe synovitis marked by synovial hypertrophy grade 3 and power doppler activity grade 3 in RA patient with increased serum CXCL13 level of 803 pg/ml. CXCL13: serum B lymphocyte chemoattractant protein13. PDUS power doppler ultrasound
Serum CXCL13 had an AUC of 0.99 at a cutoff point of 111 pg/ml with sensitivity of 96.67% and specificity of 95.0%, positive predictive value (PPV) of 96.67%, and negative predictive value of (NPV) 95.0% as a diagnostic test of RA (Fig. 3).
ROC analysis of CXCL13 as a diagnostic test for RA. This figure showed that at a cut-off point 111pg/ml CXCL13 chemokine has a sensitivity of 96.67 %, a specificity of 95%, positive predictive value (PPV) of 96.57%, and negative predictive value (NPV) of 96% for diagnosis of RA. CXCL13 serum B lymphocyte chemoattractant protein13, RA rheumatoid arthritis
Discussion
Previous articles elucidate how much chemokines and their receptors are important in RA pathogenesis. CXCL13 chemokine directs B cell chemotaxis, and it is elevated in several autoimmune diseases [18]. This study was planned to quantify the CXCL13 chemokine serum levels in recent onset RA patients and to correlate its levels with clinical and laboratory parameters of disease activity and severity, our study was extended to relate serum CXCL13 levels to more objective scores of disease activity and severity detected by GS and PD MSUS examination.
It has been previously shown that serum levels of CXCL13 levels were significantly raised in recent onset RA patients than in the healthy subjects [16, 19,20,21,22]. This is consistent with this study which showed significant increment of serum CXCL13 levels in the RA patients in contrast to the control subject. Furthermore, we have elucidated that serum CXCL13 levels were significantly elevated in active RA patients, and its levels were correlated significantly with the total DAS scores and individual disease activity parameters as morning stiffness (MS) duration, the number of swollen and tender joints, p VAS scores for global patient assessment added to ESR values.
Collectively, these results supported other investigators who found that serum CXCL13 levels were significantly associated with various measures of disease activity, such as the SJC, the disease activity global assessment, and ESR values [22,23,24].
Our results are consistent with Greisen et al. who detected raised serum CXCL13 levels in untreated early RA active patients and found that their disease activity had been reduced in those patients after treatments that supports the hypothesis that CXCL13 is a surrogate marker of disease activity and implementing a role for CXCL13 as an inflammatory marker early in the course of RA [24].
CXCL13 is superior to the conventional acute phase reactants (ESR and CRPs) in evaluating and assessing the degree of synovial inflammation. CXCL13 is constitutively generated in the lymph nodes but moreover, it originates from the inflamed synovial tissue that causes its upregulation and leads to enhancement of the lymphoid follicles′ development and germinal center reactions. On the other side, ESR and other acute phase reactants are induced by the systemic released interleukins and produced by hepatocytes only during inflammation so they are not directly linked to local joint inflammation [6, 25,26,27].
In this study, serum CXCL13 levels were significantly increased in RF and Anti-CCP Abs seropositive RA patients compared with seronegative patients. Our findings matched the previous results reported by Bugatti et al., who found that CXCL13 levels were significantly increased in Anti-CCP Abs-positive compared to seronegative patients and Jones et al, who stated that CXCL13 was associated with RF in RA patients [28, 29]. Although RF and Anti-CCP Abs help to classify and address the autoimmunity in RA but unfortunately they do not counter rapidly to the changes of disease activity and are mostly used for prognostic assessment [30].
It is widely accepted now that MSUS is beneficial and especially PD techniques used to detect the active signals within the inflamed synovium added value to the clinical examination in detecting synovitis [31]. The sensitivity of MSUS to detect the erosion compared to radiography had been evaluated before as MSUS-GS detected 6.5-fold more erosions in a study of RA patients with disease duration less than one year and 3.4-fold more erosions were distinguished by MSUS in the established RA patients compared with MRI [32].
In our study, periarticular erosions were detected in 10 patients (33.33%) by conventional X-ray and in 16 patients (46.67%) by MSUS. The mean serum CXCL13 level was increased in the RA patients with erosions but its levels were not correlated to Larsen scores. This is as per Greisen et al. whose study demonstrated that serum CXCL13 levels and radiographic progression were not significantly correlated [24] but are of controversial in comparison with others who found that there were definite association between serum CXCL13 levels and increased rates of joint destructions [28, 33]. These different results can be explained on the base that RA patients had different disease durations in the different studies.
Assessment of the inflamed joint for the degree of activity and severity by MSUS is a non-invasive, easily used, and reproducible tool that can be used for follow up the synovitis in RA patients. It was decided and agreed by the EULAR-OMERACT US taskforce consensus to characterize gray-scale US synovitis as hypoechoic SH regardless of the presence of effusion and any grade of PD signal, they concluded that effusion occurs more frequently in certain joint depending on patients’ weight and level of activity and did not add extra weighting to the definition and severity of an US- proved synovitis [15].
Consistent with previous observations, our study showed that CXCL13 levels were significantly correlated with MSUS synovial hypertrophy expressing the severity of synovitis [34, 35]. Interestingly, we have not found any significant association between CXCL13 levels and PD activity scores, which is discordant with the preceding data. Several explanations are possible for such discrepancy. First, PD examination is extremely sensitive to tissue movement and pressure, additionally, with lack of standardization of methods used in PDUS examination and various settings of PDUS, the interobserver reliability remains a concern especially in image acquisition and interpretation. Second, PD activity might be affected with different treatment regimens in the different studies.
Previously, it was reported that CXCL13 expression were significantly correlated with histological grading of synovitis in early DMARD-naïve RA patients and pathogenic significance of CXCL13 in RA synovitis had been investigated in animal model of arthritis and it was found that CXCL13 neutralization had improved the disease outcomes and histological severity of synovitis [35,36,37]. Also, Manzo et al. assessed CXCL13 expression in correlation to its circulating levels and found that circulating CXCL13 was significantly correlated with synovial CXCL13 protein (r = 0.3, P = 0.04) and mRNA (r = 0.56, P = 0.02) expression in early RA [38].
In our study, regression analysis showed that serum CXCL13 level was the only factor that is strongly associated with SH score and hence it can be used to predict the severity of the actively inflamed joints which confirmed what was stated before about its usefulness in grading the severity of synovitis in comparison to ESR, C-reactive proteins, RF, or Anti-CCP abs [16].
In our study, we found that serum CXCL13 at a cut-off point of 111 pg/ml had sensitivity of 96.67% and specificity of 95.0% as a diagnostic test of RA that is in line and supports previous researcher who concluded that serum CXCL13 levels could be helpful confirming the diagnosis of active recent onset RA when they detected elevated levels in a remarkable number of recent onset seronegative RA patients [22].
Conclusion
We concluded that serum CXCL13 is correlated with clinical disease activity and MSUS disease severity; thus, we encourage its use in monitoring the activity and severity of synovitis in recent onset RA patients. Future studies to detect the effect of disease activity control by medications on CXCL13 levels and the effect of the CXCL13 antagonist on controlling RA disease activity and severity are recommended.
Availability of data and materials
All are available
Change history
26 January 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1186/s43166-022-00112-6
Abbreviations
- CXCL13:
-
Serum B lymphocyte chemoattractant protein13
- RA:
-
Rheumatoid arthritis
- CXCR5:
-
Chemoattractant protein receptor 5
- MSUS:
-
Musculoskeletal ultrasound
- PD:
-
Power doppler
- GS:
-
Gray-scale
- US-SE:
-
Ultrasound synovial effusion
- US-SH:
-
Ultrasound synovial hypertrophy
References
Elemam NM, Hannawi S, Maghazachi AA (2020) Role of chemokines and chemokine receptors in rheumatoid arthritis. Immunotargets Ther 9:43–56
Aletaha D, Smolen JS (2018) Diagnosis and management of rheumatoid arthritis: a review. JAMA 320(13):1360–1372
Ali DMM, Fadhel SZ, Al-Ghuraibawi NHA, Al-Hakeim HK (2020) Serum chemerin and visfatin levels and their ratio as possible diagnostic parameters of rheumatoid arthritis. Reumatologia 58(2):67–75
Hughes CE, Nibbs RJB (2018) A guide to chemokines and their receptors. FEBS J 285(16):2944–2971
Zlotnik A, Yoshie O (2000) Chemokines: a new classification system and their role in immunity. Immunity 12(2):121–127
Rosengren S, Wei N, Kalunian KC, Kavanaugh A, Boyle DL (2011) CXCL13: a novel biomarker of B-cell return following rituximab treatment and synovitis in patients with rheumatoid arthritis. Rheumatology 50:603–610
Knevel R, Schoels M, Huizinga TW, Aletaha D, Burmester GR, Combe B et al (2010) Current evidence for a strategic approach to the management of rheumatoid arthritis with disease-modifying antirheumatic drugs: a systematic literature review informing the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 69(6):987–994
Brown AK, Conaghan PG, Karim Z, Quinn MA, Ikeda K, Peterfy CG et al (2008) An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum 58:2958–2967
Scirè CA, Montecucco C, Codullo V, Epis O, Todoerti M, Caporali R (2009) Ultrasonographic evaluation of joint involvement in early rheumatoid arthritis in clinical remission: power Doppler signal predicts short-term relapse. Rheumatology 48:1092–1097
Dalrymple AH, Southey-Bassols C, Bechman K, Galloway J (2019) The use of CXCl13 as a biomarker in rheumatoid arthritis: a systematic review. Rheumatology 58(3):kez110.022
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd et al (2010) 2010 rheumatoid arthritis classification criteria: an American college rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum 62:2569–2581
Prevoo ML, Ma v’t H, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL (1995) Modified disease activity scores that include twenty-eightjoint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 38:44–48
Larsen A (1995) How to apply Larsen score in evaluating radiographs of rheumatoid arthritis in longterm studies? J Rheumatol 22:1974–1975
Iagnocco A, Finucci A, Ceccarelli F, Perricone C, Iorgoveanu V, Valesini G (2015) Power Doppler ultrasound monitoring of response to anti-tumour necrosis factor alpha treatment in patients with rheumatoid arthritis. Rheumatology (Oxford) 54:1890–1896
Bruyn GA, Iagnocco A, Naredo E, Balint PV, Gutierrez M, Hammer HB et al (2019) OMERACT definitions for ultrasonographic pathologies and elementary lesions of rheumatic disorders 15 years on. J Rheumatol 46(10):1388–1393
Bugatti S, Manzo A, Benaglio F, Klersy C, Vitolo B, Todoerti M et al (2012) Serum levels of CXCL13 are associated with ultrasonographic synovitis and predict power Doppler persistence in early rheumatoid arthritis treated with non-biological disease-modifying anti-rheumatic drugs. Arthritis Res Ther 14:R34
Kragstrup TW, Vorup-Jensen T, Deleuran B, Hvid M (2013) A simple set of validation steps identifies and removes false results in a sandwich enzyme-linked immunosorbent assay caused by anti-animal IgG antibodies in plasma from arthritis patients. Springer Plus 2:263
Moussa SG, El-Hefnawy HE, El-Shishtawy HF, El Mikkawy DM, Shalaby MH (2018) Salivary CXCL13 in relation to scintigraphy in early detection of secondary Sjogren’s syndrome. Egypt Rheumatol Rehabil 45(4):153–158
Manzo A, Caporali R, Vitolo B, Alessi S, Benaglio F, Todoerti M, Bugatti S, Calliada F, Montecucco C (2012b) Subclinical remodelling of draining lymph node structure in early and established rheumatoid arthritis assessed by power Doppler ultrasonography. Rheumatology 50:1395
Rosengren S, Wei N, Kalunian KC, Kavanaugh A, Boyle DL (2011) CXCL13a novel biomarker of B-cell return following rituximab treatment and synovitis in patients with rheumatoid arthritis. Rheumatology 50:603–610
Rioja I, Hughes FJ, Sharp CH, Warnock LC, Montgomery DS, Akil M, etal (2008) Potential novel biomarkers of disease activity in rheumatoid arthritis patients: CXCL13, CCL23, transforming growth factor α, tumor necrosis factor receptor superfamily member 9, and macrophage colony-stimulating factor. Arthritis Rheum 58:2257–2267.
Allam SA, Sallam RA, Elghannam DM, El-Ghaweet AI (2019) Clinical significance of serum B cell chemokine (CXCL13) in early rheumatoid arthritis patients. Egypt Rheumatol 41:11–14
Szkudlarek M, Narvestad E, Klarlund M, Court-Payen M, Thomsen HS, Ostergaard M (2004) Ultrasonography of the metatarsophalangeal joints in rheumatoid arthritis: comparison with magnetic resonance imaging, conventional radiography, and clinical examination. Arthritis Rheum 50:2103–2112
Greisen SR, Schelde KK, Rasmussen TK, Kragstrup TW, Stengaard-Pedersen K, Hetland ML, et. al (2014) CXCL13 predicts disease activity in early rheumatoid arthritis and could be an indicator of the therapeutic 'window of opportunity. Arthritis Res Ther 24;16(5):434.
Takemura S, Braun A, Crowson C, Kurtin PJ, Cofield RH, O’Fallon WM et al (2001) Lymphoid neogenesis in rheumatoid synovitis. J Immunol 167:1072–1080
Shi K, Hayashida K, Kaneko M, Hashimoto J, Tomita T, Lipsky PE et al (2001) Lymphoid chemokine B cell-attracting chemokine-1 (CXCL13) is expressed in germinal center of ectopic lymphoid follicles within the synovium of chronic arthritis patients. J Immunol 166:650–655
Gabay C, Kushner I (1999) Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340:448–454
Bugatti S, Manzo A, Bombardieri M, Vitolo B, Humby F, Kelly S et al (2011) Synovial tissue heterogeneity and peripheral blood biomarkers. Curr Rheumatol Rep 13:440–448
Jones JD, Hamilton BJ, Challener GJ, de Brum-Fernandes AJ, Cossette P, Liang P et al (2014) Serum C-X-C motif chemokine 13 is elevated in early and established rheumatoid arthritis and correlates with rheumatoid factor levels. Arthritis Res Ther 16(2):R103
Mewar D, Coote A, Moore DJ, Marinou I, Keyworth J, Dickson MC et al (2006) Independent associations of anti-cyclic citrullinated peptide antibodies and rheumatoid factor with radiographic severity of rheumatoid arthritis. Arthritis Res Ther 8(4):R128
Naredo E, Collado P, Cruz A, Palop MJ, Cabero F, Richi P et al (2007) Longitudinal power Doppler ultrasonographic assessment of joint inflammatory activity in early rheumatoid arthritis: predictive value in disease activity and radiologic progression. Arthritis Rheum 57:116–124
Wakefield RJ, Balint PV, Szkudlarek M, Filippucci E, Backhaus M, D’Agostino MA et al (2005) Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol 32(12):2485–2487
Meeuwisse CM, van der Linden MP, Rullmann TA, Allaart CF, Nelissen R, Huizinga TW et al (2011) Identification of CXCL13 as a marker for rheumatoid arthritis outcome using an insilico model of the rheumatic joint. Arthritis Rheum 63:1265–1273
Ahmed SF, Badr T, Hosny SM, Aboul-Hamayed HF (2013) Assessment of synovitis in early rheumatoid arthritis by CXCL13 serum levels and power Doppler ultrasonography: correlation with disease activity. Egypt Rheumatol 35(1):21–27
Bugatti S, Manzo A, Vitolo B, Benaglio F, Binda E, Scarabelli M et al (2014) High expression levels of the B cell chemoattractant CXCL13 in rheumatoid synovium are a marker of severe disease. Rheumatology (Oxford) 53(10):1886–1895
Klimatcheva E, Pandina T, Reilly C, Torno S, Bussler H, Scrivens M et al (2015) CXCL13 antibody for the treatment of autoimmune disorders. BMC Immunol 12, 16:6
Finch DK, Ettinger R, Karnell JL, Herbst R, Sleeman MA (2013) Effects of CXCL13 inhibition on lymphoid follicles in models of autoimmune disease. Eur J Clin Investig 43:501–509
Manzo A, Bugatti S, Vitolo B, Benaglio F, Binda E, Scarabelli M et al (2014) THU0546 Serum CXCL13 as a biomarker of disease activity and severity in IN rheumatoid arthritis. Comparison with acute phase reactants and the autoantibody profile. Ann Rheum Dis 73:371
Acknowledgements
Not applicable
Presentation at a meeting
As an abstract in Annals of the Rheumatic Diseases 76(Suppl 2):781.2-781,2017. Under the title: SAT0038 Serum B lymphocyte chemoattractant protein 13 (CXCL 13) and musculoskeletal ultrasonographic findings in early rheumatoid arthritis.
Conference: Annual European Congress of Rheumatology, place: Italy. Date: 14–17 June, 2017
Funding
The research was not funded. Costs were the responsibility of the authors.
Author information
Authors and Affiliations
Contributions
EAB, SAE, GAH, MAK, SHA, and MME had contributed to the conception, design of the work and definitions of the intellectual contents. EAB, MAK, SHA, and MME were concerned with literature review, run the clinical studies, collecting data, manuscript preparation, and editing. EAB and MAK were concerned with analysis, and interpretation of statistical data and writing the results. The manuscript has been read and approved by all the authors, the requirements for authorship have been met, and each author believes that the manuscript represents honest work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research was accepted by Research Ethics Committee (REC) of Faculty of Medicine, Benha University in accordance the 1983 Helsinki Declaration.
The study was clarified to all the participant who gave a verbal consent before participating in the study according to the ethics committee for scientific research in our faculty to date .
The reference number is not available as this paper was done from a MD thesis that was registered on 18 May 2014 and at that time no reference numbers for registration the ethical committee was available. It was discussed in our department in 2017. For any details, please contact responsible person: Professor Dr. Nermeen Adly, telephone: +201000071033, e-mail: nmadly1@hotmail.com.
All subjects signed an informed consent before participation.
Consent for publication
Not applicable.
Competing interests
All authors declare conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1186/s43166-022-00112-6"
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baraka, E.A., Egilla, S.A., Hamad, G.A. et al. RETRACTED ARTICLE: Does serum B lymphocyte chemoattractant protein13 (CXCL13) level correlate with parameters of disease activity and severity in rheumatoid arthritis? Clinical and musculoskeletal ultrasonographic assessment. Egypt Rheumatol Rehabil 47, 42 (2020). https://doi.org/10.1186/s43166-020-00041-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43166-020-00041-2