Abstract
Background
Probiotic Enterococcus strains of human origin were microencapsulated by spray drying using whey protein and maltodextrin as an encapsulating agent. The obtained encapsulates were characterized for stability, viability, and physiological properties.
Results
The microcapsules were prepared from probiotic Enterococcus strains that were previously isolated from human vagina and infants’ meconium. The microcapsules revealed similar particle sizes and morphologies. The highest hygroscopicity was observed in the microcapsules produced with strain E. rivorum S22C (0.17 ± 1.15) g water/kg powder/min. E. canintestini S18A revealed highest dissolution time in water (703 ± 2 s). The DSC thermogram revealed excellent thermal stability of all microcapsules. The physicochemical and morphological characteristics of the microcapsules were acceptable with regard to residual water content, particle mean size, and thermophysical properties and storage stability under room temperature conditions, with a low inactivation rate of Enterococcus strains. All the microcapsules revealed the recommended count of probiotic cells, low moisture content with low water activity. Observation under a scanning electron microscope revealed spherical-shaped partially collapsed structures measuring between 9 and 14 μm with surface concavities.
Conclusions
The microcapsule probiotic strains of Enterococcus microencapsulated by spray drying using whey protein and maltodextrin revealed properties of acceptable standards. These strains can have future potential as developing probiotic animal feed and food industry.
Similar content being viewed by others
1 Background
Probiotics are live organisms that are incorporated in foods and beverages to increase the immunity of the host [1]. They are especially important in the food and beverage industry due to their health benefits. They represent about 20% of the economic value of fermented foods throughout the world [2]. Due to the increasing demand for probiotic in functional forms, various approaches have been explored as per the consumer’s demands. Food Agriculture Organisation and the International Dairy Federation have suggested a minimum concentration of 106–107 per gram to be present in the sample until the date of the product expiration [1, 3].
There are several factors involved in the loss of viability of probiotic cells, which include acidity of products, acid produced during storage, the oxygen level in products, permeation of oxygen through the package, and production of antimicrobial substances. Many strategies have been explored by the researchers to improve the viability of probiotic organisms like the selection of acid and bile resistant strains, encapsulation, stress adaptation using oxygen impermeable packages, two-step fermentation, and incorporation of micronutrients such as probiotics. The survival of the strains during gastrointestinal transit can be studied in vitro in gastric juice conditions [4].
Encapsulation technique has demand in food industries due to its ability to protect sensitive food components against degradation reactions and loss of volatiles. The wall material used for encapsulation is the soul of the encapsulating process, as the properties of chosen wall material show influence on self-life, encapsulation efficiency, and encapsulation capacity of the encapsulated compound. Moreover, the encapsulation method is also one of the factors which can affect self-life, encapsulation efficiency, encapsulation capacity, and yield of encapsulation. Different researchers practice many encapsulation methods. As per literature, spray drying is one of the most popular, suitable, and effective encapsulating techniques [5].
Whey protein is used in probiotic products as encapsulating agents. To reduce the deleterious effects of heat treatment, attempts like the addition of protectants to the media are employed before drying. For instance, thermoprotectants like trehalose [6], adonitol, non-fat milk solids [7], growth-promoting factors [8], and granular starch [9] have exhibited improvement in culture viability during drying and storage conditions. Prebiotic sugars like glucose, lactose, trehalose, oligofructose, inulin, mannitol, and sorbitol are reported to have thermo-protective properties and serve as stimulant growth factors for the organisms. Also, it has been reported that the use of complex protein-carbohydrate mixtures in an appropriate proportion may help in reducing the loss during the storage. The prebiotic carriers cause a significant reduction of the melting temperatures of the microparticles that affect the cytoplasmic membranes, eventually controlling the osmotic pressure responsible for membrane ruptures.
The present investigation involves the effect of encapsulation of three previously screened potential probiotic Enterococcus strains of human origin using a protein-carbohydrate complex as wall material and spray-drying method on viability [10, 11].
2 Methods
2.1 Bacterial cell and probiotic preparation
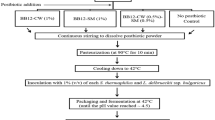
Avirulent probiotic bacterial strains of E. canintestini S18A, E. rivorum S22C, and E. rivorum S14B previously isolated from human vagina and infants’ meconium were used for the probiotic preparation by spray-drying. The bacterial strain sequences were deposited in NCBI GenBank under accession numbers KX 830979, KX 830971, and KX 830973, respectively [10,11,12]. The strains preserved in 40% glycerol stocks were inoculated in 100 ml of MRS broth and incubated for 48 h at 37 °C. The cells were harvested by centrifugation 3000 rpm for 5 min, discard the supernatant, and the cells were washed with sterile PBS at pH 7.
2.2 Preparation of the encapsulation media
The solution was made by mixing 12 g maltodextrin, 4 g d-glucose, and 4 g whey protein. The above mixture was hydrated by suspending in 100 ml of distilled water and stirred on a magnetic stirrer for 1 h at room temperature. Then, the solution was heat-treated at 90 °C for 10 min to destroy the probable microorganisms in it. This carbohydrate-protein mixture was then allowed to cool at room temperature using an ice bath. The cells were then suspended into this mixture and stirred for 5 min. Initial viable counts of cells were performed by the plate count method [13].
2.3 Spray drying
The spray dryer (JISL, Mumbai, India) was operated at 140 ± 2 °C air inlet temperature, 1400 aspiration speed, and solution federate of 3 ml/min. The outlet temperature of powder was observed 60 ± 2 °C for all formulations. Obtained dried powder was filled in airtight ziplock pouches till further use.
2.4 Spray drying efficacy
The spray-drying efficacy was checked by standard plate count procedure. Briefly, 1 g of powder was dissolved in 10 ml PBS (pH 7.0) and stirred for 30 min on a magnetic stirrer to get a homogenous suspension.
where, Nr = log cfu/ml before spray drying, Nf = log cfu/ml after spray drying
2.5 Acid and bile tolerance
The acid and bile tolerance of spray-dried bacteria was evaluated by a method suggested by Park et al. [14]. One milliliter (O.D 0.280, 50 fold dilution, 107–108 cells) of spray-dried culture enumerated in MRS broth for 24 h was inoculated into 9 mL MRS broth of pH 2 and 2.5. Similarly, the same was inoculated into 0.3% and 1 % ox-bile salts. The culture broths were then incubated at 37 °C for 4 h. The viability was checked by the plate count method, and the following formula calculated percentage viability:
Percent survival = (log cfu of cells survived/log cfu of initial cells inoculated) × 100.
2.6 Storage studies
The powder was stored at 4 °C and 25 °C in a ziplock pouch and periodically checked until 30 and 60 days for the viability of the probiotic cells. Briefly, 1 g of spray-dried powder was serially diluted in 10 mL PBS (pH 7) and plated out on sterile MRS agar, subsequently incubated at 37 °C for 72 h. The enumeration of bacteria was carried out by expressing log colony-forming units per milliliter (log cfu/ mL) [15].
2.7 Moisture content and water activity
The moisture content was calculated by weighing 2 g of powder in a pre-weighed aluminum pan and dried at 105 °C for 24 h. The moisture content was calculated by using the formula:
Percent moisture content = Wf − Wi/Wi, where Wf and Wi are final and initial weights of the powder before drying [16]. Water activity at 25 °C after the samples had been stabilized for 15 min was measured with an Aqualab 4TE analyzer by Decagon Devices, USA [15].
2.8 Differential scanning colorimetry
The glass transition temperature of the formulation was calculated by using a differential scanning colorimeter DSC-60 Plus (Shimadzu, India). Approximately 15–20 mg of dry powder was weighed in high-pressure aluminum pans. It was heated from 30–150 °C (rate 10 °C/min). The double-heating and cooling were followed by calculating thermal properties using Mettler Toledo Star [13].
2.9 Particle size analysis
The particle size analysis was carried out using a laser diffraction particle size analyzer along with Tornado dry powder system. The mean diameters of the microcapsules were measured according to the Fraunhofer theory [13].
2.10 Color measurement
Hunter Lab colorimeter (Color Quest XE, HunterLab, USA) was used for color measurements. One gram of powder was placed in plastic cuvettes, and the CIE Lab color scale was used to measure color variation ranging from 0–100 for parameters L* black to white, a* axis shows red (+) to green (−), b* axis shows yellow (+) to blue (−) [15].
2.11 Hygroscopicity
Briefly, 1 g of probiotic powder sample was placed in a desiccator equilibrated with saturated NaCl (75%) at room temperature. After 7 days, the samples were calculated for hygroscopicity using the formula:
where mf and mi express the moisture of the samples before and after storage at 75% relative humidity [15].
2.12 Dissolution
According to Fritzen-Freire et al., the dissolution capacity of the powders was determined by recording the time required for dissolution by dissolving 1 g of powder in 50 mL distilled water with magnetic stirring using a 2-mm × 7-mm stirring bar at 829 rpm [15].
2.13 Morphological characterization
The morphology of the microcapsules was analyzed using a Jeol JSM 5600 (Tokyo, Japan) focussed ion beam scanning electron microscope. A very small amount of sample powder was carefully placed on carbon tabs coated with carbon to enhance conductivity. The images were captured and recorded using secondary electron imaging at an accelerating voltage of 5 kV [13].
2.14 Statistical analysis
The data analysis was performed in the Statistical Package for Social Sciences (SPSS) software for Windows version (16.0). Post hoc tests like Duncan were approached for comparing the strains using p < 0.05 as significant value by analysis of variance (ANOVA). All the analysis was performed in triplicates except for particle size, morphology, and thermal analysis.
3 Results
3.1 Physiochemical and microbiological characteristics of the probiotic microcapsules
The viability efficacy before and after spray drying was carried out by a microbiological plate count method. The survival percentage before spray drying for samples was S18A (81.23 ± 2.07), S22C (78.07 ± 1.05), and S14B (79.45 ± 1.69), whereas the survival percentage after spray drying declined to S18A (70.65 ± 1.84 %), S22C (69.53 ± 3.01 %), and S14B (67.59 ± 2.9 %), respectively. The moisture content of the powder was S18A (6.19 ± 0.56), S22C (5.14 ± 0.82), and S14B (6.07 ± 1.06). Table 1 shows that microcapsules S18A and S14B had higher (p < 0.05) moisture content than other microcapsules. The water activity of the microencapsulated powders ranged from 0.15 to 0.2. Microcapsules S22C and S18A had the highest value with relatively small differences. The glass transition temperatures of the probiotic microcapsules are displayed in Table 1. The glass transition temperature of the powder ranged from 56 to 58 °C. Table 1 displays the color parameters for the microencapsulated powders. The positive a* value indicates red coloration, and positive b* value indicates yellow coloration of the powder. The hygroscopic property of the sample can be attributed to the presence of d-glucose and lactose in the medium, both of which have hygroscopic properties. Microcapsules of sample S22C was most hygroscopic than the others (p < 0.05). The microcapsules were also checked for their dissolution property. Samples containing strain S18A (703 ± 2) and S22C (701 ± 2.5) took more time to dissolve in water than sample S14B (692 ± 2).
3.2 Acid and bile tolerance
Figure 1 shows the relative protection levels offered by the spray-dried microcapsules to the Enterococcus cells entrapped inside, against the acidic medium at pH 2.0 and 2.5. For sample S18A, at pH 2.0, the viability percentage reduced from 9.23 log cfu/mL at the beginning to 7.56 log cfu/mL at the end of 4 h. That suggests that there was a net loss of 1.67 logs cfu/mL in cell viability at pH 2. At pH 2.5, the count was 8.49 log cfu/mL, and net loss was just 0.74 log cfu/mL. Similar results were observed for samples S22C and S14B, respectively suggesting lower tolerance at pH 2. The antimicrobial action of gastric juice was comparatively lesser on the spray-dried microcapsules.
Figure 1 shows the protective actions of spray-dried microcapsules against bile salts encountered by probiotic bacteria in simulated intestinal conditions. Spray-dried entrapped microparticles showed excellent tolerance against bile salt. The initial plate count at bile concentration 1% was 9.86 and finally declined after 4 h to 8.77, 8.57, and 8.6 log cfu/mL for samples S18A, S22C, and S14B, respectively. The initial plate count at bile concentration 0.3% decreased after 4 h to 9.12, 8.97, and 8.87 log cfu/mL for samples S18A, S22C, and S14B respectively. The spray-dried particles offered better protection to the cells and the viability.
3.3 Storage studies
The viability rate or inactivation of the probiotic powders was determined after storage at 4 °C and 25 °C for up to 60 days. The net loss with storage at 4 °C ranged from 0.05 to 0.07% after 30 days and 0.1–0.15% at the end of 60 days. On the other hand, the net loss with storage at 25 °C was more comparative (0.13–0.2 %) at 30 days and (0.3–0.4%) at 60 days. Thus, storing the powder at refrigeration temperature is preferable to prevent the inactivation of probiotic organisms. The inactivation rate was high at room temperature as compared to the refrigerated samples. The spray drying process gave rise to a partially collapsed structure with deflated, ball-like spherical particles. Many particles were irregular in size, aggregated into a large capsule, and had convex and concave deflated shapes. The size of the microcapsules ranged from 9–14 μm (Fig. 2).
4 Discussion
Microencapsulation of probiotic cells was studied as a method to improve the stability of microorganisms by protecting them from adverse environments [17, 18]. Spray drying is widely used for microencapsulation of probiotic bacteria because it has advantages like relatively low cost, ability to scale up to large throughput, and ease of operation. The survival efficacy of probiotics after spray drying and storage depends upon several factors, such as the outlet temperatures, species/strain, and incorporation of appropriate carrier agents into the drying medium [19]. For encapsulation matrix purposes, polysaccharides, proteins, and combinations thereof have been explored as carrier agents for microencapsulation. Polysaccharides with prebiotic properties such as glucose, lactose, and maltodextrin have been used to protect probiotic bacteria during spray drying and under storage conditions [15, 20, 21]. Maltodextrin is a polysaccharide produced by the acidic or enzymatic hydrolysis of starch. It has a nutritional value of only 4 calories/gram. It is a polymer of d-glucose chains linked by glycosidic α-(1-4) and α-(1-6) bonds and is formed by linear and branched carbohydrates with different equivalents of dextrose [22]. Maltodextrins have been widely used in the food industry as they provide different benefits such as texture improvement, reduction in floury taste, sweetness modifying agents, controlling non-enzymatic browning, decreasing the freezing point of mixtures, and as carrier materials [23]. Proteins have high nutritional value and excellent functional properties, which makes them a great carrier agent for microencapsulation [24]. Whey proteins are one of the widely studied carriers in the encapsulation of probiotics. Most of the reported studies included whey proteins in the form of whey protein concentrate or whey protein isolate [25, 26]. Recently, milk proteins have become popular as encapsulating material for probiotic organisms. The whey protein molecules are known to have unique dissociating and aggregating properties under varied physical parameters. This property is especially useful in preparing particles in the diameters ranging from 40 to 2 mm [27]. Rosenberg et al. suggested that whey protein when used in combination with carbohydrates, acted as an emulsifying agent forming films while the carbohydrates served to form the encapsulating network [28].
In present study, the probiotic microcapsules of whey and maltodextrin revealed interesting properties. The spray drying temperature at 60 °C ensured a survival rate of 85–90% of Enterococcus strains. The earlier understanding was that the viability of the probiotic may decrease during convective thermal processing. This may cause cellular injuries resulting from the combined effect of heat and mechanical stress. Some reasons are the denaturation of DNA/RNA, ribosomal damage, dehydration of cytoplasmic membranes, lipid peroxidation, water removal, and rupture of cell membranes [29,30,31].The moisture content of all the samples was below 4 g/100 g as recommended for stability during the prolonged storage of the probiotic powders [17]. The water activity values of the microencapsulated powders were within the normal range limit as recommended for atomized products and microbiologically stable [32]. The glass transition temperature of the powders suggests that the matrices can be stored under chilling or room temperature conditions efficiently while retaining their glassy state, despite the plasticizing effect of d-glucose and free water in the products. Our results were similar to the results obtained by Behboudi-Jobbehdar et al. while working with microencapsulated L. acidophilus NCIMB 701748 encapsulated with whey protein and maltodextrin [13, 33]. The lower luminosity of the microcapsules and their tendency towards yellowish color may be attributed to the presence of whey protein-like ingredients used for encapsulation [15]. Augustin et al. have reported similar results as for the color difference (ΔE* = 2.6) obtained for the white standard [34]. The threshold for color difference normally perceived is ΔE* = 3 [35].
The powders were hygroscopic, and this might be related to the protein content of whey in the composition of the microcapsules. It results due to the denaturation of the whey proteins during the spray drying process, which expose the hydrophobic amino acid residues usually hidden inside the proteins [36]. The dissolution property results agreed with the results obtained by Fávaro-Trindade et al. who revealed that the microcapsules took much time to dissolve in water [37]. The long dissolution time ensured good control of the release of the probiotic in aqueous solutes in the gastrointestinal tract. This property can be attributed to the greater exposure of hydrophobic and sulfhydryl groups by the denaturation of whey proteins during the spray drying process resulting in increased protein-oil interactions, thus facilitating dissolution in medium [38].
Spray drying is known to cause shrinkage in particle volume with a reduction in surface pore diameters. Hence, the acids could easily penetrate through the wet surfaces of wet microcapsules, as compared to the dried ones, and thus imparted a lethal effect on the bacterial cell membranes. The bile salts are known to affect the viability of entrapped cells by the action of detergent-like property that might cause emulsification of the entrapped and surface ingredient, thereby releasing the enterococci cells into bile salt solution [39]. Several researchers have reported that the lipophilic nature of bile salts might cause bacterial cell mortality. The detrimental action is caused by the accumulation of bile salts on the cell membrane, resulting in a structural and functional alteration of the cell membrane and permeability to protons, causing cell death. The authors found severe damage to B. animalis cell surface caused by the action of bile salts. As a measure to protect the cells during intestinal transit, they also suggested employing the pre-adaptation phase by slowly increasing the concentration of bile solution [40].
The microcapsules showed excellent viability when stored at refrigerated temperatures than room temperature. The storage temperature conditions might have an impact on the stability of the probiotics via two mechanisms. The primary reason might be the increased temperatures leading to the increased metabolic activity of the cells during storage involving chemical or enzymatic reactions, e.g., lipid oxidation. The second reason might be the water activity (molecular mobility), which causes the rubbery state of the matrix.
The scanning electron microscopy scans revealed that the microcapsules were hollow and had a deflated structure which is a usual occurrence in many spray-dried powders. The collapsed structure can be attributed to the presence of the whey protein, the heat transfer, and water diffusion rates inside-out of the spherical particles. These factors critically affect the microstructure of the particles along with the inlet and outlet temperatures of the feed flow rate [41]. The size of the microcapsules were in agreement with the values of 10–100 μm as proposed by Fang et al. in his studies of spray-dried probiotic powders [36]. Also, the particle size below 40 μm is known to give the expected mouth-feel in feed formulations [26]. The concave structures are probably formed due to the shrinkage caused during the early stage of the drying process [41]. The external surface of the particle showed a wrinkled appearance with some cracks. Such imperfections like wrinkles and cracks are caused during the slow film formation on the tiny droplets during drying. The authors suggested that the cracks may be formed to facilitate the escape of heat transfer inside-out after drying, preventing heat damage to the entrapped organism leading to the survival of bacteria. The hollow structure or the void formation may be related to the desorption of gases during the drying process, expansion with the formation of steam within the droplet, or due to the entry of air during atomization. Rajam et al. 2012 also observed the hollow structures in his encapsulation studies of Lactobacillus plantarum with whey protein [25].
5 Conclusions
In the present investigation, we studied the microencapsulation efficacy spray-drying of the probiotic powders containing Enterococcus strains. Using a protein-carbohydrate mixture resulted in better survival of probiotics during spray drying. Strains E. canintestini S18A and E. rivorum S22C revealed unusual probiotic characteristics suggesting their potential probiotic use. All microencapsulated probiotics had acceptable physicochemical properties like color, low moisture content, glass transition temperature, and hygroscopicity. It is also observed that the obtained encapsulates had an excellent thermal stability. Studies on storage at room and refrigerated temperature conditions showed that all encapsulated probiotics had excellent storage stability. The acid and bile tolerance studies revealed the viability efficacy of the strains. The promising results ensure that by using acceptable techno-functional properties, yield, and shelf life, high-quality probiotic powders can be produced. Future studies will involve the screening and effect of various protein-carbohydrate mixtures as wall materials with varying concentrations for each strain.
Availability of data and materials
Not applicable.
Abbreviations
- MRS:
-
Man-Rogosa-Sharpe
- PBS:
-
Phosphate-buffered saline
- DNA:
-
Deoxyribose nucleic acid
- RNA:
-
Ribose nucleic acid
- PEG:
-
Polyethylene glycol
- PCR:
-
Polymerase chain reaction
- Tris-HCl:
-
Tris (hydroxymethyl) aminomethane (THAM) hydrochloride
- NaCl:
-
Sodium hydrochloride
- BLAST:
-
Basic local alignment search tool
References
FAO & WHO. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Fao Who 2001:1–34. doi:https://doi.org/10.1201/9781420009613.ch16.
McKay L. Applications for biotechnology: present and future improvements in lactic acid bacteria. FEMS Microbiol Lett 1990. doi:https://doi.org/10.1016/0378-1097(90)90694-L.
Kailasapathy K (2002) Microencapsulation of probiotic bacteria: technology and potential applications. Curr Issues Intest Microbiol 3:39
Mani-López E, Palou E, López-Malo A (2014) Probiotic viability and storage stability of yogurts and fermented milks prepared with several mixtures of lactic acid bacteria. J Dairy Sci. https://doi.org/10.3168/jds.2013-7551
Anandharamakrishnan C, Ishwarya SP (2015) Selection of wall material for encapsulation by spray drying. Spray Dry Tech Food Ingred Encapsulation. https://doi.org/10.1002/9781118863985.ch4
Conrad PB, Miller DP, Cielenski PR, De Pablo JJ (2000) Stabilization and preservation of lactobacillus acidophilus in saccharide matrices. Cryobiology. https://doi.org/10.1006/cryo.2000.2260
Selmer-Olsen E, Sørhaug T, Birkeland SE, Pohrson R (1999) Survival of lactobacillus helveticus entrapped in ca-alginate in relation to water content, storage and rehydration. J Ind Microbiol Biotechnol. https://doi.org/10.1038/sj.jim.2900693
Desmond C, Ross RP, O’Callaghan E, Fitzgerald G, Stanton C (2002) Improved survival of lactobacillus paracasei NFBC 338 in spray-dried powders containing gum acacia. J Appl Microbiol. https://doi.org/10.1046/j.1365-2672.2002.01782.x
Crittenden R, Laitila A, Forssell P, Mättö J, Saarela M, Mattila-Sandholm T et al (2001) Adhesion of Bifidobacteria to granular starch and its implications in probiotic technologies. Appl Environ Microbiol. https://doi.org/10.1128/AEM.67.8.3469-3475.2001
Bhagwat A, Annapure US (2019) In vitro assessment of metabolic profile of enterococcus strains of human origin. J Genet Eng Biotechnol 17:11. https://doi.org/10.1186/s43141-019-0009-0
Bhagwat A, Nandanwar YS, Warke R, Annapure US (2019) In vitro assessment of physiological properties of enterococcus strains of human origin for possible probiotic use. Asian J Pharm Clin Res 12
Bhagwat A, Annapure US (2019) Maternal-neonatal transmission of enterococcus strains during delivery. Beni-Suef Univ J Basic Appl Sci 8:25. https://doi.org/10.1186/s43088-019-0029-5
Behboudi-Jobbehdar S, Soukoulis C, Yonekura L, Fisk I (2013) Optimization of spray-drying process conditions for the production of maximally viable microencapsulated L. acidophilus NCIMB 701748. Dry Technol 31:1274–1283. https://doi.org/10.1080/07373937.2013.788509
Park YS, Lee JY, Kim YS, Shin DH (2002) Isolation and characterization of lactic acid bacteria from feces of newborn baby and from dongchimi. J Agric Food Chem. https://doi.org/10.1021/jf011174i
Fritzen-Freire CB, Prudêncio ES, Amboni RDMC, Pinto SS, Negrão-Murakami AN, Murakami FS (2012) Microencapsulation of bifidobacteria by spray drying in the presence of prebiotics. Food Res Int. https://doi.org/10.1016/j.foodres.2011.09.020
Dave RI, Shah NP (1998) Ingredient supplementation effects on viability of probiotic bacteria in yogurt. J Dairy Sci. https://doi.org/10.3168/jds.S0022-0302(98)75839-4
Heidebach T, Först P, Kulozik U (2010) Influence of casein-based microencapsulation on freeze-drying and storage of probiotic cells. J Food Eng. https://doi.org/10.1016/j.jfoodeng.2010.01.003
Maciel GM, Chaves KS, Grosso CRF, Gigante ML (2014) Microencapsulation of lactobacillus acidophilus La-5 by spray-drying using sweet whey and skim milk as encapsulating materials. J Dairy Sci. https://doi.org/10.3168/jds.2013-7463
Lian WC, Hsiao HC, Chou CC (2002) Survival of bifidobacteria after spray-drying. Int J Food Microbiol. https://doi.org/10.1016/S0168-1605(01)00733-4
Corcoran BM, Ross RP, Fitzgerald GF (2004) Stanton C. Comparative survival of probiotic lactobacilli spray-dried in the presence of prebiotic substances:1024–1039. https://doi.org/10.1111/j.1365-2672.2004.02219.x
Avila-Reyes S V., Garcia-Suarez FJ, Jiménez MT, San Martín-Gonzalez MF, Bello-Perez LA. Protection of L. rhamnosus by spray-drying using two prebiotics colloids to enhance the viability. Carbohydr Polym 2014. doi:https://doi.org/10.1016/j.carbpol.2013.11.033.
Saavedra-Leos Z, Leyva-Porras C, Araujo-Díaz SB, Toxqui-Terán A, Borrás-Enríquez AJ (2015) Technological application of maltodextrins according to the degree of polymerization. Molecules. https://doi.org/10.3390/molecules201219746
Udomrati S, Gohtani S (2014) Tapioca maltodextrin fatty acid ester as a potential stabilizer for tween 80-stabilized oil-in-water emulsions. Food Hydrocoll. https://doi.org/10.1016/j.foodhyd.2014.08.015
Doherty SB, Auty MA, Stanton C, Ross RP, Fitzgerald GF, Brodkorb A (2012) Survival of entrapped lactobacillus rhamnosus GG in whey protein micro-beads during simulated ex vivo gastro-intestinal transit. Int Dairy J. https://doi.org/10.1016/j.idairyj.2011.06.009
Rajam R, Karthik P, Parthasarathi S, Joseph GS, Anandharamakrishnan C (2012) Effect of whey protein - alginate wall systems on survival of microencapsulated lactobacillus plantarum in simulated gastrointestinal conditions. J Funct Foods. https://doi.org/10.1016/j.jff.2012.06.006
Duongthingoc D, George P, Katopo L, Gorczyca E, Kasapis S (2013) Effect of whey protein agglomeration on spray dried microcapsules containing saccharomyces boulardii. Food Chem. https://doi.org/10.1016/j.foodchem.2013.04.093
Wei YH, Chen WC (2005) Enhanced production of prodigiosin-like pigment from Serratia marcescens SMΔR by medium improvement and oil-supplementation strategies. J Biosci Bioeng. https://doi.org/10.1263/jbb.99.616
Sheu TY, Rosenberg M (1998) Microstructure of microcapsules consisting of whey proteins and carbohydrates. J Food Sci. https://doi.org/10.1111/j.1365-2621.1998.tb15770.x
Fu N, Chen XD (2011) Towards a maximal cell survival in convective thermal drying processes. Food Res Int. https://doi.org/10.1016/j.foodres.2011.03.053
Corcoran B, Stanton C, Fitzgerald G, Ross R (2008) Life under stress: the probiotic stress response and how it may be manipulated. Curr Pharm Des. https://doi.org/10.2174/138161208784480225
Teixeira P, Castro H, Mohácsi-Farkas C, Kirby R (1997) Identification of sites of injury in lactobacillus bulgaricus during heat stress. J Appl Microbiol. https://doi.org/10.1046/j.1365-2672.1997.00221.x
Tonon RV, Brabet C, Pallet D, Brat P, Hubinger MD (2009) Physicochemical and morphological characterisation of açai (Euterpe oleraceae Mart.) powder produced with different carrier agents. Int J Food Sci Technol. https://doi.org/10.1111/j.1365-2621.2009.02012.x
Phase transitions in foods. 2016. doi:https://doi.org/10.1016/c2012-0-06577-5.
Augustin M, Hippolyte MT. Screening of biosurfactants properties of cell-free supernatants of cultures of Lactobacillus spp. isolated from a local fermented milk (Pendidam) of Ngaoundere (Cameroon). 2012;2:974–85.
Augustin MA, Sanguansri L, Margetts C, Young B. Microencapsulation of food ingredients. Food Aust 2001.
Fang Y, Rogers S, Selomulya C, Chen XD (2012) Functionality of milk protein concentrate: effect of spray drying temperature. Biochem Eng J. https://doi.org/10.1016/j.bej.2011.05.007
Favaro-Trindade CS, Santana AS, Monterrey-Quintero ES, Trindade MA, Netto FM (2010) The use of spray drying technology to reduce bitter taste of casein hydrolysate. Food Hydrocoll. https://doi.org/10.1016/j.foodhyd.2009.10.012
De Castro-Cislaghi FP, Silva CDRE, Fritzen-Freire CB, Lorenz JG, Sant’Anna ES (2012) Bifidobacterium Bb-12 microencapsulated by spray drying with whey: survival under simulated gastrointestinal conditions, tolerance to NaCl, and viability during storage. J Food Eng. https://doi.org/10.1016/j.jfoodeng.2012.06.006
Ding WK, Shah NP (2009) An improved method of microencapsulation of probiotic bacteria for their stability in acidic and bile conditions during storage. J Food Sci. https://doi.org/10.1111/j.1750-3841.2008.01030.x
Kurdi P, Kawanishi K, Mizutani K, Yokota A (2006) Mechanism of growth inhibition by free bile acids in lactobacilli and bifidobacteria. J Bacteriol. https://doi.org/10.1128/JB.188.5.1979-1986.2006
Teixeira MI, Andrade LR, Farina M, Rocha-Leao MHM (2004) Characterization of short chain fatty acid microcapsules produced by spray drying. Mater Sci Eng C. https://doi.org/10.1016/j.msec.2004.08.008
Acknowledgements
We would like to thank the University Grants Commission- Basic Science Research (UGC-BSR), India for providing funds required for the project.
Funding
This work was funded by the University Grants Commission- Basic Science Research (UGC-BSR), India. The funds were used to buy media and chemicals required for the project.
Author information
Authors and Affiliations
Contributions
AB performed the experiments and wrote the manuscript. PB assisted in setting up the experiments. USA supervised the work and edited the manuscript. To this end, all authors have read and approved the final manuscript for publication.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The present study was not subject to any conflict of interest with any individual or group of persons in terms of financial benefits or constraints. Therefore, we hereby declare that we have no knowledge of plights associated with the publication of this research work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bhagwat, A., Bhushette, P. & Annapure, U.S. Spray drying studies of probiotic Enterococcus strains encapsulated with whey protein and maltodextrin. Beni-Suef Univ J Basic Appl Sci 9, 33 (2020). https://doi.org/10.1186/s43088-020-00061-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-020-00061-z