Abstract
Background
The accurate non-invasive diagnosis of spontaneous bacterial peritonitis (SBP) in patients with decompensated liver cirrhosis has not been achieved yet. The aim of the study was to obtain an unmistakable diagnosis of SBP using a new simple serum bioscore, made by combined measurement of procalcitonin (PCT), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP), which we called the PEC index. This cross-sectional analytic study comprised 178 cirrhotic patients with ascites (60 patients with SBP and 118 patients with sterile ascites), after excluding non-SBP infection, during the period from March 2019 until September 2019. In all participants, serum levels of PCT, ESR, and CRP were measured, and PEC index was calculated [PEC index = PCT × (ESR + CRP)].
Results
Patients with SBP (n = 60) had significantly higher serum PEC index than those with sterile ascites (n = 118) (41.0/31.2–93.0 vs. 9.9/5.9–15.0, P < 0.001). PEC index distinguished culture positive cases significantly (P < 0.001). Using receiver operating characteristic (ROC) statistics, the sensitivity and specificity of PCT, at a cutoff value of 0.590 ng/mL, for SBP diagnosis, were 81.67% and 93.33%, respectively (area under the curve [AUC] = 0.879; 95% confidence interval [CI] 0.809–0.948). The sensitivity and specificity of ESR, at a cutoff value of 27.0 mm/hour, were 73.33% and 61.67%, respectively (AUC = 0.679; 95% CI 0.581–0.776). The sensitivity and specificity of CRP, at a cutoff value of 21.0 mg/L, were 93.33% and 51.67%, respectively (AUC = 0.736; 95% CI 0.639–0.833). While, the sensitivity and specificity of PEC index, at a cutoff value of 20, were highest (98.33% and 96.67%, respectively, AUC = 0.977; 95% CI 0.940–0.996).
Conclusion
Serum PEC index makes an accurate noninvasive diagnosis of SBP, after excluding other infections.
Similar content being viewed by others
Background
Spontaneous bacterial peritonitis (SBP) is the commonest life-threatening infection encountered in cirrhotic patients with ascites. It accounts for more than half of all infections [1,2,3].The outpatient prevalence of SBP is 1.5–3.5% and exceeds 10% in hospitalized patients [4, 5].
The immune dysfunction in decompensated cirrhotic patients (DCPs) along with vulnerability of gut mucosa leading to translocation of bacteria and bacterial endotoxins from bowel lumen into ascitic fluid (AF), underlie the pathogenesis of SBP [1, 6,7,8].
SBP precipitates several other complications of cirrhosis, e.g., impairment of hepatic status, hepatic encephalopathy, worsening of coagulopathy, variceal bleeding, renal failure, and even death [9]. In former decades, SBP was associated with > 90% mortality that has been reduced nowadays to ~ 20% with the development of prompt diagnosis and appropriate therapy [9, 10].
Typically, SBP presents with abdominal pain and tenderness associated with fever. However, it may present with other local symptoms and signs of peritonitis as vomiting, and ileus; other manifestations of systemic inflammation as hypothermia, chills, tachycardia, tachypnea, and shock; worsening of liver or kidney functions; or hepatic encephalopathy [11]. SBP may also be asymptomatic in 10% of cases [4].
Though, a positive AF culture for a pathogen is the gold standard for SBP diagnosis, about 60% of cases with clinical manifestations indicative of SBP and increased AF polymorphonuclear leukocytic (PMNL) count have negative cultures. Consequently, an AF PMNL count ≥ 250/μL is considered for SBP diagnosis, regardless of culture results [12,13,14,15]. In considerable number of cases, the absence of typical clinical characteristics of SBP makes its identification difficult [16].Therefore, an early non-invasive diagnosis of SBP in DCPs is sometimes recommended, especially in cases with irrelevant clinical manifestations, those newly admitted to hospital, or those with unexplained shock or deterioration of their liver functions [2, 3, 16].
Several non-invasive methods were tried in many studies for SBP diagnosis, as alternatives to diagnostic paracentesis, with variable accuracies, e.g., clinical scores [17], fecal calprotectin [18], and numerous serum inflammatory cytokines and chemokines such as monocyte chemotactic protein-1, interleukin-10 [19], human neutrophil peptide [20], platelet indices [21], macrophage inflammatory protein-1 beta [22], interferon-γ-induced protein-10, tumor necrosis factor-α, and interleukin-6 [23]. However, none of these methods was accurate enough to replace diagnostic paracentesis.
Procalcitonin (PCT) is a 116-amino acid polypeptide precursor of calcitonin with a molecular weight of 13 KDa produced by extra-thyroidal cells (e.g., monocytes) [24]. It has been proposed in highly cited studies as a potentially valuable serum biomarker to diagnose bacterial infections in general [24,25,26] and SBP in particular [27,28,29,30]. Normally, serum PCT level is undetectable (< 0.01 ng/mL), and it rapidly increases in case of infection [26].
Although the reported average estimate of sensitivity and specificity of serum PCT for SBP diagnosis in different clinical trials were relatively high (83% and 92%, respectively) [27], this performance was insufficient to make an acceptable accurate diagnosis. In order to achieve a more reliable diagnostic accuracy of PCT, we have tried a combined measurement of serum PCT with serum erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) to formulate a novel serum index for SBP diagnosis that we called, the PEC index.
Methods
Study design and sitting
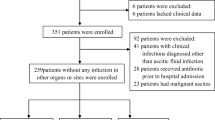
This cross-sectional analytic study was carried out on 178 consecutive hospitalized cirrhotic patients with ascites admitted to internal medicine and tropical medicine departments, in collaboration with the Clinical Pathology Department, Faculty of Medicine, Zagazig University Hospital, Egypt, from March 2019 until September 2019.
Participants
Out of 257 consecutive hospital admissions of potentially eligible cirrhotic patients with ascites, 62 patients who had non-SBP infections were excluded and 7 patients died before the sampling procedures. Eligibility for the study was confirmed in 188 patients; of them, only 178 patients accepted to participate in the study.
Inclusion criteria
-
1.
Adult patients ≥ 18 years
-
2.
All consecutive hospitalized DCPs with moderate to severe ascites (that was detected clinically and confirmed with abdominal ultrasonography) who were admitted for different purposes like clinically suspected SBP, variceal bleeding, and hepatic encephalopathy. Participants were classified into an SBP group (either symptomatic or asymptomatic) (n = 60) and a sterile ascites group (n = 118).
The diagnosis of SBP was confirmed by an AFPMNL count ≥ 250/mm3 with or without a positive ascitic fluid culture for pathogenic bacteria. Absence of both criteria meant the ascites was sterile [14, 15].
Exclusion criteria
-
1.
Patients with a diagnosed infection other than AF infection, e.g., upper and lower respiratory tract infection, urinary tract infection, and otitis media
-
2.
Patients with HCC or associated pancreatic disease, as these conditions could affect the components of PEC index.
-
3.
Patients who received antibiotics 10 days prior to hospital admission.
-
4.
Patients with AF culture positivity and AF PMNL count < 250/mm3 (bacterascites)
-
5.
Patients who refused to be enrolled in the study or refused to sign the consent.
Study tools
All included patients were subjected to thorough clinical assessment, abdominal ultrasound scanning, routine laboratory examination, e.g., complete blood picture, liver function and kidney function tests, coagulation profile and viral markers, and determination of serum levels of ESR (first hour), CRP (by Cobas 8000, Roch, Germany) and PCT, and peritoneal fluid examination.
Serum PCT measurement
This was done by the electrochemiluminescence immunoassay (ECLIA) on Cobas e 411 immunoassay analyzers, Roch, Germany, acting via the sandwich principle. The analyzer automatically calculates the analyte concentration of each sample in ng/ml with a measuring range of 0.02 to 100 ng/ml and the coefficient of variability of 8% [31].
Peritoneal fluid examination technique
Sterile bedside diagnostic paracentesis was done using a 23-G needle attached to a 20 cc syringe after applying local anesthesia. Then, aspirated ascitic fluid was collected into two tubes and analyzed within 2 h of aspiration. The first tube for culture and sensitivity and second tube with ethylene diamine tetraacetic acid were to be analyzed for biochemistry and leukocyte counts.
PEC index
This is a new serum bioscore, innovated in this study, which was calculated by the formula; PEC index = PCT × (ESR + CRP). This formula was chosen on a statistical base, after repeated trials and errors while trying several different formulae.
Statistical analysis
Statistical analysis was performed with SPSS software, version 22.0 (SPSS Inc., Chicago, IL, USA). The continuous variables were expressed as means ± standard deviation (SD) and the categorical variables as count numbers and proportions. The suitable test was used, e.g., Student’s t test, Mann–Whitney U test, Pearson’s chi-squared test, Fisher’s exact test, and Pearson’s correlation test. The result was considered significant if P ≤ 0.05. In order to test the diagnostic accuracy of various markers as well as the novel PEC index for diagnosis of SBP, we used receiver operating characteristic (ROC) statistics and determined sensitivity, specificity, and area under the curve (AUC) for each.
Results
Comparison between SBP group (n = 60) and sterile ascites group (n = 118) regarding demographic, clinical, and laboratory characteristics revealed significantly higher serum PCT, ESR, and CRP, as well as significantly higher AF LDH and PMNL count in SBP group (P < 0.001, for all parameters). Also, PEC index was significantly higher in SBP group than in sterile ascites group (41.0/31.2–93.0 vs. 9.9/5.9–15.0, P < 0.001) (Table 1).
AF bacterial culture positivity was found in 41.67% (25/60) of SBP group, but not in sterile ascites group at all (0/60) (Table 1). The commonest pathogens encountered were Escherichia coli in 68.0% (17/25) and Klebsiella in 16.0% (4/25) of culture positive cases. Positivity of AF culture among patients with SBP was significantly associated with higher levels of serum PCT, PEC index, AF LDH, and AF PMNL count and with lower AF glucose level (P < 0.001, for all) (Table 2).
ROC statistics were used to assess diagnostic accuracy of tested serum markers in SBP diagnosis (Figs. 1 and 2). The sensitivity and specificity of PCT, at a cutoff value of 0.590 ng/mL, were 81.67% and 93.33% (AUC = 0.879; 95% confidence interval [CI] 0.809–0.948). The sensitivity and specificity of ESR, at a cutoff value of 27.0 mm/h, were 73.33% and 61.67% (AUC = 0.679; 95% CI 0.581–0.776). The sensitivity and specificity of CRP, at a cutoff value of 21.0 mg/L, were 93.33% and 51.67% (AUC = 0.736; 95% CI 0.639–0.833) (Fig. 1 and Table 3). Serum PEC index, at a cutoff value of 20, had much higher sensitivity and specificity for SBP diagnosis (98.33% and 96.67%, respectively, AUC = 0.977; 95% CI 0.940–0.996), with an overall diagnostic accuracy of 97.50% (Fig. 2 and Table 3).
Figures 3 and 4 and Table 3 show the diagnostic performances of PEC index and its components in discrimination between cases with culture-negative SBP and those with sterile ascites. PCT, at a cutoff value of 0.305 ng/mL, was associated with a better diagnostic accuracy (AUC = 0.825; 95% CI 0.742–0.907) than that of ESR, at a cutoff value of 27 mm/h (AUC = 0.649; 95% CI 0.521–0.776) and that of CRP at a cutoff value of 23.5 mg/L (AUC = 0.704; 95% CI 0.613–0.794) (Fig. 3). PEC index, at a cutoff value of 20, was associated with the best diagnostic performance (AUC = 0.970; 95% CI 0.934–1.0) (Fig. 4).
Discussion
The search for a non-invasive and readily available biomarker for unmistakable diagnosis of SBP in cirrhotic patients with ascites, is still under clinical trials [22, 23, 29, 30, 32, 33]. The current work, to the best of our knowledge, is the first to characterize the use of indexed combined measurement of serum PCT, ESR, and CRP for this purpose, after exclusion of non-SBP bacterial infections.
In this study, all SBP patients (n = 60) had AF inflammatory response due to bacterial infection as was identified by AF PMNL count ≥ 250/HPF. Other biochemical findings in AF analysis confirmed the presence of infection like high LDH and low glucose. These findings were in accordance with that of Badawy et al. [34] and EL-Motasem et al. [35].
In addition, serum levels of acute phase reactants; ESR, CRP, and PCT in the SBP group of our patients (31/23–36 mm/h, 27/24–29 mg/L, and 0.691/0.604–1.690 ng/mL, respectively) were significantly higher than in sterile ascites group (25/19–30 mm/h, 20/11–25 mg/L, and 0.259/0.159–0.350 ng/mL, respectively) (P < 0.001, for all). Likewise, Viallon et al. [36], Such et al. [37], Papp et al. [38], and Wu et al. [39] described elevations of inflammatory mediators in the setting of SBP. This could be explained by activation of cytokine synthesis and innate immunity in response to circulating bacterial endotoxins [40].
As a pro-inflammatory cytokine, PCT is elevated in acute bacterial infections and reaches the highest serum level in severe infections and sepsis as it is enhanced by systemic inflammatory response. It is not elevated by viral infection or autoimmune inflammation [24,25,26]. Serum PCT measurement has an established role in differentiation between bacterial infections and other inflammatory conditions [25, 41]; however, its use as a diagnostic biomarker for SBP has been reported frequently in the last decade, with conflicting results [22, 27,28,29,30, 38, 39].
In this work, with the exception that CRP was more sensitive than PCT for SBP diagnosis (93.33% versus 81.67%, respectively), the sensitivity and specificity of serum PCT at a cutoff value of 0.590 ng/mL for SBP diagnosis (81.67% and 93.33%, respectively, AUC: 0.879) were relatively higher than that of ESR at a cutoff value of 27.0 mm/h (73.33% and 61.67%, respectively, AUC 0.679) and that of CRP at a cutoff value > 21.0 mg/L (93.33% and 51.67%, respectively; AUC 0.736). PCT appears to be superior in detecting septic conditions to other pro-inflammatory markers like ESR and CRP, because of its earlier increase upon infection and its better specificity [36, 38, 39, 41].
A previous meta-analysis by Yang et al. [27] including18 studies on 1827 DCPs, published between 2000 and 2014, investigated the diagnostic performance of PCT as a marker of SBP. They reported a relatively good diagnostic performance with a summary estimate of sensitivity and specificity of 83.0% and 92.0%, respectively, which came very close to our results. On the other hand, Lesińska et al. [22] reported that serum PCT could not distinguish patients with and without SBP. The small sample size (n = 32) of their study [22] could explain this contradictory result.
Positive results of AF bacterial cultures, in this study, were found only in 41.6% (25/60) of SBP patients. Nearly the same result was frequently reported before [12, 14, 15]. The predominance of gram-negative over gram-positive bacteria among culture positive cases, in our study, goes with other studies [1, 42]. In the current work, serum PCT, opposite to ESR and CRP, was significantly elevated in culture positive cases of SBP (P < 0.001). This comes in accordance with the study of Cekin et al. [43], but it does not come in agreement with the studies of EL-Gendy et al. [44] or Cai et al. [28] who reported the lack of sensitivity of serum PCT to differentiate the culture results in SBP patients.
In this research, the newly advocated PEC index [calculated as PCT × (ESR + CRP)] was significantly higher in SBP group than in sterile ascites group (P < 0.001), and it was significantly more elevated in culture-positive than in culture-negative cases of SBP (P < 0.001).
In the present study, at a cutoff value of 20, the sensitivity and specificity of serum PEC index for SBP diagnosis (98.33% and 96.67%, respectively, AUC 0.977) were much higher than that of serum PCT, ESR, and CRP. Likewise, the sensitivity and specificity of serum PEC index, at a cutoff value of 20, to distinguish cases with culture-negative SBP from sterile ascites cases (97.1% and 96.6%, respectively, AUC 0.970), were much better than that of PEC components. The superiority of PEC index over any of its components, for diagnosis of SBP and even of its culture-negative subtype, could be explained simply by the mathematical compensatory effect of combining several markers with different degrees of sensitivity and specificity, helping escaping false positive and false negative results.
Furthermore, the above mentioned diagnostic accuracy of PEC index in SBP is better than that of other promising serum markers that were reported in the last few years [32, 33]. Although serum homocysteine had relatively high sensitivity and specificity for distinguishing SBP (95.1% and 89.3%, respectively; AUC 0.932) [32], it is less accurate than PEC index. A d-dimer cutoff value of 1500 ng/mL was determined optimal for ruling out SBP due to high sensitivity (96.8%); however, this marker was not useful for confirming SBP due to low specificity (40.6%) [33].
Similar to our study design, Wang et al. [45] have conducted a recently published study on 259 consecutive cirrhotic patients with ascites admitted to a Chinese military hospital to investigate the efficacy in SBP diagnosis of combined measurement of PCT, mean fluorescence intensity of mature neutrophils (sNFI) and difference in hemoglobin concentration between newly formed and mature red blood cells (dCHC). Of these, 51/259 (19.7%) had culture-positive SBP, 58/259 (22.4%) had culture-negative SBP, and 150/259 (57.9%) had sterile ascites. The total bioscore of those three markers used by Wang et al. [45], at a cutoff value of ≥3.40, had a sensitivity of 92.6%, a specificity of 95.3% and an AUC of 0.937 (95% CI 0.901–0.994, P < 0.001), which are less than that of PEC index innovated in our study.
Some limitations to our study are to be mentioned. First is the relatively small sample size. Second, we did not test for serum markers other than PEC index components to help making head to head comparison. Finally, it would have been more appropriate if we added one more group of DCPs with infections other than SBP to assess specificity of PEC index for SBP.
Conclusion
The novel serum PEC index seems sufficient to make a fairly accurate non-invasive diagnosis of SBP in cirrhotic patients with ascites, provided that non-SBP bacterial infections are excluded.
Recommendations
To validate the clinical use of serum PEC index for SBP diagnosis, further larger trials are needed that should involve assessment of PEC index in non-SBP infections and its comparison with other serum biomarkers.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to confidential and institutional ethical issues but are available from the corresponding author on reasonable request.
Abbreviations
- AF:
-
Ascitic fluid
- AUC:
-
Area under the curve
- CRP:
-
C-reactive protein
- DCPs:
-
Decompensated cirrhotic patients
- ESR:
-
Erythrocyte sedimentation rate
- PCT:
-
Procalcitonin
- PEC index:
-
Index of combined measurement of PCT, ESR and PCT
- PMNL:
-
Polymorphonuclear leukocytes
- ROC:
-
Receiver operating characteristic
- SBP:
-
Spontaneous bacterial peritonitis
References
Wiest R, Krag A, Gerbes A (2012) Spontaneous bacterial peritonitis: recent guidelines and beyond. Gut 61:297–310
Acharya SK (2013) Difficult to treat spontaneous bacterial peritonitis. Trop Gastroenterol 34:7–13
Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P et al (2014) Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol 60:1310–1324
Evans LT, Kim WR, Poterucha JJ, Kamath PS (2003) Spontaneous bacterial peritonitis in asymptomatic outpatients with cirrhotic ascites. Hepatology 37:897–901
Nousbaum JB, Cadranel JF, Nahon P, Nguyen Khac E, Moreau R, Thévenot T et al (2007) Diagnostic accuracy of the Multistix 8 SG reagent strip in diagnosis of spontaneous bacterial peritonitis. Hepatology 45:1275–1281
Kalaitzakis E, Johansson J (2006) Intestinal permeability in cirrhotic patients with and without ascites. Scand J Gastroenterol 4l:326–330
Gines P, Fernandez J, Durand F, Saliba F (2012) Management of critically-ill cirrhotic patients. J Hepatol 56(Suppl 1):S13–S24
Wiest R, Lawson M, Geuking M (2014) Pathological bacterial translocation in liver cirrhosis. J Hepatol 60:197–209
Tandon P, Garcia-Tsao G (2008) Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis 28:26–42
Garcia-Tsao G (2001) Current management of the complications of cirrhosis and portal hypertension: variceal hemorrhage, ascites, and spontaneous bacterial peritonitis. Gastroenterology 120:726–748
Piddock LJ, Planas R, Bernard B, Inadomi JM (2000) Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International ascites club. J Hepatol 32(1):142–153
Fernandez J, Navasa M (2002) Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology 35:140–148
Wong CL, Holroyd-Leduc J (2008) Does this patient have bacterial peritonitis or portal hypertension? How do I perform a paracentesis and analyze the results? JAMA 299:1166–1178
European Association for the Study of the Liver (2010) EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol 53:397–417
Runyon BA, AASLD (2013) Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology 57:1651–1653
Attar BM, George M (2014) Disease dependent qualitative and quantitative differences in the inflammatory response to ascites occurring in cirrhotics. World J Hepatol 6:85–91
Wehmeyer MH, Krohm S, Kastein F, Lohse AW, Lüth S (2014) Prediction of spontaneous bacterial peritonitis in cirrhotic ascites by a simple scoring system. Scand J Gastroenterol 49:595–603
Gundling F, Schmidtler F, Hapfelmeier A, Schulte B, Schmidt T, Pehl C et al (2011) Fecal calprotectin is a useful screening parameter for hepatic encephalopathy and spontaneous bacterial peritonitis in cirrhosis. Liver Int 31:1406–1415
Kim JK, Chon CY, Kim JH, Kim YJ, Cho JH, Bang SM et al (2007) Changes in serum and ascitic monocyte chemotactic protein-1 (MCP-1) and IL-10 levels in cirrhotic patients with spontaneous bacterial peritonitis. J Interf Cytokine Res 27:227–230
Gong Y, Cui S, Li L, Wang M, Guo N (2013) Diagnostic value of human neutrophil peptide in spontaneous bacterial peritonitis. Chin J Hepatol 21:944–948 [abstract]
Abdel-Razik A, Eldars W, Rizk E (2014) Platelet indices and inflammatory markers as diagnostic predictors for ascitic fluid infection. Eur J Gastroenterol Hepatol 26:1342–1347
Lesińska M, Hartleb M, Gutkowski K, Nowakowska-Duława E (2014) Procalcitonin and macrophage inflammatory protein-1 beta (MIP-1β) in serum and peritoneal fluid of patients with decompensated cirrhosis and spontaneous bacterial peritonitis. Adv Med Sci 59:52–56
Abdel-Razik A, Mousa N, Elbaz S, Eissa M, Elhelaly R, Eldars W (2015) Diagnostic utility of interferon gamma-induced protein 10 kDa in spontaneous bacterial peritonitis: single-center study. Eur J Gastroenterol Hepatol 27:1087–1093
Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret GY (2006) Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: a systematic review and meta-analysis. Crit Care Med 34(7):1996–2003
O’Grady NP, Barie PS, Bartlett JG, Bleck T, Carroll K, Kalil AC et al (2008) Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med 36(4):1330–1349
Wacker C, Prkno A, Brunkhorst FM, Schlattmann P (2013) Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis 13(5):426–435
Yang SK, Xiao L, Zhang H, Xu XX, Song PA, Liu FY, Sun L (2014) Significance of serum procalcitonin as biomarker for detection of bacterial peritonitis: a systematic review and meta-analysis. BMC Infect Dis 14:452
Cai ZH, Fan CL, Zheng JF, Zhang X, Zhao WM, Li B et al (2015) Measurement of serum procalcitonin levels for the early diagnosis of spontaneous bacterial peritonitis in patients with decompensated liver cirrhosis. BMC Infect Dis 15:55
Yang Y, Li L, Qu C, Zeng B, Liang S, Luo Z, Wang X, Zhong C (2015) Diagnostic accuracy of serum procalcitonin for spontaneous bacterial peritonitis due to end-stage liver disease: a meta-analysis. Medicine (Baltimore) 94(49):e2077
Abdel-Razik A, Mousa N, Elhammady D, Elhelaly R, Elzehery R, Elbaz S et al (2016) Ascitic fluid calprotectin and serum procalcitonin as accurate diagnostic markers for spontaneous bacterial peritonitis. Gut Liver 10(4):624–631
Kim SU, Kim DY, Lee CK, Park JY, Kim SH, Kim HM et al (2010) Ascitic fluid infection in patients with hepatitis B virus-related liver cirrhosis: culture-negative neutrocytic ascites versus spontaneous bacterial peritonitis. J Gastroenterol Hepatol 25(1):122–128
Abdel-Razik A, Eldars W, Elhelaly R, Eldeeb AA, Abdelsalam M, El-Wakeel N, Aboulmagd A (2018) Homocysteine a new diagnostic marker in spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol 30(7):779–785
Mikuła T, Sapuła M, Jabłońska J, Kozłowska J, Stańczak W, Krankowska D, Wiercińska-Drapało A (2018) Significance of heparin-binding protein and d-dimers in the early diagnosis of spontaneous bacterial peritonitis. Mediat Inflamm 2018:2018
Badawy AA, Zaher TI, Sharaf SM, Emara MH, Shaheen NE, Aly TF (2013) Effect of alternative antibiotics in treatment of cefotaxime resistant spontaneous bacterial peritonitis. World J Gastroenterol 19(8):1271
El Motasem EM, Heikal AA, El Haddad HE, Hamdy A, Samie RM, El Din HS (2015) Value of different diagnostic markers in spontaneous bacterial peritonitis in HCV Egyptian cirrhotic patients. Open J Gastroenterol 5(09):119
Viallon A, Zeni F, Pouzet V, Lambert C, Quenet S, Aubert G et al (2000) Serum and ascitic procalcitonin levels in cirrhotic patients with spontaneous bacterial peritonitis: diagnostic value and relationship to pro-inflammatory cytokines. Intensive Care Med 26(8):1082–1088
Such J, Hillebrand DJ, Guarner C, Berk L, Zapater P, Westengard J et al (2001) Tumor necrosis factor-α, interleukin-6, and nitric oxide in sterile ascitic fluid and serum from patients with cirrhosis who subsequently develop ascitic fluid infection. Digest Dis Sci 46(11):2360–2366
Papp M, Vitalis Z, Altorjay I, Tornai I, Udvardy M, Harsfalvi J et al (2012) Acute phase proteins in the diagnosis and prediction of cirrhosis associated bacterial infections. Liver Int 32(4):603–611
Wu H, Chen L, Sun Y, Meng C, Hou W (2016) The role of serum procalcitonin and C-reactive protein levels in predicting spontaneous bacterial peritonitis in patients with advanced liver cirrhosis. Pak J Med Sci 32(6):1484–1488
Deschénes M, Villeneuve JP (1999) Risk factors for the development of bacterial infections in hospitalized patients with cirrhosis. Am J Gastroenterol 94:2193–2197
Luzzani A, Polati E, Dorizzi R, Rungatscher A, Pavan R, Merlini A (2003) Comparison of procalcitonin and C-reactive protein as markers of sepsis. Crit Care Med 31(6):1737–1741
Lata J, Stiburek O, Kopacova M (2009) Spontaneous bacterial peritonitis: a severe complication of liver cirrhosis. World J Gastroenterol 15(44):5505
Cekin Y, Cekin AH, Duman A, Yilmaz U, Yesil B, Yolcular BO (2013) The role of serum procalcitonin levels in predicting ascitic fluid infection in hospitalized cirrhotic and non-cirrhotic patients. Int J Med Sci 10(10):1367–1374
El-Gendy NA, Tawfeek NA, Saleh RA, Radwan EE, Ahmad EE, Mohammed RA (2014) Diagnosis of spontaneous bacterial peritonitis. Egypt J Intern Med 26(2):53
Wang H, Li Y, Zhang F, Yang N, Xie N, Mao Y, Li B (2018) Combination of PCT, sNFI and dCHC for the diagnosis of ascites infection in cirrhotic patients. BMC Infect Dis 18(1):389
Acknowledgments
Great thanks to all patients who accepted enrollment in the study as well as to all nursing staff and technicians who helped accomplishing this work.
Funding
This research did not receive any specific grant or any other financial support from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
HE had selected the idea and design of the work, had contributed in the data collection and interpretation, shared in drafting the work, and had made the final revision of data. SE had contributed in the data collection and interpretation and shared in drafting the work. AM had helped in the data collection and analysis and had performed the laboratory work. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the ethical principles of the 1975 Declaration of Helsinki, and it was reviewed and approved by Research Ethics Committee of Faculty of Medicine, Zagazig University Institutional Review Board, #5644, in 9 March 2019. A written informed consent was obtained from all the participants after explaining the aim and concerns of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Elsadek, H.M., Elhawari, S.A. & Mokhtar, A. A novel serum index for accurate diagnosis of spontaneous bacterial peritonitis in cirrhotic patients without other infections. Egypt Liver Journal 10, 10 (2020). https://doi.org/10.1186/s43066-020-0021-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43066-020-0021-8