Abstract
Background
Transarterial chemoembolization (TACE) is the recommended treatment modality for non-resectable intermediate-stage hepatocellular carcinoma; however, this stage has wide variable levels of liver functions and tumor burden making a challenge as regards the tolerability of the functioning residual liver to this type of therapy. Further stratification of this intermediate stage may help the clinical decision for the suitability of TACE, so we tried to derive a clinical decision support tool to predict the tolerability of the functioning residual liver to TACE using pre-intervention liver reserve function and liver residual volume as well as tumor burden.
Results
Two hundred and fifty-two patients with non-resectable intermediate-stage hepatocellular carcinoma (HCC) were consecutively included in this cohort study; all patients were blindly followed up for 6 weeks after TACE for manifestation of liver decompensation. For univariate logistic regression analysis, the significant predictors were age, s.albumin, s.bilirubin, prothrombin concentration (PC), tumor volume (TV), residual liver volume (RLV), and residual to liver volumetric ratio (RLVR) (sig, 0.007, 0.000, 0.000, 0.000, 0.000, 0.002, and 0.000, respectively). Multivariate logistic regression analysis illustrated that s.albumin, s.bilirubin, PC, and RLVR were the most independent significant predictors (sig, 0.000 for all with adjusted OR 0.002, 22.692, 0.827, and 0.000, respectively). The discriminatory performance of our proposed decision support score (liver tolerability score) was evaluated using the receiver operating characteristics that identified two cutoff points (≤ 0.30 and ≥ 0.83) to rule out or rule in the possibility of liver decompensation after TACE, respectively (AUC, 0.942 and sig. 0.000). Liver tolerability score stratified the intermediate stage of HCC to 3 risk grades (low, medium, and high) with significant difference as regards hazard distribution.
Conclusion
Computed tomography image-based assessment of RLVR may have a possible role in predicting future tolerability of the functioning residual tissue to TACE of HCC. Liver tolerability score (LTS) could stratify the intermediate stage HCC to 3 risk grades, so LTS may have a role in clinical decision for the suitability of TACE for intermediate stage HCC.
Similar content being viewed by others
Background
Transarterial chemoembolization (TACE) is the current standard of care for patients with intermediate-stage hepatocellular carcinoma (HCC) and relatively preserved liver function [1,2,3].
Despite the widespread implementation of surveillance programs of at-risk populations, most patients with HCC are diagnosed late when curative treatments cannot be applied. In addition, in a high proportion of cases, the disease recurs after attempts at curative therapy [4].
The rationale for TACE is based on the arterial neo-angiogenesis during HCC progression, which is leading to the tendency of HCC to have entirely arterial feeding unlike the surrounding non-tumorous liver parenchyma which receives dual portal and arterial blood supply. TACE is performed through the trans-catheter infusion of cytotoxic agent through the feeding arterial blood supply of HCC which is followed by injection of an embolized material leading to strong cytotoxic and ischemic effect of the tumor sparing the surrounding non-tumorous parenchyma [5].
However, TACE is a safe procedure in patients with intermediate stage HCC; it may increase both the morbidity and mortality in those patients with the majority of complications being reversible increase in liver transaminases and s.bilirubin. Irreversible hepatic decompensation may occur in small proportions of HCC patients after TACE; this serious complication is frequently accompanied by worsening of patient prognosis or survival [6,7,8,9,10,11,12].
The intermediate stage of HCC includes a wide range of patients according to their baseline liver function and tumor burden leading to a considerable variation of clinical benefit and triggering the need to sub-stratification strategy to this intermediate stage to tailor the therapeutic procedure based on the residual liver functions and tumor burden [13,14,15,16]. The aim of our study is to develop a clinical decision support score to predict the tolerance of the liver to maintain its function after TACE using pre-intervention liver reserve function and liver residual volume as well as tumor burden.
Methods
Ethical consideration
The present study was conducted in accordance with the Declaration of Helsinki and the ethics committee of our institute; all patients provided written informed consent. The results of the research were used only in scientific purposes and not in any other aims.
Study design
This study was a prospective cohort study. This prognostic study was conducted according to The TRIPOD (Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis) Statement [17].
The eligible population
This study was performed at the multidisciplinary HCC unit at our institute (tertiary hospital), in the period from January 2017 to June 2019 on a total of 397 cirrhotic patients complicated with non-resectable intermediate stage HCC who were planned to be treated by conventional TACE.
Exclusion and inclusion criteria
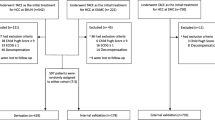
All included HCC cirrhotic patients had fulfilled the clinical practice guideline needed for conventional TACE; all patients were either Child-Pugh A or early Child-Pugh B, no portal vein thrombosis, no extrahepatic metastasis, and no bleeding tendency; all patients had no any other TACE procedures or any other treatment modalities for HCC before and up to 6 weeks after the index TACE procedure. One hundred and forty patients were excluded before recruitment; 105 patients were excluded as they had previous locoregional procedures for HCC including TACE with or without sorafenib before the index TACE, 30 patients were excluded as the index TACE were combined or followed by other treatment modality or sorafenib within 6 weeks follow-up period after the index TACE procedure, and 5 patients refused follow-up in our unit. So only 252 cirrhotic patients fulfilled the inclusion criteria in this study as illustrated in the flow diagram (Fig. 1).
Liver volumetry
-
1.
CT was performed with 320-row multidetector CT scanner (Aquilion One, Toshiba Medical Systems, Otawara, Japan) in radiodiagnosis and medical imaging department in our institute; patient laboratory data was initially revised with particular interest in the results of the renal function tests (creatinine level and clearance).
-
2.
An 18–20-gage cannula was placed into a superficial vein within the antecubital fossa or dorsum of the hand. Before the contrast material was administrated by the injector, saline injection was manually administrated at a high rate of flow, with the patient’s arms in the scanning position. This was done to ensure the successful cannulation of the vein.
-
3.
Image parameters were as follows:
-
(a)
Nominal section thickness, according to the phase
-
(b)
Rotation time, 0.5 s
-
(c)
Helical pitch, 65
-
(d)
KVp, 120
-
(a)
-
4.
The timing of injection is determined by computer-automated scanner technology (bolus tracking) with the region of interest is position just above the celiac axis and the threshold of contrast concentration is fixed 120–150 HU.
-
5.
Triphasic CT was performed following target injection of double his weight (2 ml/Kg) at a flow rate 5 ml/s. The contrast medium used was low osmolar nonionic contrast medium (Ultravist 370).
-
6.
Arterial dominant phase images were acquired 20 s after injection, portal dominant phase images at 55 s after injection, venous dominant phase image at 70 s after injection, and delayed phase images were acquired at 3 min after injection.
Image processing
-
The volume was calculated using (Vitrea Fx, Vital Images, USA) workstation.
-
Total liver volume was measured on the axial venous phase image set after we had traced, by hand, the contours of the entire liver, we calculate the whole liver volume including the caudate lobe excluding the IVC and gallbladder by manual tracing of the whole liver in each axial cut or every 3–4 cuts presuming that the liver is maintaining the same configuration. Then, after finishing the whole liver, we apply all the manually traced cuts to volume calculation software on the workstation to calculate the volume in cubic centimeters.
-
We then calculate the volume of the hepatic focal lesion on the arterial or venous phase by manual tracing of the focal lesion Then, after finishing the focal lesion, we apply all the manually traced cuts to volume calculation software on the workstation to calculate the volume in cubic centimeters as shown in Figs. 2 and 3.
-
Then, the residual liver volume was calculated by subtracting the focal lesion volume from the total liver volume (Total liver volume − the volume of the focal lesion).
-
The equation used for RLVL is (Residual liver volume/total liver volume).
Female patient aged 65 years old, with left hepatic lobe focal lesion(HCC), triphasic CT examination axial arterial (a) and delayed (b) phase showing large left hepatic lobe focal lesion segment IV and III, showing faint peripheral enhancement in arterial (a) and venous phase (not shown) with washout in delayed (b) phase. c–e CT volumetry of whole live (volume = 3002.84 ml). f CT volumetry of the focal lesion (volume = 364.07 ml).
Male patient aged 54 years old, with two right hepatic lobe focal lesions (HCC), triphasic CT examination axial arterial (a, b) and delayed (c) phase showing two right hepatic lobe focal lesions segment VIII, showing enhancement in arterial (a, b) with washout in delayed (c) phase. d–f CT volumetry of whole live (volume = 2075.05 ml). g, h CT volumetry of the focal lesions (volume = 133.69 and 39.26 ml)
TACE procedure
All TACE procedures were performed in angiography suite by well-trained and experienced interventional radiologists. After infiltration of local anesthesia, right femoral artery puncture was done by Seldinger technique to gain access to the common femoral artery; then, a 6-F vascular sheath was placed into common femoral artery, the 5F cobra head catheter was advanced over 0.035-in. guide wire under fluoroscopic guidance. Angiographic survey of the celiac and superior mesenteric artery was performed and selective catheterization of the hepatic artery was done; then, catheter advanced into the desired hepatic artery branch feeding the tumor which marked and identified by the arterial blush. Mixture of 10 cm lipiodol, doxorubicin hydrochloride (25 mg), and water-soluble contrast agent (omnipaque 350) was slowly injected through the catheter under fluoroscopic guidance. The end point of the arterial embolization was disappearance of the tumor blush at hepatic angiography performed immediately after chemoembolization.
Assessment of post TACE liver decompensation
In our study, liver decompensation was defined by addition of one or more points in the Child-Pugh score of treated patients within 6 weeks after index TACE.
Data collection
Preoperatively, each patient was evaluated to measure total liver volume (TLV), residual liver volume(RLV), and total tumor volume (TTV) using CT liver volumetry; we calculated residual to total liver volumetric ratio (RLVR), at the same time; pre-interventional liver reserve function were assessed using serum albumin, serum bilirubin, and prothrombin concentration as well as ALT and AST.
Post-operatively, each patient was followed up every 2 weeks for up to 6 weeks after index TACE procedure to trace for manifestations of liver decompensation. The research team who is responsible for post-operative data collection was blinded to the pre-operative patients’ data. TACE was done by two expert radiologists who had experience in interventional radiology 10 years. CT volumetry was done with expert radiologist who had an experience of 8 years.
Statistical analysis of the data
All collected data were summarized and statistically analyzed using the IBM SPSS, version 23 statistic software (IBM, NY, USA). For quantitative data, the median and minimum to maximum were calculated (all our variables were abnormally distributed). Qualitative data were reported as frequency and percentage.
Predictor pre-selection was based on previous well-conducted reports, clinical reasoning and statistical significant univariate logistic regression analysis. Our sample size was calculated empirically using at least 10 events per predictor.
Logistic regression analysis was performed to find the best prognostic predictors of liver decompensation after TACE. Candidate predictors considered in this analysis were patients’ demographic data (age and sex), pre-intervention liver reserve function tests (s.albumin, s.bilirubin, prothrombin concentration, ALT, and AST), and liver volumetric measures (liver volume, residual liver volume, tumor volume, and residual to liver volumetric ratio) as well as platelet count, spleen diameter, and AFP. Univariate analysis was done first for each predictor, and then the best predictors were evaluated using the multivariable logistic regression analysis by entering all significant predictors simultaneously with a stepwise backward strategy.
The predicted probability of the proposed model was derived using the regression coefficient from the multivariable logistic regression equation to build up a regression equation that is used in development of the prognostic model. Receiver operating characteristic (ROC) curves were calculated for the new prognostic model and area under the ROC curve (AUC) was computed. Our patients were divided according to the new model into risk 3 groups, low, medium, and high risk, by using cutoff points along the scale of the predicted probability of the new model; the 1st cutoff point was selected to rule in the outcome with the highest specificity and highest LR+, the 2nd cutoff point was selected to rule out the outcome with the highest sensitivity and lowest LR-, and then the hazard distribution in between different grades was analyzed using Kaplan-Meier method and log rank test. The bootstrap method was used for internal validation of our results. P values less than 0.05 were considered statistically significant.
Results
Main participants’ criteria
Table 1 of our results shows the main participants’ demographic criteria (age and sex), the pre-interventional reserve liver functions (ALT, AST, s.albumin, s.bilirubin, and prothrombin concentration (PC)), the pre-interventional liver volumetric measures (LV, TV, RLV, and RLVR) as well as other evaluated clinical and laboratory parameters (spleen diameter, AFP, s.creatinin, platelets, and Child-Pugh score). The minimum and maximum follow-up time after the index TACE procedure was 1 and 6 weeks, respectively. As regards the occurrence of worsening liver functions after the index TACE, we illustrated in Table 1 that liver decompensation occurred in 82/252 (32.5%) of our patients, while it did not occur in 170/252 (67.5%) of our patients, 5 of our patients were dropped out during follow-up with missed outcome data and excluded from analysis.
Pre-selection of proposed prognostic predictors
All the proposed predictors were evaluated separately using the univariate logistic regression analysis; the statistically significant predictors were age, s.albumin, s.bilirubin, PC, TV, RLV, RLVR, and Child-Pugh score (sig, 0.007, 0.000, 0.000, 0.000, 0.000, 0.002, and 0.000, respectively; OR, 1.055, 0.002, 22.69, 0.847, 1.002, 0.999, 0.000, and 4.646, respectively) (Table 2).
In Table 3, all the previous significant predictors were analyzed simultaneously using the multivariate logistic regression analysis with backward selection strategy to illustrate the most independent significant predictors which were s.albumin, s.bilirubin, PC, and RLVR (sig, 0.000 for all with adjusted OR 0.002, 22.69, 0.827, and 0.000, respectively).
In Table 4, the receiver operating characteristics of the independent significant predictors were evaluated, and two cutoff points were identified; the 1st cutoff point was selected to rule out the possibility of occurrence of liver decompensation after TACE and the 2nd cutoff point was selected to rule in the possibility of occurrence of liver decompensation after TACE (rule out values, ≥ 0.9, ≥ 3.5 gm/dl, ≤ 1.1 mg/dl, and ≥ 85% and rule in values ≤ 0.65, ≤ 3 gm/dl, ≥ mg/dl 1.8, and ≤ 65% for RLVR, s.albumin, s.bilirubin, and PC, respectively).
Prognostic model development
We used the multivariate logistic regression coefficient values for the identified significant predictors to build up a prognostic model that is based on pre-intervention reserve liver function (s.albumin, s.bilirubin, and PC) and RLVR, using the following equation of the predicted probability: exp(42.8 − 8.15 × s.albumin + 5.8 × s.bilirubin − 0.19 × PC − 14.8 × RLVR)/(1 + exp(42.8 − 8.15 × s.albumin + 5.8 × s.bilirubin − 0.19 × PC − 14.8 × RLVR), where s.albumin was in gm/dl, s.bilirubin was in mg/dl, and PC was in percent. We could name this prognostic model as liver tolerability (LT) score as it predict if the liver could tolerate TACE procedure without decompensation or not (Table 2).
Assessment the performance of the proposed prognostic model
The discriminatory performance of the proposed LT score was evaluated using the receiver operating characteristics that identified two cutoff points (≤ 0.30 and ≥ 0.83); the 1st cutoff point was selected to rule out the possibility of occurrence of liver decompensation after TACE for values below it with its sensitivity, specificity, LR+, and LR- were 99%, 50%, 1.95, and 0.01, respectively, and the 2nd cutoff point was selected to rule in the possibility of occurrence of liver decompensation after TACE for values above it with its sensitivity, specificity, LR+, and LR- were 50%, 99.4%, 178, and 0.15, respectively (AUC 0.942 and sig 0.000). We stratified the LT score to 3 grades (LT grades) using the previous two cutoff points for risk stratification: values ≤ 0.30 to identify LT grade 1 with mild risk, values ≥ 0.83 to identify LT grade 3 with marked risk, LT grade 2 with moderate risk for remaining values (Table 5 and Fig. 4).
The pairwise comparisons between hazard function of the different risk grades of LT score was illustrated using the Kaplan-Meier curve and analyzed using log rank test as identified in Table 6 and Fig. 5.
Discussion
It is well known that TACE is considered the most ideal treatment modality for patients with unresectable intermediate-stage HCC in different international guidelines [1,2,3]; its rationale is based on the arterial neo-angiogenesis during HCC progression that is leading to HCC tendency to be supplied entirely by arterial feeding unlike the surrounding non-tumorous liver parenchyma which receives dual portal and arterial blood supply. TACE is a safe procedure in patients with intermediate stage HCC; however, irreversible hepatic decompensation may occur in a proportion of HCC patients after the procedure; this serious complication is frequently accompanied by worsening of patient prognosis or survival [6,7,8,9,10,11,12].
The intermediate stage of HCC includes a wide range of patients according to their baseline liver function and tumor burden leading to a considerable variation as regards the tolerability of the functioning residual liver to this type of therapy triggering the need of sub-stratification strategy to this intermediate stage; this may help the clinical decision for the suitability of TACE according to patient baseline pre-interventional liver function and tumor burden [13,14,15,16]. In this respect, we tried in this study to evaluate the possible role of volumetry of the functioning non-tumorous residual liver tissue in predicting liver decompensation after TACE and at the same time we tried to develop a clinical decision support score to predict the tolerance of the liver to maintain its function after TACE using pre-intervention liver reserve function and liver residual volume as well as tumor burden.
As regards pre-interventional proposed prognostic predictors for liver decompensation after TACE, our results revealed that serum albumin, serum bilirubin, and prothrombin concentration were the most independent significant predictors for liver decompensation after TACE with good discriminatory performances (AUC, 0.803, 0.683, and 0.721 respectively; sig, 0.000 for all). These results were supported by many studies done by Kohla et al., Miksad et al., Oliveri et al., Hansmann et al., Khalid et al., Lee et al., Hsin et al., and Kadalayil et al. [18,19,20,21,22,23,24,25] which evaluated the different pre-interventional parameters of liver functions and tumor burden and they concluded that irreversible liver decompensation could be increased by poor hepatic reserve.
As regards the possible role of volumetry of functioning non-tumorous residual liver tissue, the results of our study showed that residual volume to liver volume ratio (RLVR) is the most independent significant predictor in comparable to residual liver volume, total liver volume, and tumor volume with good discriminatory performance (AUC, 0.722 and sig, 0.000); from these results we may use RLVR as a surrogate parameter for assessment of both tumor burden and non-tumorous residual volume as prognostic risk factor for liver decompensation after TACE. Kohla et al. [18] use both tumor size and number of nodules to identify the independent significant risk prediction of tumor burden to liver decompensation after TACE.
It is well known that imaging-based liver volumetry using computed tomography is an important method in preoperative assessment of cirrhotic patients who were planned for hepatic resection or liver transplantation to determine the future remnant liver volume which helps in prediction of post-operative liver dysfunction [26]. However, to our knowledge, this method of volumetry was not used in non-surgical treatment modality of HCC, so our work may be unique in using imaging-based liver volumetry for pre-operative assessment of TACE suitability in HCC.
Our rationale to use accurate residual liver volumetry in non-surgical treatment modalities of HCC is our hypothesis that post TACE hepatic function may be affected by pre-interventional reserve liver function as well as the possible impact of the intervention procedure itself on the liver non-tumorous residual volume; this liver residual volume may be affected to variable extent by the possible extra-target ischemic or toxic effect of embolization or chemotherapy of TACE as the feeding arterial blood supply of HCC may be shared with the surrounding non-tumorous liver tissue [27].
As regards the performance of our new proposed prognostic model as a clinical decision support tool for liver tolerability after TACE for HCC, after combination of serum albumin, serum bilirubin, prothrombin concentration, and RLVR in single prognostic model increased the discriminatory performance of this new proposed model to a very good level (AUC, 0.942 and sig, 0.000). We named this model as liver tolerability score (LTS) as we may use it for future prediction of the tolerability of functioning non-tumorous liver tissue to TACE of HCC. We identified two cutoff points to this model (≤ 0.30 and ≥ 0.80) for rule out and rule in, respectively. We stratified our new score to 3 risk grades (LTG), low-, moderate-, and high-risk grades using the previous two cutoff points for risk stratification with significant difference as regards the pairwise comparisons between hazard function of these different risk grades.
Limitations of our study
Our study has some limitations; the accurate assessment of liver decompensation after TACE used Child-Pugh scoring; however, this scoring system is widely used, it has some limitations itself as some parameters are subjectively assessed like encephalopathy and ascites and the co-linearity between hypo-albuminemia and ascites. The interpersonal variability as regards the experience in TACE procedures in our study may limit our results; however, we tried to treat this point as the experience of the intervention radiologists in our center was excellent and comparable. However, our results were internally validated; it needs to be externally validated in other centers to evaluate its applicability.
Conclusion
CT Image-based assessment of RLVR may have a possible role in predicting of future tolerability of the functioning residual tissue to TACE of HCC. Liver tolerability score (LTS) could stratify the intermediate stage HCC to 3 risk grades, so LTS may have a role in clinical decision for the suitability of TACE for intermediate stage HCC.
Availability of data and materials
The authors confirm that all data supporting the finding of the study are available within the article and the raw data and data supporting the findings were generated and available at the corresponding author on request.
Abbreviations
- TACE:
-
Transarterial chemoembolization
- PC:
-
Prothrombin concentration
- TV:
-
Tumor volume
- RLV:
-
Residual liver volume
- RLVR:
-
Residual to liver volumetric ratio
- HCC:
-
Hepatocellular carcinoma
- LTS:
-
Liver tolerability score
- TRIPOD:
-
Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis
References
European Association for the Study of the Liver (2018) EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 69:182–236
Heimbach JK, Kulik LM, Finn RS et al (2018) AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 67:358–380
Lencioni R, Petruzzi P & Crocetti L (2013). Division of diagnostic imaging and intervention, Pisa University School of Medicine, Pisa, Italy Semin Intervent Radiol ;30:3–11.
Lencioni R (2012) Chemoembolization for hepatocellular carcinoma. Semin Oncol 39(4):503–509
Sangro B, Salem R (2014) Transarterial chemoembolization and radioembolization. Semin Liver Dis 34:435–443
Lencioni R, de Baere T, Soulen MC et al (2016) Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology 64:106–116
Lo CM, Ngan H, Tso WK et al (2002) Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 35:1164–1171
Lu W, Li YH, Yu ZJ, , et al (2007). A comparative study of damage to liver function after TACE with use of low-dose versus conventional-dose of anticancer drugs in hepatocellular carcinoma. Hepatogastroenterology ;54:1499–1502.
Dorn DP, Bryant MK, Zarzour J et al (2014) Chemoembolization outcomes for hepatocellular carcinoma in cirrhotic patients with compromised liver function. HPB (Oxford) 16:648–655
Garwood ER, Fidelman N, Hoch SE (2013) Morbidity and mortality following transarterial liver chemoembolization in patients with hepatocellular carcinoma and synthetic hepatic dysfunction. Liver Transpl 19:164–173
Chan AO, Yuen MF, Hui CK et al (2002) A prospective study regarding the complications of transcatheter intraarterial lipiodol chemoembolisation in patients with hepatocellular carcinoma. Cancer 94:1747–1752
Caturelli E, Siena DA, Fusilli S et al (2000) Transcatheter arterial chemoembolization for hepatocellular carcinoma in patients with cirrhosis: evaluation of damage to nontumorous liver tissue long-term prospective study. Radiology 215:123–128
Raoul J-L, Sangro B, Forner A et al (2011) Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev 37:212–220
Park J-W, Chen M, Colombo M et al (2015) Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int 35:2155–2166
Bolondi L, Burroughs A, Dufour J-F et al (2012) Heterogeneity of patients with intermediate (BCLC B) hepatocellular carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis 32:348–359
Hucke F, Pinter M, Graziadei I et al (2014) How to STATE suitability and START transarterial chemoembolization in patients with intermediate stage hepatocellular carcinoma. J Hepatol 61:1287–1296
Moons KG, Altman DG, Reitsma JB et al (2015) Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 162:W1–W73
Kohla MAS, Abu Zeid MI, Al-Warraky M et al (2015) Predictors of hepatic decompensation after TACE for hepatocellular carcinoma. BMJ Open Gastro 2:e000032
Miksad RA, Ogasawara S, Xia F et al (2019) Liver function changes after transarterial chemoembolization in US hepatocellular carcinoma patients: the LiverT study. BMC Cancer 19:795
Oliveri RS, Wetterslev J & Gluud C (2011). Transarterial (chemo)embolisation for unresectable hepatocellular carcinoma. Coch Database Syst Rev , Issue 3. Art. No.: CD004787.
Hansmann J, Evers MJ, Bui JT et al (2017) Albumin-bilirubin and platelet-albumin-bilirubin grades accurately predict overall survival in high-risk patients undergoing conventional transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol 28:1224–1231
Khalid MA, Achakzai IK, Hanif FM et al (2019) To determine the prognostic value of the albumin–bilirubin grade (ALBI) in patients underwent transarterial chemoembolization for unresectable hepatocellular carcinoma. Gastroenterol Hepatol Bed Bench 12(2):110–115
Lee SK, Song MJ, Kim SH et al (2019) Comparing various scoring system for predicting overall survival according to treatment modalities in hepatocellular carcinoma focused on plateletalbumin-bilirubin (PALBI) and albumin-bilirubin (ALBI) grade: A nationwide cohort study. PLoS ONE 14(5):e0216173
Hsin IF, Hsu CY, Huang HC et al (2011) Liver failure after transarterial chemoembolization for patients with hepatocellular carcinoma and ascites: incidence, risk factors, and prognostic prediction. J Clin Gastroenterol 45(6):556–562
Kadalayil L, Benini R, Pallan L et al (2013) A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann Oncol 24:2565–2570
Lim MC, Tan CH, Cai J et al (2014) CT volumetry of the liver: where does it stand in clinical practice? Clin Radiol 69:887–895
Sun Z, Li G, Ai X et al (2011) Hepatic and biliary damage after transarterial chemoembolization for malignant hepatic tumors: incidence, diagnosis, treatment, outcome and mechanism. Crit Rev Oncol Hematol 79(2):164–174
Acknowledgements
To all the participants for their cooperation and patience
Funding
No funding. Not applicable for this section.
Author information
Authors and Affiliations
Contributions
WY suggested the research idea, ensured the original figures and data in the work, minimized the obstacles to the team of work, correlated the study concept and design, and had the major role in analysis. RE supervised the study with significant contribution to design the methodology, manuscript revision, and preparation. HA correlated the clinical data of patient and matched it with the findings, and drafted and revised the work. TG collected data in all stages of manuscript and performed data analysis. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted according to the guidelines of the ethics committee of Tanta University and was approved by our institutional review board. All patients gave written informed consent to be imaged in our study. Ethics committee’s reference number is not applicable.
Consent for publication
All participants included in the research gave written consent to publish the data included in the study. Authors accepted to publish the paper.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elsawy, A.A., Dawoud, M.M., Elarabawy, R.A. et al. Role of residual liver volumetry and function in prediction of liver tolerability after transarterial chemoembolization for hepatocellular carcinoma in cirrhotic patients: deriving a clinical decision support score. Egypt J Radiol Nucl Med 51, 152 (2020). https://doi.org/10.1186/s43055-020-00265-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-020-00265-4