Abstract
Background
Tinnitus, sensory neural hearing loss (SNHL), and vertigo are common audio-vestibular symptoms. Many diseases are associated with these symptoms; however, the exact cause is not always identified. Some studies show that the etiology could be related to the presence of a vascular loop in contact with the 8th cranial nerve. Three-dimensional (3D) constructive interference in steady state (CISS) is a fully refocused gradient-echo magnetic resonance imaging (MRI) sequence that has high sensitivity in evaluation of the cranial nerves. This high sensitivity is a result of its inherent ability to accentuate the T2 values between cerebrospinal fluid (CSF) and adjacent anatomical or pathological structures. We aimed to evaluate the association of audio-vestibular symptoms with the presence of vascular loops and vascular contact in cerebellopontine angle (CPA) and the internal auditory canal (IAC) using 3Tesla MRI. The study included 98 patients (196 ears); 51 females and 47 males with audio-vestibular dysfunction symptoms in isolation or combined; 40 patients with tinnitus, 50 with sensory neural hearing loss, and 32 with vertigo. The healthy control group with no symptoms in either ear, n = 60 (120 ears): 32 females and 28 males. The non-symptomatic ears in the patients were added to the healthy control group. All MRI examinations were performed by using a 3 T (Magnetom Verio 3 T; Siemens Medical Solutions, Erlangen, Germany).
Results
No statistically significant association was detected between the presence of different grades of vascular loop or types of vascular contact and any of the studied audio-vestibular symptoms.
Conclusion
No possible role of the presence of vascular loop/contact was identified in causing tinnitus, deafness, or vertigo as evaluated by 3D-CISS sequence. Therefore, presence of vascular loops in contact with the 8th cranial nerve is not certainly considered pathological but possibly to be a normal anatomical coincidental finding.
Similar content being viewed by others
Background
Tinnitus, sensorineural hearing loss (SNHL), and vertigo are common audio-vestibular symptoms and they are well-known classic triad in inner ear disease involving the membranous labyrinth [1]. However, in absence of inner ear disease, the exact cause is not always recognized. It has been proposed that compression of the vestibulocochlear nerve (8th cranial nerve) by a vascular loop of the anterior inferior cerebellar artery (AICA) could be the causative factor resulting in the otologic symptom. This pathology is described as vascular compression syndrome (VCS) which is caused by direct contact between a blood vessel and a cranial nerve [2,3,4]. Initially, the hypothesis of VCS was suggested by McKenzie in 1936 and later, discussed by Jannetta in 1975, to refer to cranial nerve dysfunction [2].
Various explanations were assumed to explain the impaired nerve’s function as an effect of vascular compression. Early in 1945, Sunderland et al. assumed that the proximity between the AICA and the nerves within the narrowed space of the IAC possibly produce nerve conduction disturbance due to the applied mechanical pressure via atheromatous, tortuous, or pulsating vessels [5]. The pulsatile vascular compression may result in nerve demyelination and/or fixation of the artery to the nerve by arachnoid adhesions [6]. It was also proposed that the arterial elongation and brain “sag” related to the aging process may result in cranial nerve cross-compression in the CPA [7]. Impaired blood flow through the vascular loop as a direct result of neurovascular compression was suggested to result in reduced vascular perfusion of the cochlea and vestibule leading to dysfunction [8].
An improvement in dysfunctional hyperactivity of the 8th cranial nerve was detected after microvascular decompression, which favored relation to the existence of a vascular loop [2, 9]. Though the concept of vascular compression has been adopted for hemifacial spasm and trigeminal neuralgia, contradictory results have been reported about the relationship between VCS and neuro-otologic symptoms [3, 10, 11].
In literature, audio (tinnitus, SNL)-vestibular (vertigo) symptoms triad often occur in combination or in isolation, with different insinuations for causative pathology as there is a wide range of conditions causing these symptoms [12]. Researchers have tried to establish a consensus on the clinical categorization of these symptoms that should be implemented as guidelines for deciding the appropriateness of the required imaging [13].
In routine clinical practice, the diagnostic potentials of conventional radiography in revealing the underlying causes for facial and audio-vestibular dysfunction were limited. Meanwhile, computed tomography (CT) contribution to evaluate membranous labyrinthine diseases and small lesions of the internal auditory canal is hindered. Magnetic resonance imaging (MRI) is currently the most informative imaging technique in evaluating patients with audio-vestibular dysfunctions [11, 14, 15]. MRI has allowed better visualization of the CPA and IAC and is the method of choice for evaluating the 8th CN in patients with audio-vestibular symptoms. Preference of MRI techniques is contributed to the superior contrast resolution and multiplanar imaging capability, which make detection of small lesions of the membranous labyrinth and IAC within reach. MRI is used to exclude retrocochlear pathologies especially in patients with asymmetrical sensorineural hearing loss, unilateral tinnitus, or vestibular findings [14].
The purpose of our study was to evaluate the association of audio-vestibular symptoms with the presence of vascular loops and vascular contact in the CPA and IAC using 3Tesla magnetic resonance imaging.
Methods
Patients groups
This retrospective study included 98 patients (196 ears): 51 females and 47 males; age range, 11-73 years; mean 47.6 ± 15 years with audio-vestibular dysfunction symptoms in isolation or combined; 40 patients with tinnitus, 50 with sensory neural hearing loss and 32 with vertigo. The healthy control group with no symptoms in either ear, n = 60 (120 ears); 32 females and 28 males; age range, 19-88 years; mean 45.6 ± 11.7 years (Table 1). The non-symptomatic ears in the patients were added to the healthy control group. In the groups of tinnitus and SNHL, both sides of patients and controls were evaluated separately as symptomatic and asymptomatic ear. Exclusion criteria included the patients with neuritis, tumors at the CPA, previous CPA surgery, or temporal bone trauma.
Imaging and evaluation
The vascular loops of the AICA were graded as previously described by Chavda in McDermott et al. [16] as follows: grade I—when an AICA vascular loop borders the internal auditory meatus (internal acoustic pore), lying only in the CPA but not entering the IAC; grade II—when the loop insinuates itself into the internal auditory meatus but occupies 50% or less of the canal; or grade III—when the loop occupies more than 50% of the canal. In addition, we also categorized the presence of vascular contact with the 8th cranial nerve into type I—no contact, type II—contact without nerve angulation, and type III—contact with 8th cranial nerve angulation.
All MRI examinations were performed using a 3 T (Magnetom Verio 3 T; Siemens Medical Solutions, Erlangen, Germany). The imaging protocol consisted of axial T2-weighted images of the whole brain and coronal and axial T1-weighted images of the CPA before and after administration of intravenous contrast medium. Three-D constructive interference steady state (CISS) imaging of the CPA was performed. The healthy control group was examined with the 3D CISS sequence in addition to the routine cranial MR imaging protocol.
The 3-dimensional (3-D) constructive interference in steady state (CISS) sequence parameters consisted of TR 6.6/TE 3.0/excitation 1, flip angle of 70°, matrix of 256 × 512, and field of view of 140 × 140 mm signal-to-noise ratio (SNR) 1.00. An axial slab was imaged and divided into 72 sections (slice thickness, 0.4 mm). Voxel size was 0.5 × 0.4 × 0.4 mm. The average acquisition time was 6 min.
For all patients, 3-D CISS sequences produced detailed images of the CPA and the IAC with visualization of the cisternal and intracanalicular segments of the facial nerve and the various components of the vestibulocochlear nerve complex.
Statistical analysis
Data were analyzed using IBM’s statistical package for the social sciences (SPSS) software, version 20.0. (Armonk, NY: IBM Corp). Parametric quantitative data were expressed using mean, standard deviation (SD), and median. Significance level was set at 5%. Qualitative data were analyzed using chi-square test.
Results
The included 98 patients (196 ears) were divided into symptomatic and asymptomatic ears according to the clinical data: tinnitus, SNHL, and vertigo (Table 2). Sixty controls (120 ears) were included in the asymptomatic category.
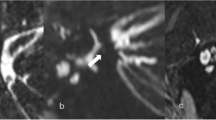
According to MRI finding, analysis of the relation between tinnitus (n = 42 ears) and vascular loop grades I-III (Fig. 1) showed grade I in n = 31 (73.8%), grade II in n = 7 (16.7%), and grade III in n = 4 (9.5%) (Table 2). The asymptomatic sides were evaluated in the patients (n = 115 ears) and healthy controls (n = 120 ears). The patients presented as grade I n = 111 (72.1%), grade II n = 39 (25.3%), and grade III n = 4 (2.6%). The healthy control presented as grade I n = 75 (62.5%), grade II n = 37 (30.8%), and grade III n = 8 (6.7%). The relation between tinnitus and vascular loop (grades I-III) showed no significant association between the presence or absence of tinnitus and any of the studied vascular loop grades (p = 0.15). The relation between tinnitus and vascular contact types I-III (Fig. 2) showed type I in n = 22 (52.4%), type II in n = 18 (42.8%), and type III in n = 2 (4.8%)
(Table 2). The asymptomatic sides in the patients showed type I n = 111 (72.1%), type II n = 38 (24.7%), and type III n = 5 (3.2%). The healthy controls presented as type I n = 53 (44.1%), type II n = 62 (51.7%), and type III n = 5 (4.2%). The relation between tinnitus and vascular contact (types I-III) showed no significant association between the presence or absence of tinnitus and any of the studied vascular contact types (p = 0.65).
The relation between SNHL (n = 34 ears) and vascular loop (grades I-III) showed that grade I was present in n = 25 (73.5%), grade II in n = 8 (23.5%), and grade III in n = 1 (3%) (Table 2). The asymptomatic sides were evaluated in the patients n = 162 and healthy controls n = 120. The patients presented as grade I n = 115 (71%), grade II n = 39 (24%), and grade III n = 8 (5%). The relation between SNHL and vascular loop (grades I-III) showed no significant association between the presence or absence of deafness and any of the studied vascular loop grades (p = 0.70). As for the relation between SNHL and vascular contact (types I-III), patients showed type I in n = 20 (58.8%), type II in n = 13 (38.3%), and type III in n = 1 (2.9%) (Table 2). The asymptomatic sides in the patients presented as type I n = 112 (69.1%), type II n = 47 (29%), and type III n = 3 (1.9%). The relation between SNHL and vascular contact (types I-III) showed no significant association (p = 1.0).
Studying the relation between vertigo and vascular loop (grades I-III) showed that patients complained of vertigo (n = 64 ears) presented as follows; grade I n = 45 (70.3%), grade II n = 18 (28.1%), and grade III n = 1 (1.6%) (Table 2). The asymptomatic patients (n = 132 ears) presented as grade I n = 88 (66.7%), grade II n = 37 (28%), and grade III n = 7 (5.3%). The relation between vertigo and vascular loop (grades I-III) showed no significant association between the presence or absence of vertigo and any of the studied vascular loop grades (p = 0.33). As for the relation between vertigo and vascular contact (types I-III), the patients presented as follows type I in n = 42 (65.6%), type II in n = 20 (31.3%), and type III in n = 2 (3.1%) (Table 2). The asymptomatic patients presented as type I n = 100 (75.7%), type II n = 31 (23.5%), and grade III n = 1 (0.8%). The relation between vertigo and vascular contact (types I-III) showed no significant association between the presence or absence of vertigo and any of the studied vascular contact types (p = 0.68).
Discussion
The diagnosis of the etiology of audio-vestibular symptoms is a clinical challenge; nevertheless, the use of MRI in diagnosis has increased the recognition of the underlying pathologic conditions. The 3-D CISS sequence provides a more comprehensive analysis of the CPA, IAC, root exit zone, and cisternal and intracanalicular segments of the cranial nerves [17]. Adoption of the role of VCS as a causative pathology for hemifacial spasm and trigeminal neuralgia was widely accepted. Meanwhile, its causal relationship with the neuro-otologic symptoms as tinnitus, deafness, or vertigo has been argued.
The exact pathophysiology of VCS is still to be elucidated. On the basis of the concept suggested by Jannetta et al. [2] which supported the role of neurovascular compression in causing cranial nerve dysfunction, several attempts were conducted to study a relationship between vascular compression and a number of clinical conditions [4, 18]. It was proposed that the arterial pulsatile compression causes mechanical irritation and focal demyelination which results in the development of these audio-vestibular symptoms. The other hypothesis is that the neurovascular compression disturbs the normal blood flow with decreased vascular perfusion of the nerve.
The present study was designed to evaluate the association of audio-vestibular symptoms with the presence of vascular loops and vascular contact in CPA and the IAC using the 3D CISS sequence done by 3 Tesla magnetic resonance imaging machine. Based on the results of the current study, it was concluded that no possible role of the presence of vascular loop in causing tinnitus, SNHL, or vertigo using 3D-CISS sequence as an assessment tool. Therefore, the presence of vascular loops in contact with the 8th cranial nerve is not certainly considered pathological but possibly to be a normal anatomical coincidental finding. Hence, this radiological finding alone should not be used to qualify patient for further invasive microvascular decompression surgery and is not suggested to be applied as a sole stratification criterion for any unnecessary intervention and its relation to cranial nerve should be cautiously considered.
In attempt to address the neurovascular conflict involving the 8th cranial nerve, and in accordance to our findings, several studies corroborated our data. Sirikci et al. reported no relationship between cochleovestibular symptoms and the type of vascular compression [14]. Furtherly, they suggested that MR modalities could be the preferred procedure in studying this anatomical relationship precisely. Similarly, several researches demonstrated the presence of neurovascular contact in both symptomatic and asymptomatic groups with no statistically significant association detected [3, 19, 20]. Additionally, several trials applied microvascular decompression techniques as an attempt to relief VCS of 8th cranial nerve and reported that variable percentages of patients did not improve after the microvascular decompression which raised the doubt about this clinical radiological relationship [21, 22]. Meanwhile, Bae et al. showed that the AICA loop anatomic location in the IAC and CPA in the symptomatic and asymptomatic sides of subjects with tinnitus and controls without tinnitus did not show statistically significant differences. However, the 8th cranial nerve angulation was significantly higher on the symptomatic sides of patients with tinnitus than in the other groups [23]. McDermott et al. reported similar results concerning tinnitus, where they showed no association between tinnitus and the presence of vascular loops, though they showed a relationship between AICA loops and unilateral hearing loss [16].
Nevertheless, other studies were not in accordance with the present study findings [10, 24, 25]. These studies supported the concept of VCS of the 8th CN based on the symptomatic improvement after microvascular decompression surgery of 8th CN. Furtherly, they even recommended early microvascular decompression before permanent damage of the nerve. Moreover, these studies showed a correlation between the presence of vascular loops in the IAC and pulsatile tinnitus and attributed the tinnitus to direct transmission of arterial pulsations to the cochlea via a resonance effect in the petrous bone. However, in a contradictory note, McLaughlin et al. study that assessed the efficacy of microvascular decompression after 4400 surgeries for various cranial nerves reported that variable percentages of patients did not benefit from the intervention [21].
In relative accordance to our study, an interesting multicenter study was proposed by Di Stadio et al. investigating the correlation between specific characteristics of the vascular loop with audio-vestibular symptoms using MRI scanning of CPA of asymptomatic patients [26]. In Di Stadio study, the assessed loop metrics were the depth of loop penetration into IAC, caliber of the vessel, position, number, and length of contacts between vessel and nerve. In accordance with our data, Di Stadio et al. concluded the absence of correlation between the presence of vertigo or tinnitus and a single neurovascular contact, while significantly correlated with an increased number of neurovascular contacts. The results were explained in the context of the multiplicity of mechanisms recruited by the vestibular system to maintain the balance including the visual and motor system which can lessen the incidence of these symptoms [27].
Stratification criteria for patients with cochleovestibular compression syndrome was proposed by De Ridder et al. who developed diagnostic criteria that are now widely adopted [28]. These criteria are (1) unilateral paroxysmal tinnitus; (2) co-existent ipsilateral symptoms including hemifacial spasms, otalgia, vertiginous spells, or hearing losses at tinnitus frequencies; (3) MRI findings evidencing vestibular conflict; and (4) abnormal auditory brainstem responses( ABR) [28]. Thus, clinical evaluation should be considered as more consistent diagnostic evidences than radiologic imaging of neurovascular compression of the cochlear nerve.
The underlying rationale for selection of 3D CISS over the conventional MRI is the high gradient amplitude. High strength magnets are employed to reach the maximum intensity within a short period of time [29]. CISS as a high resolution heavily T2WI 3D sequence with a very high CSF-tissue contrast provides optimal images for evaluation of neurovascular relationships allowing visualization of small vessels and enables the best assessment of an accurate relationship between nerve roots or their branches and adjacent vessels with a minimal signal loss due to CSF pulsations. Any desired imaging plane can be obtained by the multiplanar reconstructive technique [30].
However, CISS sequence is limited by the long image-acquisition times [30]. Furtherly, CISS sequences may be constrained by banding artifact as a result of magnetic field inhomogeneity causing pseudolesions to appear in the IAC assessment [31]. The deficiency of contrast between soft tissues and even between soft tissues and bone can obscure the lesions on CISS images [32]. CISS as a T2W sequence although of high diagnostic accuracy, can miss small CPA lesions which could be the occult cause for the patient’s symptoms and requires contrast-enhanced studies to be detected [33]. A study proposed by Abele et al. suggested an unenhanced MR imaging protocol utilizing axial CISS and coronal T2WI as a screening protocol to identify small IAC lesions ≤ 10 mm with 100% sensitivity [34].
In an attempt to minimize the bias in our study, we designed it to include a control group of healthy persons along with unaffected healthy ears in the patients. The current study was non-invasive and performed with no contrast administration, hence, saving health and financial burden. Using high strength MRI machine in the current study offers increased diagnostic sensitivity and specificity due to its higher SNR which is beneficial in improved visualization of small anatomical structures at higher field strengths. The high-field MR imaging (3 T and higher) has the potential to significantly improve clinical care in various neurologic disorders. Further studies recruiting a larger number of patients and control groups are recommended to confirm our data making it possible to apply our results for the general population.
Conclusion
Using CISS sequence can offer better visualization and further clarification of the relation between vascular and neurological structures in CPA and IAC. There is a diverse variability in vascular anatomy at CPA and IAC with nondependent association between location of the vascular loop and clinical profile. Therefore, the presence of vascular loops in contact with the 8th cranial nerve is not always considered pathological but likely to be a normal anatomical variant. Subsequently, this radiological finding alone should not be used to qualify patient for further invasive microvascular decompression surgery.
Availability of data and materials
The data used or analyzed during the study are available from the corresponding author on reasonable request.
Abbreviations
- AICA:
-
Anterior inferior cerebellar artery
- ABR:
-
Auditory brainstem responses
- CPA:
-
Cerebellopontine angle
- CSF:
-
Cerebrospinal fluid
- CT:
-
Computed tomography
- CISS:
-
Constructive interference in steady state
- IAC:
-
Internal auditory canal
- MRI:
-
Magnetic resonance imaging
- SNHL:
-
Sensory neural hearing loss
- SNR:
-
Signal-to-noise ratio
- VCS:
-
Vascular compression syndrome
References
Ciuman RR (2013) Inner ear symptoms and disease: pathophysiological understanding and therapeutic options. Medical science monitor: international medical journal of experimental and clinical research 19:1195–1210
Jannetta PJ (1975) Neurovascular cross-compression in patients with hyperactive dysfunction symptoms of the eighth cranial nerve. Surg Forum 26:467–469
Makins AE, Nikolopoulos TP, Ludman C, O’Donoghue GM (1998) Is there a correlation between vascular loops and unilateral auditory symptoms? Laryngoscope 108:1739–1742
Markowski J, Gierek T, Kluczewska E, Witkowska M (2011) Assessment of vestibulocochlear organ function in patients meeting radiologic criteria of vascular compression syndrome of vestibulocochlear nerve--diagnosis of disabling positional vertigo. Medical science monitor : international medical journal of experimental and clinical research 17:CR169–CR173
Sunderland S (1945) The arterial relations of the internal auditory meatus. Brain J Neurol 68:23–27
Applebaum EL, Valvassori GE (1984) Auditory and vestibular system findings in patients with vascular loops in the internal auditory canal. The Annals of otology, rhinology & laryngology Supplement 112:63–70
Jannetta PJ (1980) Neurovascular compression in cranial nerve and systemic disease. Ann Surg 192:518–525
Brunsteins DB, Ferreri AJ (1990) Microsurgical anatomy of VII and VIII cranial nerves and related arteries in the cerebellopontine angle. Surgical and radiologic anatomy : SRA 12:259–265
Jannetta PJ (1997) Outcome after microvascular decompression for typical trigeminal neuralgia, hemifacial spasm, tinnitus, disabling positional vertigo, and glossopharyngeal neuralgia (honored guest lecture). Clin Neurosurg 44:331–383
Nowe V, De Ridder D, Van de Heyning PH, Wang XL, Gielen J, Van Goethem J et al (2004) Does the location of a vascular loop in the cerebellopontine angle explain pulsatile and non-pulsatile tinnitus? Eur Radiol 14:2282–2289
Schick B, Brors D, Koch O, Schafers M, Kahle G (2001) Magnetic resonance imaging in patients with sudden hearing loss, tinnitus and vertigo. Otology & neurotology: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 22:808–812
Bisdorff A, Von Brevern M, Lempert T, Newman-Toker DE (2009) Classification of vestibular symptoms: towards an international classification of vestibular disorders. Journal of vestibular research : equilibrium & orientation 19:1–13
Expert Panel on Neurologic I, Sharma A, CFE K, Aulino JM, Chakraborty S, Choudhri AF et al (2018) ACR appropriateness criteria((R)) hearing loss and/or vertigo. Journal of the American College of Radiology : JACR 15:S321–SS31
Sirikci A, Bayazit Y, Ozer E, Ozkur A, Adaletli I, Cuce MA et al (2005) Magnetic resonance imaging based classification of anatomic relationship between the cochleovestibular nerve and anterior inferior cerebellar artery in patients with non-specific neuro-otologic symptoms. Surgical and radiologic anatomy : SRA 27:531–535
Sonmez G, Basekim CC, Ozturk E, Gungor A, Kizilkaya E (2007) Imaging of pulsatile tinnitus: a review of 74 patients. Clin Imaging 31:102–108
McDermott AL, Dutt SN, Irving RM, Pahor AL, Chavda SV (2003) Anterior inferior cerebellar artery syndrome: fact or fiction. Clin Otolaryngol Allied Sci 28:75–80
De Foer B, Kenis C, Van Melkebeke D, Vercruysse JP, Somers T, Pouillon M et al (2010) Pathology of the vestibulocochlear nerve. Eur J Radiol 74:349–358
Cavusoglu M, Ciliz DS, Duran S, Ozsoy A, Elverici E, Karaoglanoglu R et al (2016) Temporal bone MRI with 3D-FIESTA in the evaluation of facial and audiovestibular dysfunction. Diagnostic and interventional imaging 97:863–869
de Abreu JL, Kuniyoshi CH, Wolosker AB, Borri ML, Antunes A, Ota VK et al (2016) Vascular loops in the anterior inferior cerebellar artery, as identified by magnetic resonance imaging, and their relationship with otologic symptoms. Radiol Bras 49:300–304
Gultekin S, Celik H, Akpek S, Oner Y, Gumus T, Tokgoz N (2008) Vascular loops at the cerebellopontine angle: is there a correlation with tinnitus? AJNR Am J Neuroradiol 29:1746–1749
McLaughlin MR, Jannetta PJ, Clyde BL, Subach BR, Comey CH, Resnick DK (1999) Microvascular decompression of cranial nerves: lessons learned after 4400 operations. J Neurosurg 90:1–8
Brookes GB (1996) Vascular-decompression surgery for severe tinnitus. The American journal of otology 17:569–576
Bae YJ, Jeon YJ, Choi BS, Koo JW, Song JJ (2017) The role of MRI in diagnosing neurovascular compression of the cochlear nerve resulting in typewriter tinnitus. AJNR Am J Neuroradiol 38:1212–1217
De Ridder D, Ryu H, Moller AR, Nowe V, Van de Heyning P, Verlooy J (2004) Functional anatomy of the human cochlear nerve and its role in microvascular decompressions for tinnitus. Neurosurgery. 54:381–388 discussion 8-90
De Ridder D, De Ridder L, Nowe V, Thierens H, Van de Heyning P, Moller A (2005) Pulsatile tinnitus and the intrameatal vascular loop: why do we not hear our carotids? Neurosurgery 57:1213–1217 discussion -7
Di Stadio A, Dipietro L, Ralli M, Faralli M, Della Volpe A, Ricci G et al (2020) Loop characteristics and audio-vestibular symptoms or hemifacial spasm: is there a correlation? A multiplanar MRI study. Eur Radiol 30:99–109
Thompson TL, Amedee R (2009) Vertigo: a review of common peripheral and central vestibular disorders. Ochsner J 9:20–26
De Ridder D, Heijneman K, Haarman B, van der Loo E (2007) Tinnitus in vascular conflict of the eighth cranial nerve: a surgical pathophysiological approach to ABR changes. Prog Brain Res 166:401–411
Casselman JW, Kuhweide R, Deimling M, Ampe W, Dehaene I, Meeus L (1993) Constructive interference in steady state-3DFT MR imaging of the inner ear and cerebellopontine angle. AJNR Am J Neuroradiol 14:47–57
Roser F, Ebner FH, Danz S, Riether F, Ritz R, Dietz K et al (2008) Three-dimensional constructive interference in steady-state magnetic resonance imaging in syringomyelia: advantages over conventional imaging. J Neurosurg Spine 8:429–435
Bangerter NK, Hargreaves BA, Vasanawala SS, Pauly JM, Gold GE, Nishimura DG (2004) Analysis of multiple-acquisition SSFP. Magn Reson Med 51:1038–1047
Hingwala D, Chatterjee S, Kesavadas C, Thomas B, Kapilamoorthy TR (2011) Applications of 3D CISS sequence for problem solving in neuroimaging. The Indian journal of radiology & imaging 21:90–97
Hentschel MA, Kunst HPM, Rovers MM, Steens SCA (2018) Diagnostic accuracy of high-resolution T2-weighted MRI vs contrast-enhanced T1-weighted MRI to screen for cerebellopontine angle lesions in symptomatic patients. Clinical otolaryngology : official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery 43:805–811
Abele TA, Besachio DA, Quigley EP, Gurgel RK, Shelton C, Harnsberger HR et al (2014) Diagnostic accuracy of screening MR imaging using unenhanced axial CISS and coronal T2WI for detection of small internal auditory canal lesions. AJNR Am J Neuroradiol 35:2366–2370
Acknowledgements
None
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
MZ conceived of the study, participated in its design, supervised the selection of cases and controls, drafted and finalized the manuscript. NA helped in reviewing cases and carried the statistical analysis. All authors read and approved the final manuscript and accepted the publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study has been carried out following the approval of the Ethics Committee of the Faculty of Medicine Alexandria University in accordance with Helsinki Declaration (reference number: 0304444).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zidan, M.A., Almansor, N. Presence of vascular loop in patients with audio-vestibular symptoms: is it a significant finding? Evaluation with 3-tesla MRI 3D constructive interference steady state (CISS) sequence. Egypt J Radiol Nucl Med 51, 114 (2020). https://doi.org/10.1186/s43055-020-00238-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-020-00238-7