Abstract
Background
Accurate radiologic assessment of treatment response in lymphomas is important for helping the clinicians to properly evaluate effectiveness of treatment and consequently guide therapeutic management of these patients. Imaging tools based on anatomic response are suboptimal in detecting functional changes in tumors resulting after early effective treatment. Recently, with the development of 18F-FDG PET/CT, both functional and anatomic information has been integrated for assessing treatment response in both solid tumors and hematologic malignancies. The aim of this study was to compare therapeutic response in lymphoma patients using both RECIST 1.1 and PERCIST 1.0 criteria.
Results
Among the included 33 lymphoma patients, RECIST 1.1 and PERCIST 1.0 classifications of therapeutic response were concordant in 20 patients (60.6%) and discordant in 13 patients (39.4%), with a tendency of RECIST 1.1 to downgrade the tumor response in 12/13 patients (P value < 0.001).
Conclusion
The recently applied PERCIST 1.0 is efficient and more accurate than RECIST 1.1 to assess treatment response in lymphoma patients, which is subsequently affecting further management of these patients.
Similar content being viewed by others
Background
Lymphomas show different response to treatment rather than other solid tumors. Post-therapeutic residual lesions are frequently detected in both non-Hodgkin and Hodgkin lymphomas. Lymphomatous masses often do not regress completely after therapy because of necrotic debris and residual fibrosis [1]. Anatomic response criteria in lymphoma patients often underestimate the chemotherapeutic effect [2]. The currently accepted standard anatomic response classifications are Response evaluation criteria in solid tumor (RECIST) and RECIST 1.1; however, they do not have the ability to detect functional changes in tumors resulting after early effective treatment [3]. 18F-Fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) is a hybrid imaging modality which allows simultaneous metabolic and anatomical imaging of tumors during a single diagnostic session [4,5,6,7]. Assessments of treatment response of different cancers with 18F-FDG PET/CT can be obtained with high efficiency and tumor tracer uptake is expected to decline over time. So, it is important to report the change in standardized uptake value (SUV) in initial and follow-up scans as the PET biologic predictive value of PET is much greater than that of anatomic studies [8,9,10]. This is done by using PET response criteria in solid tumor (PERCIST criteria) depending on standardized uptake value changes by the tumoral mass [11]. Aim of this work was to compare between anatomical post-therapeutic response by contrast-enhanced CT with applied RECIST 1.1 criteria and combined qualitative and quantitative metabolic response by 18F-FDG PET/CT with applied PERCIST 1.0 criteria in lymphoma patients.
Methods
Patients
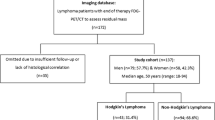
Our prospective study was conducted in the period between September 2018 and January 2020. Thirty three patients with biopsy-proven lymphoma (including both Hodgkin and Non-Hodgkin types) who were referred to perform baseline pre-treatment and end of therapy PET/CT imaging studies were enrolled in this study. Approval of the institutional review board and written informed consents from all patients were obtained before the start of this study.
Inclusion criteria
Any patient presented with lymphoma who did not receive any therapy, aged 18 years or older with no sex predilection were included.
Exclusion criteria
Any patient showing normal initial study or with concomitant malignancy, patients known to have contraindications for radiation (e.g., pregnant females), patients with renal impairment or had high blood glucose levels at the time of the study, and patients who underwent any surgical intervention or received radiotherapy as a line of treatment were excluded.
Patient preparation
Procedure time was 2 weeks after the end of chemotherapy. Patients were fasting for a minimum of 6 h before the scan with good hydration. Exercises were avoided for a minimum of 2 h (ideally 48 h) before the scan. Kidney function tests were reviewed and confirmed to be within normal limit with pre-scanning blood glucose level estimation (accepted between 150 and 200 mg/dL) and administration of antecubital intravenous cannula.
Technique of 18F-FDG PET/CT scan
The radioactive tracer (18F-FDG) was injected intravenously in a dose of 0.06–0.08 mCi/kg body weight. All patients were kept in a warm temperature room and asked to rest without vigorous activity and void just before imaging. Scanning by a hybrid PET/CT scanner (Discovery IQ five-ring machine class I IPX0 used with 16 slices of CT, GE [General Electric Company], Milwaukee, WI, USA, 2016) was performed 60 min after injection. The patient was positioned supine on the table. Initial single-phase contrast material-enhanced helical CT was performed following injection of 125 mL of a low-osmolarity iodinated contrast medium (Optiray 350) at a rate of 4 mL/s by using a power injector. A whole body CT study (from the head to mid-thigh) scanning was obtained using 110 mA, 110 kV, 0.5 s tube rotation time, and 3.3-mm section thickness. After CT scanning, PET scan covering the same field of view was obtained immediately. Six to seven bed positions are planned in the three-dimensional acquisition mode for scanning the entire patient with 3–5-min acquisition at each bed position. Images were transferred to a dedicated workstation to be reconstructed and displayed in axial, coronal, and sagittal planes.
Image analysis
Images were analyzed by two experienced radiologists having five and seven years’ experience in PET/CT imaging. Analysis of CT images was done by visual inspection with selection of target lesions and measuring their dimensions. Analysis of PET/CT images was done by visual inspection, comparing PET and CT data, viewing fused PET/CT images, and quantitative calculation of FDG uptake corrected to lean body mass (SULpeak). They used PERCIST 1.0 criteria on PET/CT interpretation and RECIST 1.1 criteria on CT interpretation (Table 1).
Statistical analysis
Analysis of data was done using SPSS (Statistical package for social science) program version 23. To describe the studied sample, quantitative data were presented as minimum, maximum, mean, and standard deviation. Qualitative data were presented as count and percentage. The chi-square statistic is used for testing relationships between categorical variables. The Wilcoxon signed-rank test is a non-parametric statistical hypothesis test used to compare two related samples, matched samples, or repeated measurements on a single sample to assess whether their population mean ranks differ (i.e., it is a paired difference test). One-way analysis of variance (ANOVA) test was used to compare parametric quantitative data between more than two groups. Kruskal–Wallis test was used to compare non-parametric quantitative data between different groups.
Results
The study included 33 patients (24 females and 9 males) ranged in age from 18 to 85 years with mean age of 41.91 ± 16.63 years. Fifteen patients presented with histopathologically proven non-Hodgkin lymphoma, 17 patients had Hodgkin lymphoma, and 1 patient with Gut-associated lymphoid tissue (GALT). The most presented pathological subtype was NHL-DLBCL (36.4%) and HL-NS (30.3%), the rest of presented data are summarized in Table 2.
At baseline staging (Table 3), most of the patients were of stages II and III (representing 72.7%). Sixteen patients showed only nodal disease (48%), 13 patients showed both nodal and extra-nodal disease, and only 4 patients showed only extra-nodal disease (12%). The most affected extra-nodal organ was the spleen (21.2 %).
According to CT-based assessment of therapeutic response (RECIST 1.1), 8 patients showed complete response (CR; 24.2%), 18 patients showed partial response (PR; 54.5%), 3 patients showed stationary disease (SD; 9.1%), and 4 patients showed progressive disease (PD; 12.1%). So, the responders (PR and CR) represented 78.8% and the non-responders (SD and PD) represented 21.2% (Tables 4 and 5). There was no significant correlation between response criteria with patient’s age or sex.
According to PET-based assessment of therapeutic response (PERCIST 1.0), 18 patients showed complete metabolic response (CMR; 54.5%), 9 patients showed partial metabolic response (PMR; 27.3%), 2 patients showed stationary metabolic disease (SMD; 6.1%), and 4 patients showed progressive metabolic disease (PMD; 12.1%). So, the responders (PMR and CMR) represented 81.8% and the non-responders (SMD and PMD) represented 18.2% (Tables 6 and 7). There was no significant correlation between response criteria with patient’s age or sex.
Thus, RECIST 1.1 and PERCIST classifications of therapeutic response were discordant in 13 patients representing 39.4% (Table 8). Of 11 patients classified as PR according to RECIST 1.1, ten were classified as CMR according to PERCIST 1.0 (Fig. 1) as the sum of the longest diameters’ reduction of residual target lesions was more than 30% yet showed 100% reduction in the SULpeak, while one patient was classified as SMD according to PERCIST 1.0 (Fig. 2). Two patients were classified as SD according to RECIST 1.1, yet, they were classified as PMR according to PERCIST 1.0 (as the decrease in the sum of the longest diameters of the target lesions was less than 30%, while the decrease in the SULpeak of the target lesions was more than 30%). So, PERCIST 1.0-based therapeutic response was upgraded in twelve patients and downgraded in one patient.
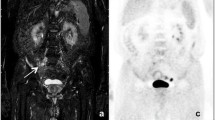
A 51-year-old female patient, recently diagnosed case of NHL (large B cell type). Initial PET/CT study revealed large hypermetabolic mass at the left axilla and axillary tail of left breast in whole body PET MIP image (a), measuring about 16 x 16 x 15 cm with central breaking down in axial CECT image (b) and achieving 19 SULpeak in corresponding fused PET/CT image (c). Follow-up PET/CT study revealed smaller metabolically inactive lesion in whole body PET MIP image (f) that measured 10 x 9.5 x 9.3 cm in axial CECT image (d) denoting PR according to RECIST 1.1, yet showing no FDG uptake in corresponding fused PET/CT image (e) denoting CMR according to PERCIST 1.0
A 43-year-old male patient, recently diagnosed case of T-cell NHL. Initial PET/CT study revealed hypermetabolic nodal and splenic lesions in whole body PET MIP image (a), the largest and most active lesions are seen at left axillary group as in axial CECT image (b), achieving up to 20 SULpeak in corresponding fused PET/CT image (c). Follow-up PET/CT study showed residual active lesions in whole body PET MIP image (f). The left axillary nodal lesions showed size regression in axial CECT image (d) denoting PR according to RECIST 1.1, yet with almost stationary FDG uptake (21 SULpeak) in fused PET/CT image (e) denoting SMR according to PERCIST 1.0
On the other hand, both RECIST 1.1 and PERCIST 1.0 classifications of therapeutic response were concordant in 20 patients representing 60.6%. Eight patients (24.2%) had CR by RECIST 1.1 and CMR by PERCIST 1.0 (Fig. 3). Seven patients had PR by RECIST 1.1 and PMR by PERCIST 1.0 (21.2%). One patient (3%) showed SD by RECIST 1.1 and SMD by PERCIST 1.0 (Fig. 4). Four patients presented with newly developed lesions and were classified as PD and PMD by both RECIST 1.1 and PERCIST 1.0 classifications, respectively (12.1%).
A 35-year-old male patient, newly diagnosed case of gastric lymphoma. Initial PET/CT study including whole body PET MIP (a), axial CECT (c), and corresponding fused PET/CT (d) images revealed irregular hypermetabolic gastric soft mass infiltrating the related left hepatic lobe and achieving 22.8 SULpeak. Follow-up PET/CT study revealed complete resolution of the mass in whole body PET MIP (b) as well as axial CECT (e) and corresponding fused PET/CT (f) images denoting CR and CMR according to RECIST 1.1 and PERCIST 1.0, respectively. However, the whole body image (b) showed another newly developed metabolically active lesion at upper lobe of left lung that was a pneumonic consolidative patch in axial images (g) and (h).
A 20-year-old female patient, recently diagnosed case of HL. Initial PET/CT study revealed hypermetabolic mediastinal mass in whole body PET MIP image (a), formed of enlarged amalgamated retrosternal/prevascular mediastinal in axial CECT image (b) and achieving 5.5 SULpeak in corresponding fused PET/CT image (c). Follow-up PET/CT study still showed the hypermetabolic mediastinal mass in whole body PET MIP image (f) that was of stationary size in axial CECT image (d) denoting SD according to RECIST 1.1, and almost showing stationary FDG uptake (6 SULpeak) in fused PET/CT image (e) denoting SMR according to PERCIST 1.0
Discussion
On the follow-up of cancer patients to evaluate response to treatment, CT was the modality of choice with RECIST criteria as a widely used and accepted tool for assessment, yet it is depending on the changes in tumoral size which may show some inter-observer variability especially in irregular lesions. Also, CT has the inability to detect the changes in activity which occurred in response to treatment [12]. It is difficult to distinguish necrotic tissue or fibrotic scar from residual tumor on CT scans [13]. Response evaluation protocols that are depending on morphological changes are still limited as changes in tumor dimensions are not true markers of therapeutic efficacy because tumor tissue consists of different components that are not all completely regressed over time [14]. This is why some studies found a discrepancy between the CT-based RECIST and the histopathological results among a significant number of the studied cases during follow-up after neoadjuvant chemotherapy [15].
So, more satisfactory methods of evaluation are needed to accurately measure tumor responses quantitatively. 18F-FDG PET thought to overcome these limitations and became a well-established quantitative method for the staging, follow-up, and detection of recurrence in patients with several malignancies [5, 11, 16]. With the development of 18F FDG PET/CT, which is integrated anatomic and metabolic imaging, a modified PET-based criteria (PERCIST) have been reported for assessing treatment response in both solid tumors and hematologic malignancies [3].
In our study, we investigated the concordance between the metabolic criteria and morphologic criteria for the assessment of end therapeutic response to chemotherapy in patients with malignant lymphoma determined using RECIST 1.1 and PERCIST 1.0 criteria.
We included 33 patients who were recently diagnosed with malignant lymphoma. Their end of treatment response was evaluated using RECIST 1.1 and PERCIST 1.0 criteria. Results showed a considerable discrepancy in the assessment of tumor responses between the morphologic criteria (RECIST 1.1) and metabolic criteria (PERCIST 1.0) with discordance in 39.4% of patients (13 patients) and P value < 0.001.
There is tendency for RECIST to downgrade the tumor response (ten patients classified as PR according to RECIST 1.1 were classified as CMR according to PERCIST 1.0, and two patients that classified as SD according to RECIST 1.1 were classified as PMR according to PERCIST 1.0). This lead to avoiding unnecessary anticancer treatments including chemotherapy or radiotherapy, which means that PERCIST 1.0 criteria were more sensitive and reliable than RECIST 1.1 for the detection of therapeutic response.
In a retrospective study conducted by Baratto et al. and compared therapeutic response in 38 patients with non-Hodgkin lymphoma by RECIST 1.1 and PERCIST criteria, evaluating both early treatment response as well as end of therapy concluded that the Deauville and PERCIST criteria were the most reliable for predicting end-of-treatment response, reporting an accuracy of 81.6%, being consistent with our results [1].
Our study results are also matched with other studies which included lymphoma and other different solid tumors. In a study done by Yanagawa et al. on 46 patients with esophageal cancer who received chemotherapy, 56.5% of patients showed discordant therapeutic response between RECIST 1.1 and PERCIST with Wilcoxon signed-rank test, P < 0.0001 [17]. Other studies done by Ding et al. and Shang et al. which included 44 and 35 patients with non-small cell lung cancer, 34.1% and 62.9% of patients showed discordant response after application of RECIST 1.1 and PERCIST [14, 18]. A 61.5% discordant rate was achieved by Bang et al. in a study conducted on 39 patients with colorectal cancer after targeted therapy [19]. Also, in a study done by Riedl et al. (included 65 patients with breast cancer who received therapy and evaluated with RECIST 1.1 and PERCIST criteria), a 52.3% discordant rate was found [20]. Studies conducted by Agrawal et al. and Aras et al. included 43 and 60 patients with different types of solid tumors (breast cancer, lung cancer, PNET, head and neck cancer, sarcoma, non-Hodgkin lymphoma, GIT cancers, and others), 20% and 18.3% of patients showed contradictory response when evaluated using RECIST 1.1 and PERCIST criteria [21, 22].
These results mean that PERCIST is used among international observers more consistently, as it provides the needed standardization of the PET protocol. This is very important because availability of consistent criteria leads to accurate comparisons between different studies and facilitates advances in cancer treatment. Moreover, the use of computer-assisted reading application that is fully customized for use with PERCIST makes the procedure of evaluation easier and helps ensure more precise results.
There are few limitations of our study. First, the corresponding pathological results to our imaging findings has not been evaluated and further studies are needed to investigate the correlation of these findings with pathological examinations. Second, adequate follow-up of patients were not achieved to correlate our results with the patients’ progression-free survival or overall survival. The main limitation was the small sample size due to the high cost of the technique, and future studies with larger number of patients may be needed to obtain more accurate results.
Conclusion
The recently applied PET/CT-based criteria (PERCIST 1.0) is efficient and more accurate than CT-based criteria (RECIST 1.1) to assess treatment response in patients with lymphoma, which subsequently help in decision making and affect further management of these patients.
Availability of data and materials
All data generated or analyzed during this study are included in this article.
Abbreviations
- 18F FDG:
-
18F-Fluorodeoxyglucose
- PET/CT:
-
Positron emission tomography/ computed tomography
- HL:
-
Hodgkin lymphoma
- NHL:
-
Non-Hodgkin lymphoma
- RECIST:
-
Response evaluation criteria in solid tumor
- PERCIST:
-
PET response criteria in solid tumor
- CR:
-
Complete response
- CMR:
-
Complete metabolic response
- PR :
-
Partial response
- PMR:
-
Partial metabolic response
- SD:
-
Stationary disease
- SMD:
-
Stationary metabolic disease
- PD:
-
Progressive disease
- PMD:
-
Progressive metabolic disease
References
Baratto L, Jegede O, Hong F, Kahl B, Evens A, Quon A (2016) Comparison between LUGANO, EORTC, PERCIST and IHP criteria at interim PET in patients with follicular non-Hodgkin’s lymphoma. Eur J Nucl Med Mol Imaging 43(20):S30–S31
Maffione AM, Paolini R, Rodella E, Ambrosio C, Rampin L, Lisato LC, Rubello D (2015) Response criteria for malignant lymphoma. Nucl Med Commun 36(4):398–405
Moghbel MC, Kostakoglu L, Zukotynski K, Chen DL, Nadel H, Niederkohr R, Mittra E (2016) Response assessment criteria and their applications in lymphoma: part 1. J Nucl Med 57(6):928–935
Ali SA, Abd Elkhalek YI (2016) Added value of combined 18F-FDG PET/CT for detection of osseous metastases in cancer patients. EJRNM 47(2):453–458
Fletcher JW, Djulbegovic B, Soares HP et al (2008) Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med 49:480–508
Ali SA, Abd Elkhalek YI (2016) Value of integrated PET/CT in detection of hepatic metastatic deposits. EJRNM 47(2):459–465
Ali SA, Hamed MA (2017) The diagnostic efficacy of whole body 18F FDG PET CT in detection of unexpected second primary malignancy in cancer patients. EJRNM 48(3):671–676
Baratto L, Davidzon G, Moghbel M, Hatami N, Iagaru A, Mittra E (2018) Comparison between different PET and CT-based imaging interpretation criteria at interim imaging in patients with diffuse large B-cell lymphoma. Clin Nucl Med 43(1):1–8
Mansour MG, Ali SA (2016) Transarterial chemoembolization using drug eluting microspheres in refractory colorectal liver metastases with 18F-FDG PET/CT follow-up to assess therapeutic response. EJRNM 47(4):1467–1472
Ali SA, Amin DH, Abdelkhalek YI (2020) Efficiency of whole-body 18F-FDG PET CT in detecting the cause of rising serum AFP level in post-therapeutic follow-up for HCC patients. Jpn J Radiol 38(5):472–479
Wahl R, Jacene H, Kasamon Y et al (2009) From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 50:122S–150S
Sheikhbahaei S, Mena E, Yanamadala A et al (2017) The value of FDG PET/CT in treatment response assessment, follow-up, and surveillance of lung cancer. AJR 208(2):420–433
Suzuki C, Jacobsson H, Hatschek T et al (2008) Radiologic measurements of tumor response to treatment: practical approaches and limitations. Radiographics 28:329–344
Shang J, Ling X, Zhang L et al (2016) Comparison of RECIST, EORTC criteria and PERCIST for evaluation of early response to chemotherapy in patients with non-small-cell lung cancer. Eur J Nucl Med Mol Imaging 43:1945–1953
William W, Pataer A, Kalhor N et al (2013) Computed tomography RECIST assessment of histopathological response and prediction of survival in patients with resectable non-small cell lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 8:222–228
Ali SA, Abdelkawi MM, Hussien NM (2019) Delayed post-diuretic 18F-FDG PET/CT: can it help in determination of the best clinical decision for muscle invasive UB cancer patients? EJRNM 50:111
Yanagawa M, Tatsumi M, Miyata H et al (2012) Evaluation of response to neoadjuvant chemotherapy for esophageal cancer: PET response criteria in solid tumors versus response evaluation criteria in solid tumors. J Nucl Med 53:872–880
Ding Q, Cheng X, Yang L et al (2014) PET/CT evaluation of response to chemotherapy in non-small cell lung cancer: PET response criteria in solid tumors (PERCIST) versus response evaluation criteria in solid tumors (RECIST). J Thorac Dis 6:677–683
Bang JI, Lim Y, Paeng JC et al (2017) Comparison of quantitative methods on FDG PET/CT for treatment response evaluation of metastatic colorectal cancer. Nucl Med Mol Imaging 51:147–153
Riedl CC, Pinker K, Ulaner GA et al (2017) Comparison of FDG-PET/CT and contrast-enhanced CT for monitoring therapy response in patients with metastatic breast cancer. Eur J Nucl Med Mol Imaging 44:1428–1437
Agrawal A, Purandare N, Shah S, Puranik A, Banavali S, Rangarajan V (2014) Response assessment in metronomic chemotherapy: RECIST or PERCIST. Indian J Nucl Med 29:74–80
Aras M, Erdil TY, Dane F et al (2016) Comparison of WHO, RECIST 1.1, EORTC, and PERCIST criteria in the evaluation of treatment response in malignant solid tumors. Nucl Med Commun 37:9–15
Acknowledgements
Not applicable
Funding
This work has not received any funding.
Author information
Authors and Affiliations
Contributions
MM carried out the PET/CT studies and collected the data. SA, AY, and AM participated in the design of the study. MM performed the statistical analysis, and SA drafted the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Research Ethics Committee (REC) of Ain Shams University, Faculty of Medicine (FMASU M D 221/ 2018) and written informed consent was obtained from all patients to participate in the study.
Consent for publication
Written informed consent was obtained from all patients for publication of the study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tawfik, M.M.H., Monib, A.M., Yassin, A. et al. Comparison between RECIST and PERCIST criteria in therapeutic response assessment in cases of lymphoma. Egypt J Radiol Nucl Med 51, 82 (2020). https://doi.org/10.1186/s43055-020-00203-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-020-00203-4