Abstract
Background
Diffusion tensor imaging (DTI) is a novel MRI technique which can provide information of the compressed tissue’s microstructural properties. The evaluation of visual function is important for the clinical management of patients with suprasellar mass lesion. Compression of the optic nerve and chiasm due to pituitary mass lesions often leads to impaired visual function. The evaluation and prognosis of visual function are important for patient management. The aim of this work is to evaluate the feasibility of DTI in the evaluation of the optic pathway in patients with suprasellar masses. Fifty patients were included in the study, 24 cases with suprasellar masses and 26 cases without suprasellar masses as a control group. All patients underwent detailed history taking, MRI of the sella with coronal and sagittal T1, and coronal T2 sequences and DTI. The region of interests (ROIs) were set on the optic chiasm as well as the anterior and posterior optic tracts. For each ROI, axial diffusivity (AD), radial diffusivity (RD), fractional anisotropy (FA), and apparent diffusion coefficient (ADC) values were calculated. DTI parameters of cases group were compared with normal control group parameters.
Results
The result of this study showed that in cases with suprasellar masses, there were significantly lower mean AD and ADC values and higher mean FA value than control group with no masses.
Conclusion
The DTI is a useful technique in the assessment of optic pathway compression degree by suprasellar masses. This will provide the clinicians and surgeons with precise information about the degree of the visual field compromise in correlation with the clinical data.
Similar content being viewed by others
Background
Pituitary adenoma is a primary tumor of pituitary gland and the most common suprasellar mass. It represents from 10 to 25% of all intracranial neoplasms .Pituitary adenoma is broadly classified according to its size into pituitary microadenoma which measures less than 10 mm, and pituitary macroadenoma which measures more than 10 mm. Majority of the patients is manifested due to mass effect, and the most common structure to be affected is the optic chiasma. The anatomical pathway of the optic nerve describes the transmission of special sensory information from the retina of the eye to the primary visual cortex of the brain, as it receives impulses from the photoreceptors of the eye and transmits it to the primary visual cortex [1].
The optic nerve (cranial nerve II) is not a true cranial nerve but a fiber tract of the brain made of retinal ganglion cell axons which, as they leave the optic disc, become myelinated by oligodendrocytes which unite the optical nerves of every eye to form the optic chiasma. The optic chiasma is a flattened band of fibers with characteristic X-shaped morphology situated at the junction of the floor and anterior wall of the third ventricle. The optic chiasma lies in the subarachnoid space of the suprasellar cistern above the sella turcica and the pituitary gland. The optic tracts emerge from the chiasma to the lateral geniculate body and the primary visual cortex [2].
The conventional MRI shows a role to evaluate visual impairment in suprasellar masses patients. Impairment of the vision is associated with hyperintensity on T2W of the optic pathway ventral to the suprasellar mass. However, hyperintensity of the optic nerve is usually evaluated by only visual analysis. Optical nerve imaging needs 1.5 T or 3 T technology. The challenge in MR imaging for the optic nerve is to conduct high-resolution studies with small fields of view on one hand and cover the whole brain on the other hand with maintaining a satisfactory time frame for the entire duration of the MRI study [3].
Diffusion tensor imaging (DTI) is a technique of MRI that is based into the directional properties of the diffusion of water molecules thereby demonstrating fiber tracts and their structure alteration due to pathology. DTI can be used efficiently in comprehensive, non-invasive, functional anatomy mapping of the nerve fibers. The structural properties as the myelination and axonal density can be assessed by diffusion tensor measures as fractional anisotropy (FA), axial diffusivity (AD), and radial diffusivity (RD) [4].
The aim of this work is to evaluate the feasibility of DTI in the evaluation of the optic pathway in patients with suprasellar masses.
Methods
Fifty patients were included in this retrospective case-control study. This includes 25 cases with suprasellar masses and 25 as a control group without suprasellar masses. The control group was age and sex matched. In the cases group, the age ranged from 23 to 45 years with the mean value of the age was 34.96 years. The study included patients with suprasellar masses who had been subsequently experiencing visual symptoms and visual field defects, which was confirmed by clinical examination and previous investigations. The research started on October 2018 and all patients signed an informed consent. Subjects refused to participate in the research and subject with contraindication to MRI as having pacemaker, aneurysm clips, joint replacement or any other electronic or magnetically activated implants, as well as claustrophobic subjects were excluded. All participants underwent careful history taking in the form of history visual disturbances as blurring of vision, history of systemic diseases, and present history of current illness. Previous investigations and visual field assessment were also considered.
The images are interpreted by two neuroradiologists who have medical doctorate degree and are experienced in neuroimaging for more than 10 years duration with 100% agreement inbetween. Statistical analysis of the present study was conducted using the mean, standard deviation, and chi-square test by SPSS V.22.
This study was performed using 1.5 T MRI scanner (GE) and Toshiba Medical Systems with no specific preparation of the patients was needed. The following MRI pulse sequences were included: 1-multiplanar T1-weighted fast spin-echo sequence (T1WFSE) coronal and sagittal coronal T1WFSE: TR = 578 m/s, TE = 9.6 m/s, field of view = 250 mm, acquisition matrix = 95 × 120, slices = 12, scan time = 2:35 min, slice thickness = 2.8 mm, and interslice gap = 0.3 mm. Sagittal T1WFSE: TR = 723 m/s, TE = 8.3 m/s, field of view = 250 mm, acquisition matrix = 95 × 120, slices = 11, scan time = 3:35 min, slice thickness = 3 mm, and interslice gap = 0.3 mm. 2-multiplanar T2-weighted fast spin-echo sequence (T2WFSE) coronal with thin cuts: TR = 3116 m/s, TE = 100 m/s, field of view = 250 mm, acquisition matrix = 95 × 120, slices = 12, scan time = 1:59 min, slice thickness = 2.8 mm, and interslice gap = 0.3 mm.
In all control group subjects, the standard MRI protocol was carried out. The DTI acquisition was performed in all subjects after anatomical imaging in the coronal plane covering the sella turcica with the following parameters: TR = 2000 ms, TE = 68 ms, FOV = 220 × 220 mm, acquisition matrix = 200 × 200, slices = 10, scan time = 6:26 min, and slice thickness = 3 mm without gap.
The DTI protocol was performed with b = 0 and b = 1000 s/mm2 in 12 motions probing gradient directions. ROIs were set at the optic chiasma on two subsequent slices and the anterior and posterior optic tracts on each slice. In cases group of patients with suprasellar mass lesions compressing the optic chiasma, ROIs were set on the optic chiasma at the site of compression. Fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD), and apparent diffusion coefficient (ADC) values were calculated for each ROI.
Results
The study included 25 patients (17 females and 8 males) and an age and sex matched control group. In both groups, the minimum age was 23 years and maximum age was 45 years. Based on visual assessment of the conventional T1WI and T2WI sequences, there was no significant signal intensity change of the compressed part of the optic pathway (Figs. 1 and 2).
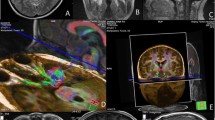
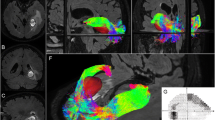
A case of 37-year-old male patient presented with headache and visual disorder. MRI of the sella coronal T1 (a) and sagittal T1 (b), coronal T2 (c), and DTI of the optic pathway were done and ROI delineation on DTI maps. Color ROI are drawn on anterior optic tracts (d), the optic chiasm (e), and the posterior optic tracts (f) showing enhancing sellar soft tissue mass measuring 1.2 × 1.1 × 1.1 mm at three dimensions with right para sellar extension where it extends within the right cavernous sinus encasing right internal carotid artery and DTI examination shows the following: FA = 0.321, 0, 308, 0.311; ADC = 1.50, 1.52, 1.57 μm2/ms; AD =2.3, 2.44, 2.4 μm2/ms; and RD = 1.1, 1.55, 1.2 μm2/ms of the anterior optic tracts, the optic chiasm, and the posterior optic tracts respectively. The increased value of FA and decreased values of ADC, AD, and RD more at the anterior optic tracts denoting compression more at the anterior optic tracts
A 45-year-old male patient presented with headache and visual disorder. MRI of the sella coronal T1 (a), sagittal T1 (b), coronal T2 (c), and DTI of the optic pathway were done and ROI delineation on DTI maps. Color ROI are drawn on anterior optic tracts (d), the optic chiasm (e), and the posterior optic tracts (f) showing enhancing sellar soft tissue mass measuring 24 × 26 mm with compression on the optic chiasm and DTI examination shows the following: FA =0.311, 0,443, 0.303; ADC = 1.6, 1.33, 1.7μm2/ms; AD = 2.2, 2.3, 2.4μm2/ms; RD = 1.2, 1.4, 1.1μm2/ms of the anterior optic tracts, the optic chiasm, and the posterior optic tracts respectively. There are increased value of FA and decreased values of ADC, AD, and RD
According to the statistical analysis, the mean value of the age was 34.96 years, standard deviation was 6.94, and the p value of age was 0.284 (Table 1). The AD value of the optic chiasm of the cases group (n = 24) was 1.91 μm2/ms ranging from 1.1 to 2.8 (Figs. 1, 2, and 3). The AD value of the optic chiasm of the control group (n = 26) was 2.52 μm2/ms ranging from 2 to 2.8 (Fig. 4 ) (Table 2).
A case of 29-year-old female patient presented with headache and amenorrhea. MRI of the sella coronal T1 (a) and sagittal T1 (b), coronal T2 (c), and DTI of the optic pathway were done and ROI delineation on DTI maps. Color ROI are drawn on d anterior optic tracts, e the optic chiasm, and f the posterior optic tracts showing non-enhancing focal lesion in the right side of the pituitary gland measuring 9 × 6 mm in diameter and DTI examination shows the following: FA =0.272, 0, 202, 0.204; ADC = 1.95, 1.97, 1.9 μm2/ms; AD = 2.2, 2.3, 2.2μm2/ms; and RD = 1.1, 0.9, 1.1 μm2/ms of the anterior optic tracts, the optic chiasm, and the posterior optic tracts respectively
A control case of 21-year-old female patient presented with headache showing normal MRI study of sella. MRI of the sella coronal T1 (a) and sagittal T1 (b), coronal T2 (c), and DTI of the optic pathway were done and ROI delineation on DTI maps. Color ROI are drawn on anterior optic tracts (d), the optic chiasm (e), and the posterior optic tracts (f) showing that the pituitary gland is contained within the sella with concave upper border. No evidence of pituitary focal lesions and normal appearance of suprasellar cistern. DTI examination shows the following: FA = 0.089, 0.26, 0.192; ADC = 2.89, 2.03, 1.98μm2/ms; AD = 2.4, 2.5, 2.6 μm2/ms; and RD = 1.6, 1.4, 1.8 μm2/ms of the anterior optic tracts, the optic chiasm, and the posterior optic tracts respectively
The RD value of the optic chiasm of the cases group (n = 24) was 1.11 μm2/ms ranging from 0.6 to1.55 (Figs. 1, 2, and 3). The RD value of the optic chiasm of the control group (n = 26) was 0.95 μm2/ms ranging from 0.6 to1.44 (Fig. 4 ) (Table 3).
The FA value of optic chiasm of the cases group (n = 24) was 0.31 ranging from 0.25 to 0.44 (Figs. 1, 2, and 3). The FA value of the optic chiasm of the control group (n = 26) was 0.22 ranging from 0.15 to 0.27 (Fig. 4) (Table 4).
The ADC value of the optic chiasm of the cases group (n = 24) was 1.65 μm2/ms ranging from 1.22 to 1.99 (Figs. 1, 2, and 3). The ADC value of the optic chiasm of the control group (n = 26) was 2.10 μm2/ms ranging from 1.26 to 2.51 (Fig. 4) (Table 5).
A comparison between the studied groups according to AD, RD, FA, ADC, values of anterior optic tracts showed that there is no significant difference in the DTI parameters (Table 6). A comparison between the studied groups according to AD, RD, FA, ADC, values of posterior optic tracts showed that there is no significant difference in the DTI parameters (Table 7).
A correlation analysis between DTI parameters and visual field disorder confirmed that the optic chiasm of patients with visual field disorder showed significant lower AD and ADC values and higher FA value than those without the disorder while RD showed no significant difference (Table 8).
The results showed that in cases with suprasellar masses, there was significantly lower mean AD and ADC values and higher mean FA value than control group with no masses.
Discussion
DTI is a new MRI technology which may give data on the compressed tissue’s microstructural properties. This study assessed if DTI can accurately evaluate optic pathway in patients with suprasellar masses. DTI is based on the anisotropic properties of water molecule motion in white matter fiber. The motion of water molecules with the axis of the fiber is relatively free, while it is more limited if it is perpendicular to it. Accordingly, this technique can track the fibers on a pixel by pixel base and can measure the anisotropic properties by parameters as fraction anisotropy (FA). DTI is a non-invasive technique that detects structural changes. Although there is plethora of reports on DTI in brain disorders, there are only few studies which detect changes in white matter structure. Barker’s group (Wieshmann [4] and Eriksson [5]) was the first to find that DTI may detect precisely displacements in the white matter fibers and found that the compression of white matter fibers leads to increase in anisotropy.
Another study by Witwer et al. used directionally color-coded maps or directionally encoded color (DEC) mapping of DTI to track white matter displacement. Also, Assaf et al. [6] have found that DTI parameters can help tracking white matter displacement in 2D DEC-FA maps.
Compression by a mass causes structural changes as loss of the myelin sheath, axonal degeneration, cell or neuronal degeneration, and gliosis. White matter compression by a tumor leads to increase in FA values, and another cause of the DTI measures change may be the edema like changes which leads to distension of Virchow-Robin spaces adjacent to the optic tract [6].
Group of reports studied the MRI findings of the optic pathways in multiple diseases. Tokumaru et al. [7] found that hyperintense signal of the optic chiasma due to the pituitary macroadenoma was associated with visual acuity impairment.
The change of the DTI measures of optic pathway, due to compression by masses lesions, may be a good prognostic factor of the visual function. DTI measures as the FA, AD, and RD may show structural criteria as the degree of myelination and axonal density. These measures give data about the white matter microstructure that cannot be evaluated by conventional MRI. Anik et al. [8] reported that if after endoscopic pituitary surgery, the mean FA value is reduced and the mean diffusivity value is elevated of the optic pathway, it is an indicator of there is no improvement.
This study hypothesized that the suprasellar masses compressing the optic pathway cause changes in the DTI parameters values. The studied patients revealed that, on using DTI, when compared values in cases with suprasellar masses and control cases without masses, significant changes were found in the FA, AD, and ADC values. The FA values of the optic chiasma in cases group with mass compression increased compared to the control group. The AD and ADC values of the optic chiasma in cases group with suprasellar mass significantly decreased compared to the control group, and this study was in accordance to Yamada et al. In their study, optic chiasma of cases with compressive suprasellar masses showed significantly decrease in mean AD, ADC, and RD values compared to mean values of patients with no masses [9].
Yamada et al. [9] found in a study performed on 24 consecutive cases with intra or parasellar masses; they performed diffusion tensor magnetic resonance imaging of optic pathway for all the patients. They revealed that AD and RD values showed significant changes in cases with masses than those of patients without masses compression. Optic chiasma of cases with visual field defect showed significant decrease in AD and RD values more than patients without the visual field defect.
Schonberg et al. [10] were studying the characters of the displaced white matter by brain masses and evaluated the different diffusion tensor measures. They found that the RD decreased, while the AD increased, and that lead to an increase in the FA with no significant difference in the ADC. The mechanism of these changes is the increase in the fiber density and tension, and another mechanism might be increasing in the homogeneity of the fibers by a tighter alignment of the fibers within the fiber bundle.
This study was in accordance to Schonberg et al. [10] in the compressive mass effect on the optic chiasm revealed significant increase in FA value, but this study disagreed with them as it detected that on compression of optic chiasma, there were significant decrease in the AD and ADC values.
Singman et al. [11] were studying the indirect traumatic effect of optic neuropathy. The affected optic pathway revealed that mean FA value progressively decreased, and mean RD value progressively increased.
The DTI parameters of the control cases without suprasellar mass compressing the optic pathway revealed lower FA and higher AD, RD, and ADC values in comparison with other studies. There are a lot of factors explaining these differences as the partial volume effect of the surrounding CSF, the manual setting of ROIs position, signal to noise ratio, imaging orientations, spatial resolution, and CSF and fat suppression ratio [9].
In terms of the correlation between clinical, visual field assessment, and radiological findings, this study confirmed that there are significant changes in the DTI parameters in patients with visual field disorder, which makes DTI a crucial and essential tool in the evaluation of patients with suprasellar masses. DTI will provide the clinicians with accurate numbers that reveal to which degree the optic pathway is affected. For this reason, DTI is highly recommended when performing MRI for suprasellar masses.
Regarding our present study limitations, there are two limitations. The first one, and the main drawback in DTI analysis, is the length of the acquisition time as the duration of the examination is 6 min 26 s, and the second one is related to the ROIs’ size, which were small and may have included contamination from the surrounding structures.
Conclusion
In conclusion, this study found that the DTI is a useful technique in the assessment of optic chiasm compression degree in patients with suprasellar masses by detection of changes in different DTI parameters in comparison to control patients. This will provide the clinicians and surgeons with precise information about the degree of the visual field compromise in correlation with the clinical data.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- MRI:
-
Magnetic resonance imaging
- DTI:
-
Diffusion tensor imaging
- ROI:
-
Region of interest
- AD:
-
Axial diffusivity
- RD:
-
Radial diffusivity
- FA:
-
Fractional anisotropy
- ADC:
-
Apparent diffusion coefficient
- T:
-
Tesla
- T1WFSE:
-
T1-weighted fast spin-echo sequence
- T2WFSE:
-
T2-weighted fast spin-echo sequence
- TR:
-
Time of repetition
- TE:
-
Time to echo
- DEC:
-
Directionally encoded color
- CSF:
-
Cerebrospinal fluid
References
Chaidas K, Toma S, Hopkins C (2014) Anatomical variation of the extracranial course of the optic nerve in the floor of the sphenoid sinus. J Laryngol Otol. 128(5):481
Tayebi A, Borba L, Lawton MT et al (2019) Interdural course of the ophthalmic artery in the optic canal. J Neurosurg 4:1–7
Becker M , Masterson K , Delavelle J et al (2010) Imaging of the optic nerve. European journal of radiology 74(2):299-313 ·
Wieshmann UC, Symms MR, Parker GJ et al (2000) Diffusion tensor imaging demonstrates deviation of fibres in normal appearing white matter adjacent to a brain tumour. J Neurol Neurosurg Psychiatry 68(4):501–503
Eriksson BM, Sjölin J, Claesson K et al (2001) Circulating soluble vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 in immunocompetent and renal transplant patients: correlation with cytomegalovirus disease and renal function. Scand J Infect Dis. 33(5):350–354
Witwer BP, Moftakhar R, Hasan KM et al (2002) Diffusion-tensor imaging of white matter tracts in patients with cerebral neoplasm. J Neurosurg. 97(3):568–575
Assaf BA, Mohamed FB, Abou-Khaled KJ (2003) Diffusion tensor imaging of the hippocampal formation in temporal lobe epilepsy. AJNR Am J Neuroradiol. 24(9):1857–1862
Tokumaru AM, Sakata I, Terada H et al (2006) Optic nerve hyperintensity on T2-weighted images among patients with pituitary macroadenoma: correlation with visual impairment. AJNR Am J Neuroradiol 27(2):250–254
Anik I , Anik Y , Cabuk Be al (2018) Visual outcome of an endoscopic endonasal transsphenoidal approach in pituitary macroadenomas: quantitative assessment with diffusion tensor imaging early and long-term results. World Neurosurg. 112:e691-e701.
Yamada M, Emmanuele V , Sanchez-Quintero MJ et al (2016) Can compromise mitochondrial replacement by nuclear transfer in human oocytes. Cell Stem Cell. 2;18(6):749-754.
Singman EL, Daphalapurkar N, White H et al (2016) Indirect traumatic optic neuropathy. Mil Med Res. 11;3:2.
Acknowledgements
Not applicable
Funding
This study had no funding from any resource.
Author information
Authors and Affiliations
Contributions
1. N. H.: data collection and analysis, reviewing literature, share in statistical analysis, manuscript editing, and prepare figures and tables
2. M. A.: suggest and develop the research idea, reviewing literature, data collection and analysis, perform statistical analysis, write and revise the manuscript
3. H. H.: data collection and analysis, reviewing literature, share in statistical analysis, manuscript editing, and prepare figures and tables
4. A. R.: data collection and analysis, reviewing literature, share in statistical analysis, manuscript editing, and prepare figures and tables
All authors have read and approved the final manuscript. I confirm that the content of the manuscript has not been published, or submitted for publication elsewhere.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of the Faculty of Medicine at Tanta University in Egypt in December 2017. All patients included in this study gave written informed consent to participate in this research. Please note that reference number is not available.
Consent for publication
All patients included in this research gave written informed consent to publish the data contained within this study. If the patient was unconscious when consent for publication was requested, written informed consent for the publication of this data was given by their legal guardian.
Competing interests
There is no any financial or non-financial competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Hady, N.A.A., Alashwah, M.M.A., Emam, H.H. et al. Diffusion tensor magnetic resonance imaging of optic pathway in cases of suprasellar masses. Egypt J Radiol Nucl Med 51, 87 (2020). https://doi.org/10.1186/s43055-020-00179-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-020-00179-1