Abstract
Background
Various abdominal vessels can compress the adjacent structures or in turn can get compressed by them. Most of these compression syndromes present with non-specific symptoms. Unlike the common causes of acute abdomen, the various vascular compression syndromes have bizarre clinical presentations and subtle imaging findings, which can easily be missed by the physicians as well as the radiologists.
Main body of the abstract
This is a retrospective study which was done for a period of 3 years from April 2015 to April 2018 using a 64-slice CT scanner. Among 2412 cases that came for evaluation, 114 patients were diagnosed to have one of the various vascular compression syndromes. These 114 cases were further managed either conservatively or surgically depending on the pathology and the severity of the compression. The syndromes discussed in this article include median arcuate ligament syndrome (29 cases), superior mesenteric artery syndrome (23 cases), portal biliopathy (3 cases), nutcracker syndrome (6 cases), pelvi-ureteric junction obstruction due to crossing of vessels (8 cases), and retrocaval ureter and May-Thurner syndrome (45 cases).
Conclusions
The primary goal of this article is to reinforce the knowledge of the radiologists of the various vascular compression syndromes and to make them possess a high degree of vigilance to detect them. This article elaborates the imaging findings of these syndromes and the role of multidetector CT angiography in diagnosing them.
Similar content being viewed by others
Background
Various abdominal vessels can compress the adjacent structures or, in turn, can get compressed by them. It is important not to over-diagnose or treat patients that are incidentally detected as they may have anatomical factors that predispose to vascular compression who are otherwise asymptomatic. However, when symptomatic, they can lead to a diagnostic dilemma as most of these compression syndromes are easily missed. The pathogenesis of most of these syndromes is controversial and can present with non-specific symptoms like epigastric or flank pain, nausea, vomiting, hematuria, or urinary tract infection. More common causes of acute abdomen (like appendicitis and cholecystitis) have numerous classic signs and symptoms as well as tell-tale imaging features, which make their diagnosis straightforward. This is not the case for the vascular compression syndromes wherein the clinical presentations are quite vague and imaging findings are quite subtle, to be easily missed by the physicians as well as the radiologists. Multidetector computed tomography (MDCT) is the imaging modality of choice to illustrate the vascular compression in abdomen and pelvis as they have a higher spatial and temporal resolution, multiplanar two-dimensional (2D) and three-dimensional (3D) post-processing, high accuracy, easy accessibility, and non-invasive as compared to conventional angiography.
In this article, we discuss the various vascular compression syndromes of the abdomen and pelvis in terms of relevant anatomy, pathogenesis, clinical features, typical imaging findings on MDCT (Table 1), and treatment options which include surgical or endovascular techniques, especially in venous compressions.
Main text
Median arcuate ligament syndrome
Median arcuate ligament syndrome (MALS), also known as celiac artery compression syndrome or Dunbar syndrome, was first described in 1963 by Harjola [1]. The median arcuate ligament is an arch-like fibrous band that unites the diaphragmatic crura on either side of the aortic hiatus at the level of the first lumbar vertebral body. The ligament usually passes superior to the origin of the celiac artery; however, in 10–24% of the subjects, the ligament may cross anterior to the proximal portion of the celiac artery and compress the celiac axis resulting in compromised blood flow [2]. It is commonly seen in young patients aged between 20 and 40 years with a slight female predilection. The affected individual presents with chronic postprandial abdominal pain, weight loss, and epigastric bruit.
Imaging findings
The typical findings of MALS on CT angiography include focal narrowing of the proximal celiac artery with a characteristic hooked appearance, more pronounced in end-expiration/inspiration. This appearance is better appreciated in the sagittal view which helps to distinguish MALS from atherosclerotic narrowing (Fig. 1). The other findings appreciated are post-stenotic dilatation in severe stenosis and collaterals vessels such as pancreaticoduodenal arcade from the superior mesenteric artery.
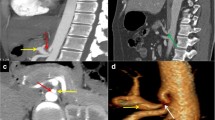
A 37-year-old case with median arcuate ligament syndrome. (a) shows a hand diagram of celiac trunk origin illustrating the compression of the origin of the coeliac trunk by the median arcuate ligament with post stenotic dilatation. Axial view of the contrast-enhanced CT study of the abdomen in arterial phase (b) shows the median arcuate ligament (arrow) compressing the origin of the coeliac trunk with post stenotic dilatation (asterisk). Sagittal reformatted MIP (c) and volume-rendered (d) images show the median arcuate ligament compressing the origin of the coeliac trunk with post stenotic dilatation. The hand diagram is self-drawn by the author
Management
There is no definitive consensus in the management of MALS. The definitive treatment for a clinical and radiologically proven case of MALS is a surgical or laparoscopic division of median arcuate ligament to restore normal blood flow in the celiac artery (Fig. 2) [3]. However, complex surgical procedures such as the vascular reconstruction of the celiac axis, aortoceliac bypass, and reimplantation of the celiac artery may be required in some patients [4].
Intraoperative image of the same case in Fig. 1 with median arcuate ligament syndrome. The median arcuate ligament is first identified (a) and is dissected bluntly to release (b and c) the pressure on the celiac trunk origin
Superior mesenteric artery syndrome
SMA syndrome also known as Wilkie’s syndrome was first described in 1927 as a rare cause of obstruction of the third part of the duodenum due to compression between the SMA and the aorta. The prevalence of SMA syndrome is around 0.13–0.3% with a female predilection and nearly two-thirds of affected individuals are between 10 and 39 years of age [5].
The SMA arises at the L1–2 level and courses anteriorly and inferiorly, forming an angle with the aorta known as the aorto-mesenteric angle (AMA). The third portion of the duodenum crosses between the aorta and the proximal SMA at approximately the L3 level. Normally, the third portion of the duodenum is surrounded by retroperitoneal fat, which provides a “cushion” for the duodenum between the anterior SMA and posterior aorta and helps maintain a wide aorto-mesenteric distance (AMD) and aorto-mesenteric angle (AMA). Various studies have reported the normal range of the AMD and AMA and to be 25° to 60° and 10–28 mm, respectively [6].
Symptoms include postprandial epigastric pain and fullness, nausea, vomiting, weight loss, and anorexia. The pain may classically be relieved by lying on prone or left lateral decubitus position [7].
Imaging findings
CT is best performed in the late angiographic phase for the simultaneous optimal depiction of the vascular anatomy and the bowel wall. In SMA syndrome, both the AMA and AMD are reduced with a value of 6° to 15° and 2 to 8 mm, respectively (Figs. 3 and 4) [6]. The proximal duodenum and stomach are dilated due to obstruction.
A 27-year-old male with superior mesenteric artery syndrome. (a) shows hand diagram of aorto mesenteric junction in sagittal view illustrating the normal aorto mesenteric angle and the reduced aorto mesenteric angle in SMA syndrome. Axial view (b) and reformatted sagittal view (c) contrast-enhanced CT study of the abdomen in arterial phase shows the third part of the duodenum (asterisk) being compressed between the superior mesenteric artery (arrow) and the aorta (arrowhead)
Same patient as in Fig. 3 with SMA syndrome. Axial view (a) and sagittal view (b) of the abdomen in the late arterial phase shows reduced aortomesenteric distance measuring (AMD—8.3 mm). The second part of the duodenum and the stomach is moderately dilated
Management
The treatment is usually conservative and consists of fluid and electrolyte balance, small liquid meals, nasojejunal feeding to bypass the level of obstruction, and mobilization of the patient into left lateral decubitus or prone position [8]. Surgical options include duodenojejunostomy, gastrojejunostomy, or lysis of the ligament of Treitz with derotation of the bowel—Strong’s procedure [9].
Portal biliopathy
Portal biliopathy or portal ductopathy refers to the biliary obstruction that is associated with cavernous transformation of the portal vein due to portal vein thrombosis [10]. The patients are usually asymptomatic. Rare clinical presentations with jaundice, cholangitis, or choledocholithiasis could also be seen. Stricture formation is a well-known complication [11].
Imaging findings
CT clearly depicts the cavernous transformation of the portal vein, marked dilatation of the intra- and extrahepatic portions of the parabiliary and peribiliary plexuses, and gallbladder varices (Fig. 5) [12]. MDCT can show secondary biliary ductal dilatation caused by the portal collaterals thus excluding a cholangiocarcinoma or extrinsic compression by metastatic adenopathies as the cause of obstruction [13].
A 51-year-old case with portal biliopathy. (a) shows hand diagram in coronal view illustrating the dilated intrahepatic biliary radicles secondary to compression of the common bile duct by the dilated and serpigenous portal cavernoma. Coronal and axial views (b and c) contrast-enhanced CT study of the abdomen in venous phase shows cavernous transformation of the portal vein (asterisk) at the porta hepatis causing compression with proximal dilatation of the common bile duct (arrow). (d) shows dilated intrahepatic biliary radicle involving the left lobe of the liver (arrow). The hand diagram is self-drawn by the author
Management
Asymptomatic portal biliopathy does not require treatment. In a symptomatic case, the treatment ranges from endoscopic treatment which includes balloon catheter dilation, endoscopic papillotomy, and stent insertion. When endoscopic decompression fails, transjugular intrahepatic portosystemic shunt (TIPS) could be considered. Biliary intestinal bypass is the next treatment of choice, when TIPS fails to significantly reduce symptoms [14].
Nutcracker syndrome
The term “nutcracker phenomenon” refers to the entrapment of the left renal vein between the superior mesenteric artery and the abdominal aorta, leading to impaired venous outflow from the left kidney as well as the gonadal vein. When associated with clinical symptoms, this entity is termed as “nutcracker syndrome” [15]. The condition was first described as ‘left renal vein entrapment syndrome’ by Grant in 1937 and can occur in any age group with a slight female predominance [16]. Gender prevalence is, however, controversial [17].
The pubertal growth spurt can be attributed as a causative factor, as the increase in height can lead to narrowing of the angle between the superior mesenteric artery and aorta [18]. When this angle becomes lesser than 35°, as evident on a CT, it supports the diagnosis of anterior nutcracker syndrome. The much less common posterior nutcracker syndrome occurs due to compression of an abnormal retro-aortic left renal vein between the abdominal aorta and the vertebral body.
These patients can have a highly variable presentation—from being asymptomatic to severe pelvic congestion. The commonest complaint reported by far is microscopic/macroscopic hematuria, which is a result of increased venous pressure in the collecting system. The other symptoms include pelvic pain, flank pain, varicocele, ovarian vein syndrome [19].
Imaging findings
Visualization of the compressed left renal vein along with distended gonadal veins and pelvic congestion guide to the diagnosis (Figs. 6 and 7). The “beak sign” on CT due to the compressed part of the left renal vein and the left renal vein diameter ratio more than or equal to 4.9 (between the hilar and aorto-mesenteric parts) are of high diagnostic value. However, the gold standard investigations include phlebography and intravascular ultrasound [20].
27-year-old male with nutcracker syndrome. (a) shows hand diagram in sagittal view shows the compressed left renal vein between the SMA and the aorta with dilated left gonadal vein draining into the left renal vein in en face view. Axial view (b) contrast-enhanced CT study of the abdomen in venous phase shows the left renal vein (dot) being compressed by the superior mesenteric artery (arrowhead) and the aorta (left arrow). Reformatted sagittal image (c) clearly depicts the compressed left renal vein between the SMA and the aorta. The left renal vein (long arrow) proximal to the compression is moderately dilated. The hand diagram is self-drawn by the author
Same case as in Fig. 6 with nutcracker syndrome. Reformatted axial MIP image (a) clearly depicts the compressed left renal vein between the SMA and the aorta. The axial section at the level of pelvis (b) shows the dilated and tortuous left gonadal vein
Management
Patients with mild symptoms are managed conservatively—advised weight gain, ACE inhibitors alacepril and aspirin. In non-responsive cases and patients with persisting symptoms, surgical management has been opted, the first line being renal vein transposition along with hybrid repair [21].
Pelvi-ureteric junction obstruction due to crossing of vessels
Obstruction at the junction of the renal pelvis and the proximal ureter, commonly seen as hydronephrosis, can occur due to intrinsic or extrinsic causes. Intrinsic causes being more common in infants and young children, the crossing of vessels is the most common extrinsic cause for PUJ obstruction in elder children and adolescents. As evident from literature, the reported incidence of this condition in early childhood is between 11 and 18.5% and that in late childhood is between 49 and 58% [22]. The common presentations can be flank pain, nausea, vomiting, and flank/abdominal mass due to an enlarged kidney.
Imaging features
The most common vessel attributed to the compression is an aberrant branch of a renal vessel that crosses the pelviureteric junction anteriorly. As a result, there will be moderate to marked hydronephrosis. MDCT in the late-arterial phase allows depiction of both arteries and veins. MDCT with MPR and 3D VR provides an excellent depiction of crossing vessels (Figs. 8 and 9), with a reported positive predictive value of 100% [23]. When available, a functional magnetic resonance urography (fMRU) is of higher value in providing both anatomical—crossing aberrant vessel and functional information [24].
28-year-old male with hydronephrosis secondary to PUJ obstruction due to anterior crossing of the accessory right renal artery. (a) shows hand diagram in coronal view illustrating this condition. Axial and sagittal views (b and c) of contrast-enhanced CT study of the abdomen in arterial phase show the dilated pelvicalyceal system due to the compression at the level of pelviureteric junction due to anterior crossing of the accessory right renal artery (arrow). The hand diagram is self-drawn by the author
Same case as in Fig. 8 with PUJ obstruction. Coronal reformatted MIP image (a) in the arterial phase shows the main and accessory right renal arteries seen superior and inferior to the dilated pelvis, respectively. Coronal reformatted MIP image in the delayed phase (b) and volume-rendered 3D image (c) shows the site of PUJ obstruction (arrow) with secondary hydronephrosis
Management
Anderson-Hyne’s dismembered pyeloplasty is the most preferred surgical procedure, which involves excising a small segment of the ureter and transposing the aberrant vessel behind the PUJ. The other options include the Hellstrom technique and Chapman technique [25].
Retrocaval ureter
Retrocaval ureter also referred to as circumcaval ureter or pre-ureteral vena cava is a rare congenital anomaly with the right ureter passing posterior to the inferior vena cava, resulting in hydroureteronephrosis [26]. It is considered as a developmental anomaly of the IVC rather than that of the ureter. The onset of symptoms is usually in the fourth decade and males predominate by a ratio of 3:1. The usual clinical manifestations are right flank pain, hematuria, recurrent urinary infections, and recurrent pyelonephritis [27]. Long-standing HUN can lead to cortical scarring and progressive renal failure.
Imaging features
MDCT and IVU are the two commonest imaging investigations done to evaluate the retrocaval ureter. However, MDCT is preferred, with or without contrast, as it can demonstrate accurately other causes of hydroureteronephrosis. In MDCT, the proximal ureter can be distinctly seen to course posterior to the IVC (Fig. 10) and emerge to the right of the aorta, coming to lie anterior to the right iliac vessels. Evidence of moderate to marked right HUN above the segment abutting the IVC can distinctly be seen using the multiplanar reformats (Fig. 11). Retrocaval ureters are classified into two clinical types: type 1 (commonest, moderate to severe HUN) and type 2 (less severe form) [28].
26-year-old male with retrocaval ureter. (a) shows hand diagram in coronal view illustrating the retrocaval course of the right ureter with secondary hydroureteronephrosis. Axial and coronal views (b and c) of contrast-enhanced CT study of the abdomen in delayed phase show the compressed ureter (arrowhead and asterisk) in between the inferior vena cava and right psoas. The hand diagram is self-drawn by the author
Same patient as in Fig. 10 with retrocaval ureter. Coronal reformatted MIP (a) and volume-rendered 3D (b) images show the narrowed right proximal ureter (left arrow) coursing posterior the IVC with mild dilatation of the pelvicalyceal system and the proximal ureter
Management
Treatment options include observation for those who are asymptomatic, reconstructive surgery (Fig. 12) for those with hydronephrosis or nephrectomy if there is cortical atrophy. During surgery, the retrocaval segment is often resected as it remains aperistaltic [27].
May-Thurner syndrome
May-Thurner syndrome (MTS) (also called iliac vein compression syndrome) is a condition that arises as a result of compression of the left common iliac vein by the right common iliac artery. It is also one of the reasons postulated for the increased incidence of deep vein thrombosis in the left lower limb [29]. MTS is particularly prevalent in younger and middle-aged women (mean age = 42 years) [30]. MTS can be suspected in a patient who has unilateral left lower limb swelling and pain without preceding trauma or evidence of infection. Venous ulcerations, varicose veins, and recurrent DVT are the commonest complications of MTS.
Imaging features
Catheter venography was considered the gold standard in diagnosing this condition; however, cross-sectional imaging is currently being preferred as one can obtain additional extra-vascular information. A visualization of greater than 50% stenosis in the luminal diameter of the vein is considered an adequate indicator of LCIV compression related to MTS (Fig. 13) [31]. Complications include thrombosis of the left iliac and proximal femoral veins. Also, CT venography also demonstrates the presence of pelvic collaterals and the presence of intraluminal iliac spurs.
65-year-old female with May-Thurner syndrome. (a) shows the hand diagram in axial view illustrating the compression of left common iliac vein by the right common iliac artery against the vertebral body. Axial view (b) of contrast-enhanced CT study of the abdomen in venous phase shows the compression and resultant thrombosis of the left common iliac vein. Multiple arrows in c illustrate the course of thrombosed left common iliac, external iliac, and common femoral veins as a result of proximal compression of the left common iliac vein. The hand diagram is self-drawn by the author
Management
Conservative and invasive/surgical treatments are now being considered outdated. The use of endovascular techniques in the treatment of MTS patients is considered successful and carries lesser risk than invasive surgical treatments. Angioplasty has been found to be associated with low long-term patency rates.
Conclusion
Vascular compression syndromes are silent and occult causes of acute abdomen with non-specific signs and symptoms. These conditions are easily missed unless the radiologists are aware of them and are on the look-out. Contrast-enhanced computed tomography is the imaging modality of choice to detect their presence. This article elaborates the imaging findings of the various vascular compression syndromes and emphasizes the radiologists in having a high degree of vigilance to detect these conditions, as the clinical presentations are quite vague and imaging findings are quite subtle. Early diagnosis and management leads to avoidance of inadvertent complications and decreased patient stay in the hospital.
Availability of data and materials
The data are taken solely from our institution. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACE:
-
Angiotensin-converting enzyme
- AMA:
-
Aorto-mesenteric angle
- AMD:
-
Aorto-mesenteric distance
- fMRU:
-
Functional magnetic resonance urography
- HUN:
-
Hydroureteronephrosis
- IVC:
-
Inferior vena cava
IVU
Intravenous urogram
- LCIV:
-
Left common iliac vein
- MALS:
-
Median arcuate ligament syndrome
- MDCT:
-
Multidetector computed tomography
- MTS:
-
May-Thurner syndrome
- PUJ:
-
Pelvi-ureteric junction
- SMA:
-
Superior mesenteric artery
- TIPS:
-
Transjugular intrahepatic portosystemic shunt
References
Lamba R, Tanner DT, Sekhon S, McGahan JP, Corwin MT, Lall CG (2014) Multidetector CT of vascular compression syndromes in the abdomen and pelvis. Radiographics 34(1):93–115
Tembey RA, Bajaj AS, Wagle PK, Ansari AS (2015) Real-time ultrasound: key factor in identifying celiac artery compression syndrome. Indian J Radiol Imaging 25(2):202–205
Duffy AJ, Panait L, Eisenberg D, Bell RL, Roberts KE, Sumpio B (2009) Management of median arcuate ligament syndrome: a new paradigm. Ann Vasc Surg 23:778–784
Hongsakul K, Rookkapan S, Sungsiri J, Tubtawee T (2012) A severe case of median arcuate ligament syndrome with successful angioplasty and stenting. Case Rep Vasc Med 2012:129870. https://doi.org/10.1155/2012/129870
Welsch T, Büchler MW, Kienle P (2007) Recalling superior mesenteric artery syndrome. Dig Surg 24(3):149–156
Singal R, Sahu PK, Goel M, Gupta S, Gupta R, Gupta A et al (2010) Superior mesenteric artery syndrome: a case report. N Am J Med Sci 2(8):392–394
Desai MH, Gall A, Khoo M (2015) Superior mesenteric artery syndrome - a rare presentation and challenge in spinal cord injury rehabilitation: a case report and literature review. J Spinal Cord Med 38(4):544–547
Agrawal GA, Johnson PT, Fishman EK (2007) Multidetector row CT of superior mesenteric artery syndrome. J Clin Gastroenterol 41(1):62–65
Merrett ND, Wilson RB, Cosman P, Biankin AV (2009) Superior mesenteric artery syndrome: diagnosisand treatment strategies. J Gastrointest Surg 13(2):287–292
Dhiman RK, Behera A, Chawla YK, Dilawari JB, Suri S (2007) Portal hypertensive biliopathy. Gut 56(7):1001–1008
Walser EM, Runyan BR, Heckman MG, Bridges MD, Willingham DL, Paz-Fumagalli R, et al (2011) Extrahepatic portal biliopathy: proposed etiology on the basis of anatomic and clinical features. Radiology 258:1,146-153.
Ozkavukcu E, Erden A, Erden I (2009) Imaging features of portal biliopathy: frequency of involvement patterns with emphasis on MRCP. Eur J Radiol 71(1):129–134
Besa C, Cruz JP, Huete A, Cruz F (2012) Portal biliopathy: a multitechnique imaging approach. Abdom Imaging 37(1):83–90
Jeon SJ, Min JK, Kwon SY, Kim JH, Moon SY, Lee KH et al (2016) Portal biliopathy treated with endoscopic biliary stenting. Clin Mol Hepatol 22(1):172–176
Kurklinsky AK, Rooke TW (2010) Nutcracker phenomenon and nutcracker syndrome. Mayo Clin Proc 85(6):552–559
Ananthan K, Onida S, Davies AH (2017) Nutcracker syndrome: an update on current diagnostic criteria and management guidelines. European Journal of Vascular and Endovascular Surgery 53(6):886–894
He Y, Wu Z, Chen S, Tian S, Tian L, Li D et al (2014) Nutcracker syndrome—how well do we know it? Urology 83(1):12–17
Mahmood SK, Oliveira GR, Rosovsky RP (2013) An easily missed diagnosis: flank pain and nutcracker syndrome. BMJ Case Rep. 2013:bcr2013009447. https://doi.org/10.1136/bcr-2013-009447
Inal M, Karadeniz Bilgili MY, Sahin S (2014) Nutcracker syndrome accompanying pelvic congestion syndrome; color doppler sonography and multislice ct findings: a case report. Iran J Radiol 11(2):e11075
Ananthan K, Onida S, Davies AH (2017) Nutcracker syndrome: an update on current diagnostic criteria and management guidelines. European Journal of Vascular and Endovascular Surgery 53(6):886–894
Stawiarski K, Wosnitzer M, Erben Y (2017) A novel hybrid left renal vein transposition and endovascular stenting technique for the treatment of posterior nutcracker syndrome. J Vasc Surg Cases Innov Tech 3(3):142–145
Rooks VJ, Lebowitz RL (2001) Extrinsic ureteropelvic junction obstruction from a crossing renal vessel: demography and imaging. Pediatr Radiol 31(2):120–124
Khaira HS, Platt JF, Cohan RH, Wolf JS, Faerber GJ (2003) Helical computed tomography for identification of crossing vessels in ureteropelvic junction obstruction: comparison with operative findings. Urology 62(1):35–39
Guven A (2016) Crossing renal vessel causing ureteropelvic junction obstruction in children. J Integr Nephrol Androl 3:31–32
Esposito C, Bleve C, Escolino M, Caione P, Gerocarni Nappo S, Farina A et al (2016) Laparoscopic transposition of lower pole crossing vessels (vascular hitch) in children with pelviureteric junction obstruction. Transl Pediatr 5(4):256–261
Kyei MY, Yeboah ED, Klufio GO, Mensah JE, Gepi-Atee S, Zakpaa L et al (2011) Retrocaval ureter: two case reports. Ghana Med J 45(4):177–180
Batura D, Saxena VK (2017) Retrocaval ureter - a rare cause of hydronephrosis. Med J Armed Forces India 53:223–225
Hassan R, Aziz AA, Mohamed SK (2011) Retrocaval ureter: the importance of intravenous urography. Malays J Med Sci 18:84–87
Cil BE, Akpinar E, Karcaaltincaba M, Akinci D (2004) Case 76: May-Thurner syndrome. Radiology 233(2):361–365
Brinegar KN, Sheth RA, Khademhosseini A, Bautista J, Oklu R (2015) Iliac vein compression syndrome: clinical, imaging and pathologic findings. World J Radiol 7:375–381
Liu Z, Gao N, Shen L, Yang J, Zhu Y, Li Z, Si Y (2014) Endovascular treatment for symptomatic iliac vein compression syndrome: a prospective consecutive series of 48 patients. Ann Vasc Surg 28:695–704
Acknowledgements
The author would like to thank his great and beloved teachers, his closest friends, and his mentors for their aids in making him complete this article.
Funding
This article did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
LS and RR did the major write up of this review article. Majority of the cases in this review article were diagnosed and followed up by PPV and VB. The work was carried under the guidance of PR who is the abdominal radiologist in our institution. PR provided us the insight and knowledge to diagnose indeterminate lesions with imaging alone. All the authors read through the rough draft and provided valuable suggestions for the final draft. PP reviewed this article for corrections and final draft. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable (retrospective study)
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sankaran, L., Ramachandran, R., Bala Raghu Raji, V. et al. The role of multidetector CT angiography in characterizing vascular compression syndromes of the abdomen. Egypt J Radiol Nucl Med 50, 55 (2019). https://doi.org/10.1186/s43055-019-0063-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-019-0063-2