Abstract
Background
The lack of early diagnostic tools and the development of chemoresistance have made ovarian cancer (OC) one of the deadliest gynaecological cancers. The tumour microenvironment is characterised by the extracellular release of high levels of ATP, which is followed by the activation of P1 adenosinergic and P2 purinergic signalling systems. The sequential hydrolysis of ATP by the ectonucleotidases CD39 and CD73 generates adenosine, which creates an immune suppressive microenvironment by inhibiting the T and NK cell responses via the A2A adenosine receptor.
Main body of the abstract
In OC, adenosine-induced pAMPK pathway leads to the inhibition of cell growth and proliferation, which offers new treatment options to prevent or overcome chemoresistance. The activation of P2Y12 and P2Y1 purinergic receptors expressed in the platelets promotes epithelial-mesenchymal transition (EMT). The inhibitors of these receptors will be the effective therapeutic targets in managing OC. Furthermore, research on these signalling systems indicates an expanding field of opportunities to specifically target the purinergic receptors for the treatment of OC.
Short conclusion
In this review, we have described the complex purinergic signalling mechanism involved in the development of OC and discussed the merits of targeting the components involved in the purinergic signalling pathway.
Similar content being viewed by others
Background
Ovarian cancer (OC) is one of the most lethal malignancies among all gynaecological cancers. Life expectancy of women with OC is no more than 5 years, which is mainly due to the increased number of chromosomal alterations in the circulating lymphocytes [1, 2]. In India, OC has the sixth-highest incidence rate among all feminine diseases, and the age-standardised incidence rate has raised by 28·6% (95% UI 19·2–41·6) from 1990 to 2016 [3]. OC predominantly metastasizes via the physiological movement of the peritoneal fluid or the circulating tumour cells in the blood [4, 5]. During this metastatic progression, the ovarian surface epithelial cells undergo epithelial-mesenchymal transition (EMT) induced by adenosine triphosphate (ATP) in the extracellular milieu [6]. ATP provokes the activation of specific purinergic receptors in the plasma membrane (Fig. 1.) [7]. Those responsible for cell communication, proliferation, differentiation, motility and cell death are mainly differentiated into the P1 receptors for adenosine and P2 receptors for ATP [8]. The latter can be further subdivided into the G-protein-coupled P2Y receptors (P2YRs) and the ionotropic P2X receptors (P2XRs) [9]. The adenosine receptors play a significant role in cancer progression and metastasis by inhibiting the immune triggering cells [10]. Another factor that helps the purinergic receptors in the progression of OC is the platelet, which mediates the circulation of tumour cells [11]. This platelet-tumour cell interaction is facilitated by the P2Y12 purinergic receptors, which leads to the formation of tumour cell-induced platelet aggregates (TCIPA) and promotes EMT during cancer progression [12]. Various agonists and antagonists are involved in the inhibition and induction of purinergic signalling, which causes alterations in the responsive cells. It has also been established that this signalling pathway, mainly the P2Y receptors, has a key function as it alters the drug pathways in the OC cells [13]. In the present biological era, many genetic mutations affecting the cell signalling pathway have been implicated in OC tumourigenesis [14]. Thus, signalling pathways are crucial in regulating specific molecular mechanisms after activation of the target molecules, such as ATPs, in treating the OC patients [15]. In this review, we aim to summarise the correlative role of ATPs, ectonucleotidases, platelets and adenosine in the purinergic signalling pathway. Further, we also intend to highlight purinergic signalling as a novel therapeutic target in treating OC.
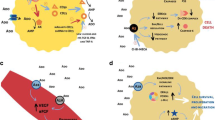
General outline of purinergic signalling in ovarian cancer. In a tumour cell, ATP is released into the extracellular milieu either through connexion or pannexin hemichannel or by ABC (ATP binding cassette) transporter proteins. Presence of ATP in the extracellular milieu activates the P2X and P2Y purinergic signalling cascade. Extracellular ATP is soon hydrolysed by ectonucleotidases CD39 (conversion of ATP/ADP to AMP) and CD73 (conversion of AMP to adenosine) generate adenosine; it will activate the purinergic P1 adenosine receptors. This extracellular adenosine is transported into the cell through nucleoside transporter proteins. P2X purinoceptor is activated by the presence of ATP and that of P2Y purinoceptor is activated by the presence of ATP, UTP, ADP, UDP and UDP-GLUCOSE
Main text
ATP, the major component in the tumour microenvironment
Each cell in the body has a storage factory for ATP, which is the universal signalling molecule in the interstitial region [16]. In the resting cells, the concentration of ATP is very low; however, in damaged or excited tissues, it becomes elevated [17]. The high levels of ATP in the damaged or infected cells are considered as warning signals that prompt the activation and release of inflammatory cytokines into these cells [18, 19]. Ca2+-mediated exocytosis, membrane transporter proteins and plasmalemmal channels are mainly responsible for the release of ATP in the interstitium. Presence of ATP in the tumour microenvironment (TME) triggers the activation of cytosolic Ca2+ channels and the purinergic receptors such as P2X and P2Y. ATP either activates the P2X7 ion channel to induce the extracellular Ca2+ influx or turns on the P2Y1, P2Y2 and P2Y11 receptors to cause intracellular Ca2+ influx from the endoplasmic reticulum (ER). Simultaneous stimulation of both P2X and P2Y results in abnormally elevated levels of Ca2+ in the cancer cells [20]. Besides, ATP also has a role in provoking the activation of various immune cells via the P2 purinergic receptors in the TME [21]. The excess ATPs in the cancer cells are quickly degraded by ectonucleotidases such as CD39 and CD73 [22]. Adenosine generated by the degradation of ATP stimulates the P1 adenosine receptors, which favours tumour cell progression [7].
Adenine nucleotides and adenosine coupled to the purinergic receptors result in a cross-talk among several other signalling systems related to cell proliferation, differentiation, migration, apoptosis, growth arrest and motility [23]. The purinergic receptors regulate different signalling systems through the activation of effectors, including adenylyl cyclase (AC), phosphatidyl inositol-specific phospholipase (PLC), phospholipase D (PLD), phospholipase A (PLA), Src and GTPases, by the production of second messengers such as cyclic AMP (cAMP), inositol trisphosphate (Ins3P), prostaglandin (PG) and nitric oxide (NO). This process in turn leads to the activation of protein kinases such as mitogen-activated protein kinases (MAPK), protein kinase C (PKC), Akt, protein kinase A (PKA), glycogen synthase kinase (GSK), calcium/calmodulin protein kinase (CaMK) and Rho-dependent kinase (RhoK), ultimately leading to gene expression in the responding cells [8].
Effect of ectonucleotidases CD39 and CD73 in ovarian cancer
Ectonucleotidases, which are expressed in almost every cell, are responsible for the hydrolytic conversion of nucleotides into various nucleosides [22]. Overexpression of these enzymes is the major cause of cell proliferation and metastasis in OC [24]. The ectonucleotidases are categorised into four major groups, namely ectonucleoside triphosphate diphosphohydrolases (NTPDases), ectonucleotide pyrophosphatase/phosphodiesterases (ENPPs), alkaline phosphatases (APs) and ecto-5′-nucleotidase (e5NT)/CD73 [25]. Among these, NTPDase1/CD39 and CD73 are critical for the regulation of immune homeostasis in cancer cells. CD39 is involved in the catalytic conversion of ATP to AMP, while CD73 dephosphorylates AMP into adenosine [26, 27]. Under hypoxic conditions, tumour-associated macrophages express elevated levels of CD39 and CD73 owing to the influence of transcription factors such as Sp1, Stat3 and Gfi-1, which results in the mass generation of immunosuppressive adenosine [28]. This stimulatory effect of adenosine can be diminished by CD39 inhibitors such as POM-1 and adenosine deaminase (ADA), which convert adenosine into inosine and decrease immunoregulatory IL-10 secretion by TAM [29]. In OC-derived spheres, upregulation of CD73 triggers a rise in the extracellular adenosine level, which potentially supresses the T and NK cells and causes tumour progression [9]. Another interesting study revealed that CD73 influences the ovarian cancer-initiating cells (OCICs) by regulating the expression of stemness and EMT-related genes, thereby resulting in tumour initiation, metastasis and chemoresistance [24]. Hence, the ectonucleotidase CD73 has a potential role in the purinergic signalling related to the initiation and metastasis of OC.
Role of purinergic receptors in ovarian cancer
Many cellular and biological responses, right from growth stimulation to apoptosis, chemotaxis to cell differentiation and even cytokine release, come under the control of purinergic signalling. This process is of two types, namely short-term and long-term. The former employs neuromodulation, neurotransmission, secretion and platelet aggregation, while the latter encompasses cell proliferation, differentiation and cell death. The receptors are differentiated into P1 and P2. The P1 adenosine receptors are G-protein-coupled receptors that consist of four subtypes, namely A1, A2A, A2B and A3. These adenosine receptors control various cellular activities by triggering the AMPK signalling pathway [30]. The P2 receptor, on the other hand, is further sub-divided into P2X and P2Y. The P2X receptor comprises seven subtypes, P2X1–7, which exhibit 30–50% amino acid sequence similarity (Table 1). The receptor has intracellular C and N terminals and two membrane-spanning regions, TM1 and TM2. While TM1 acts as the gate, TM2 serves as the lining of the ion pore. Adjacent to these regions, a large exterior loop with an ATP binding site exists. The G-protein-coupled receptors in association with the heterotrimeric G-proteins such as Gq/11, Gs and Gi are meant to regulate the calcium Ca2+ and cAMP levels in the cell by influencing phospholipase C adenylyl cyclase [37].
Purinergic receptors are considered to be the causative agents of cancer as they are implicated in various tumourigenic functions. For instance, it was asserted that P2X5 is responsible for cell differentiation, P2X7 for apoptotic death of the tumour cells and both P2Y1 and P2Y2 for tumour cell proliferation [35]. Activation of P2X7 by the agonist 2,3-O-(4-benzoylbenzoyl)-ATP (BzATP) was proven to influence cell progression in SKOV-3 and CAOV-3 in the OC cell lines [38]. The tumour cells, under hypoxic conditions, activate the P2X7 receptor, which leads to the phosphorylation of extracellular signal-regulated kinase (ERK) and serine/threonine-specific protein kinase (AKT) pathway and in turn results in enhanced cell invasion and nuclear accumulation of NF-κB [39]. Similarly, another study also established that the activation of P2X7 receptor leads to the phosphorylation of ERK in the SKOV-3 and CAOV-3 cell lines of OC. Furthermore, it was identified that the use of P2X7R inhibitor AZ10606120 reduces cell viability in OC cell lines [38]. Just like the earlier study, this work also reported that overexpression of P2X7 in the ovarian surface epithelium (OSE) causes the phosphorylation of ERK and triggers the AKT pathway, ultimately increasing the influx of Ca2+ in the OC TME [40]. Interestingly, it was stipulated that the P2X7 receptor was an oncogene mainly because of its versatile effects such as aerobic glycolysis, hypoxia-inducible factor 1-alpha (HIF-1a) activation of PI3K/AKT pathway and release of VEGF, which result in OC progression and metastasis [41, 42].
In OC, progression of the disease is also linked to tumour cell-induced platelet activation (TCIPA), which is regulated by the stimulation of the P2 (ATP) type of purinergic receptors such as P2Y1 and P2Y12. In the OC cells, the activation of P2Y1 receptor coupled with the heterotrimeric G-protein Gq results in the phosphorylation of phospholipase Cβ and the release of Ca2+. Finally, the activation of protein kinase C initiates a change in the normal shape of the platelets [43]. Many studies demonstrated that P2Y12 receptors coupled with Gi activate phosphatidylinositol-3-kinase, which results in platelet degranulation inside the cancer cells and facilitates tumour progression [44]. These degranulated platelets release several cytokines such as TGF-CXCL5 and CXCL7, which results in the control of cell pro-proliferation and pro-metastasis effects in OC [45]. Thus, focusing on the TCIPA mechanism along with purinergic signalling would enlighten the researchers on the early detection of OC.
Platelet-induced tumour progression in ovarian cancer
In OC, thrombocytosis and thrombosis are the major challenges and may be viewed as the prognostic biomarkers of the disease. These have emerged as important factors in the field of cancer pathology [46]. Almost every cancer cell exhibits high levels of platelet angiogenesis regulators such as VEGF, ANGPT-1, MMP-2, PF-4 and PDGF [47]. In OC patients, the platelets display some structural changes when compared with the controls, which are responsible for the epithelial-mesenchymal transition [48]. This alteration is initiated by the activation of P2 purinergic receptors under the elevated levels of ATP or ADP released from the platelets [49]. In cancer cells, ADP instigates P2Y1 followed by P2Y12 to create a temporary change in the shape of the platelets and their aggregation [50]. The tumour cell-derived IL-6 enhances the rate of megakaryopoiesis, which increases platelet production in the ovarian TME [51]. Interaction between the platelets and the tumour cells activates the NF-κB and TGF-β signalling pathway in the cancer cells, which results in epithelial mesenchymal transition (EMT) and induces metastasis in the tumour cells [36]. The activated platelets may cause extravasation of the tumour cells by augmenting the endothelial permeability and the signals for tumour progression via the P2Y2 receptor [52]. The P2Y12 purinergic receptor is the core factor that activates the platelet glycoprotein IIb/IIIa receptor, and it is the hotspot for the treatment of OC [53]. Another study also revealed that the inactivation of the P2Y12 receptor may lead to a marked reduction in tumour progression [54]. Cancer-associated platelets have a significant role in liquid biopsy, and platelet-based analytics serves as a major diagnostic tool and a potent biomarker in cancer [55]. Thus, high platelet counts can be used as a predictable marker to detect chemoresistance and tumour progression in the OC cells.
Role of adenosine in chemoresistance
Chemoresistance is one of the major problems observed while treating the OC patients. Thus, creating drugs based on the reprogramming of cells into iPSCs using adenosine can serve as a platform for therapeutic treatment [56]. The nucleoside regulates various metabolic activities by binding with the receptors A1R, A2AR, A2BR and A3R in the extracellular membrane [33], thereby controlling the inherent functions of tumour cells such as proliferation, apoptosis, angiogenesis, metastasis and chemoresistance [30]. This effect of adenosine is concentration-dependent, wherein low levels are responsible for cytostatic effects, while high levels lead to cytotoxic effects [34]. Adenosine is a downstream signalling factor for adenylyl cyclase (cAMP) and suppresses the immune system in the cancer cells [57, 58]. The receptors activate the ERK1/2 pathway concerned with the regulation of cell proliferation and cell death in response to different stimuli in the cancer cells [59]. ERK1/2 expression increases in the cancer stem cells (CSCs) in various human tumours [60]. In a colorectal study, it was observed that adenosine receptors induce the activation of ERK1/2 or MEK pathway, which results in the enhanced expression of multi-drug resistance-associated protein 2 (MDRP2) [61]. MDRP2 belongs to the ABC superfamily governing the efficiency of drug treatment and is involved in drug export from the cells [62]. In OC, platinum- and Taxol-based treatment is most commonly used, but resistance to these drugs limits the effectiveness of the treatment [63]. The mesenchymal nature of the OC cells is also an important reason for the potent drug resistance and tumour progression [64]. Besides, several other mechanisms may lead to cisplatin resistance, such as variations in the transport and trafficking of the drug and disturbance in the apoptosis pathway [65].
Adenosine can induce apoptosis externally via receptors A1, A2A, A2B and A3 in various cancers. In OVCAR-3 OC cells, the nucleoside mediates cell cycle arrest in the G1 phase and induces apoptosis in a caspase-3-dependent manner. Presence of the molecule in the cell may cause the downregulation of CDK4, cyclin D1 and anti-apoptotic Bcl-2 proteins. Adenosine also induces a significant increase in the level of pro-apoptotic Bax protein. Overall, adenosine induces cell cycle arrest and apoptosis, which could be determined by the increased concentration of apoptotic sub-G1 population [66]. Besides, the chemical inhibits the mTOR growth stimulatory pathway via the AMPK-dependent pathway. After conversion of adenosine to AMP by adenosine kinase, AMP-activated protein kinase (AMPK) is stimulated owing to the presence of AMP and downstream pathways, finally resulting in adenosine-induced cell growth arrest and apoptosis in the OC lines [30]. Uptake of this purine nucleoside by the intracellular nucleoside transporters activates the pAMPK signalling in an LKB1-dependent manner. AMPK phosphorylates the Raptor at S792 to inhibit mTOR1. This will result in reduced phosphorylation of pS6K, leading to cell growth arrest (Fig. 2). Application of adenosine prior to cisplatin treatment may lead to induced drug cytotoxicity in the OC cells. Moreover, the inhibitors of A1 and A2B receptors, such as SLV320 or PSB603, may be unable to suppress these effects of adenosine, thereby providing an emerging therapeutic target to eliminate chemoresistance [67]. Thus, adenosine pathways provide new treatment options to prevent or overcome chemoresistance.
Adenosine induced cytotoxicity in ovarian cancer. Adenosine generated by the presence of ectonucleotidases CD39 and CD73 activate the adenosine receptors in tumour cells. Nucleoside transporter transports this extracellular adenosine into the cell. Inside, the cell adenosine promotes apoptosis through the caspase-3-dependent pathway. Adenosine downregulates the expression of the anti-apoptotic protein Bcl-2 and G1phase cyclin D1 and CDK4 and also, it induce the expression of pro-apoptotic Bax protein. Adenosine inhibits the cell growth by the AMPK-dependent pathway. Adenosine kinase phosphorylate the adenosine and converted into AMP results in the phosphorylation of AMPK by the upstream kinase LKB1. Activation of AMPK results in the inhibition of growth stimulatory mTOR pathway and also by decreased phosphorylation of S6K. Reduced pS6K leads to the inhibition of cell growth arrest and proliferation
Conclusion
This research is still in its infancy, and the possibilities are limitless [68]. Purinergic signalling plays an important regulatory role in the development of cancer. In OC, phenomena such as epithelial mesenchymal transition, platelet-induced tumour progression and chemoresistance are controlled by this cell communication system. It involves several extracellular messengers in the form of ATP, ADP, AMP and adenosine. The purinergic receptors P2Y2, P2X5 and P2X7 involved in high-grade bladder cancer are responsible for the antitumuor effect of ATP. The P2X7 receptors, upon activation, allow Ca2+ influx to stimulate the mitochondrion-dependent apoptotic machinery [31, 69]. Ectonucleotidases CD39 and CD73 degrade this extracellular ATP into adenosine, which creates an immunosuppressive microenvironment. Adenosine present in the tumour microenvironment may cause the induction of cisplatin cytotoxicity in OC cell lines through A1 and A2BR receptors. Adenosine uptake by the intracellular nucleoside transporters activates pAMPK signalling, which leads to the inhibition of growth stimulatory mTOR1, which in turn results in cell growth arrest and enhanced cytotoxicity. Resistance to platinum-based chemotherapy is one of the major problems faced while treating the OC cases. Platelets are involved in the induction of tumour progression and chemoresistance. Platelet-tumour cell interaction through P2Y12 may lead to EMT during cancer progression. This reveals the importance of using P2Y12 receptor antagonists in the treatment of cancer. Purinergic molecules are dynamically positioned in cancer immunity, and targeting this pathway could efficaciously suppress tumour progression and metastasis and can be used as the best therapeutic target in OC.
Availability of data and materials
As this is a review article, there is no availability of data and materials.
Abbreviations
- AC:
-
Adenylyl cyclase
- ADA:
-
Adenosine deaminase
- AKT:
-
Serine/threonine-specific protein kinase
- AMP:
-
Adenosine monophosphate
- AMPK:
-
Adenosine monophosphate-activated protein kinase
- ANGPT-1:
-
Angiopoietin1
- APs:
-
Alkaline phosphatases
- ATP:
-
Adenosine triphosphate
- cAMP:
-
Cyclic adenosine monophosphate
- CaMK:
-
Calcium/calmodulin protein kinase
- CD39:
-
Ectonucleoside triphosphate diphosphohydrolase1
- CD73:
-
Ectonucleotidase
- CRISPR-CAS9:
-
Gene editing
- CSCS :
-
Cancer stem cells
- CXCL5:
-
Chemokine ligand-5
- CXCL7:
-
Chemokine ligand-7
- EGFR:
-
Epidermal growth factor receptor
- ELK-1:
-
ETS-like transcription factor-1
- EMT:
-
Epithelial-mesenchymal transition
- ENPPs:
-
Ectonucleotide pyrophosphatase/phosphodiesterases
- e5NT:
-
Ecto-5′-nucleotidase
- ER:
-
Endoplasmic reticulum
- ERK:
-
Extracellular signal-regulated kinase
- GPI-linked:
-
Glycosylphosphatidylinositol-linked
- GSK:
-
Glycogen synthase kinase
- HER2:
-
Human epidermal growth factor receptor
- HIF-1a:
-
Hypoxia-inducible factor 1 alpha
- IL-6:
-
Interleukin 6
- MAPK pathway:
-
Mitogen-activated protein kinase pathway
- MDRP2:
-
Multi-drug resistance
- Ins3P:
-
Inositol trisphosphate
- MMP-2:
-
Matrix metalloproteinase-2
- mTOR1:
-
Mammalian target of rapamycin1
- NDP kinase:
-
Nucleoside diphosphate kinases
- NO:
-
Nitric oxide
- NTPDases:
-
Ectonucleoside triphosphate diphosphohydrolases
- NF-κb:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NK cells:
-
Natural killer cells
- OC:
-
Ovarian cancer
- OCICs:
-
Ovarian cancer-initiating cells
- OSE:
-
Ovarian surface epithelium
- pAMPK:
-
PhosphoAMPK
- PDGF:
-
Platelet-derived growth factor
- PG:
-
Prostaglandin
- PI3K/AKT:
-
Phosphatidyl inositol-3-kinase/protein kinase
- PIK3CA:
-
Phosphatidyl inositol-4,5-bisphosphate 3-kinase catalytic subunit alpha
- PLA:
-
Phospholipase A
- PLC:
-
Phospholipase C
- PLD:
-
Phospholipase D
- POM-1:
-
Polyoxometalate 1
- pS6K:
-
Ribosomal protein s6 kinase beta-1
- RhoK:
-
Rho-dependent kinase
- siRNA:
-
Small interfering RNA
- TAM:
-
Tumour-associated macrophages
- TCIPA:
-
Tumour cell-induced platelet aggregates
- TGF-b1:
-
Transforming growth factor beta1
- UTP:
-
Uridine-5′-triphosphate
- VEGF:
-
Vascular endothelial growth factor
- Wnt signalling pathway:
-
Wingless/integrated signalling pathway
References
Balachandar V, Lakshman Kumar B, Sasikala K, Manikantan P, Sangeetha R, Mohana DS (2007) Identification of a high frequency of chromosomal rearrangements in the centromeric regions of prostate cancer patients. J Zhejiang Univ Sci B 8(9):638–646 https://doi.org/10.1631/jzus.2007.B0638
Mahalaxmi I, Santhy KS (2018) Role and hallmarks of Sp1 in promoting ovarian cancer. Journal of oncological sciences 4(2):102–105
Dhillon PK (2018) The burden of cancers and their variations across the states of India: the global burden of disease study 1990–2016 India state-level disease burden initiative cancer collaborators. Lancet Oncol 19:1289–1306 https://doi.org/10.1016/S1470-2045(18)30447-9
Yeung T, Leung CS, Yip K, Chi LAY, Wong STC, Mok SC (2015) Cellular and molecular processes in ovarian cancer metastasis. A review in the theme: cell and molecular processes in cancer metastasis. Am J Phys Cell Physiol 309:C444–C456 https://doi.org/10.1152/ajpcell.00188.2015
Mahalaxmi I, Mohana Devi S, Kaavya J, Arul N, Balachandar V, Santhy KS (2019) New insight into NANOG: a novel therapeutic target for ovarian cancer (OC). Eur J Pharmacol 852:51–57 https://doi.org/10.1016/j.ejphar.2019.03.003
Sarah L, Bandiera M, Huntsman D, Lstou VS, Kuo W et al (2014) Epithelio-mesenchymal transition in a neoplastic ovarian epithelial hybrid cell line. Differentiation. 72:150–116 https://doi.org/10.1111/j.1432-0436.2004.07204003.x
Stagg J, Smyth MJ (2010) Extracellular adenosine triphosphate and adenosine in cancer. Oncogene. 29:5346–5358 https://doi.org/10.1038/onc.2010.292
Burnstock G, Verkhratsky A (2010) Long-term (trophic) purinergic signalling: purinoceptors control cell proliferation, differentiation and death. Cell Death Dis 1:e9 https://doi.org/10.1038/cddis.2009.11
Junger WG (2011) Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol 11(3):201–212 https://doi.org/10.1038/nri2938
Sek K, Mølck C, Stewart GD et al (2018) Targeting adenosine receptor signalling in cancer immunotherapy. Int J Mol Sci 19:3837 https://doi.org/10.3390/ijms19123837
Miyashita T, Tajima H, Makino I (2015) Metastasis-promoting role of extravasated platelet activation in tumor. J Surg Res 193:289–294 https://doi.org/10.1016/j.jss.2014.07.037
Cooke NM, Spillane CD, Sheils O (2015) Aspirin and P2Y12 inhibition attenuate platelet-induced ovarian cancer cell invasion. BMC Cancer 15:627 https://doi.org/10.1186/s12885-015-1634-x
Wilson FH, Johannessen CM, Piccioni F et al (2015) A functional landscape of resistance to ALK inhibition in lung cancer. Cancer Cell 27(3):397–408 https://doi.org/10.1016/j.ccell.2015.02.005
Jayaramayya K, Mahalaxmi I, Mohana Devi S, Santhy KS, Balachandar V (2019) Targeting phosphoinositide-3-kinase pathway in biliary tract cancers: a remedial route? J Cell Physiol 234(6):8259–8273 https://doi.org/10.1002/jcp.27673
Ganesan H, Venkatesh B, Mahalaxmi I, Anila V, Mohana Devi S, Ssang-Goo C, Balachandar V (2019) mTOR signalling pathway - a root cause for idiopathic autism? BMB Rep 52(7):424–433
Burnstock G (2018) The therapeutic potential of purinergic signalling. Biochemical Pharmacology l151:157–165. https://doi.org/10.1016/j.bcp.2017.07.016
Yegutkin GG (2014) Enzymes involved in metabolism of extracellular nucleotides and nucleosides: Functional implications and measurement of activities. Crit Rev Biochem Mol Biol l49:473–497 https://doi.org/10.3109/10409238.2014.953627
Savio LEB, de Andrade MP, da Silva CG et al (2018) The P2X7 receptor in inflammatory diseases: angel or demon? Front Pharmacol 9:52 https://doi.org/10.3389/fphar.2018.00052
Giuliani AL, Sarti AC, Falzoni S et al (2017) The P2X7 receptor-interleukin-1 liaison. Front Pharmacol 8:123 https://doi.org/10.3389/fphar.2017.00123
Lin-Hua J, Fatema M, Xuebin Y et al (2017) ATP-induced Ca2+-signalling mechanisms in the regulation of mesenchymal stem cell migration. Cell Mol Life Sci 4(20):3697–3710 https://doi.org/10.1007/s00018-017-2545-6
Aymeric L, Apetoh L, Ghiringhelli F et al (2010) Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res 70:855–858 https://doi.org/10.1158/0008-5472.CAN-09-3566
Hausler SFM, Montalban DBI, Strohschein J et al (2010) Ectonucleotidases CD39 and CD73 on OvCA cells are potent adenosine-generating enzymes responsible for adenosine receptor 2A-dependent suppression of T cell function and NK cell cytotoxicity. Cancer Immun 60:1405–1418 https://doi.org/10.1007/s00262-011-1040-4
Zimmermann H (2006) Nucleotide signaling in nervous system development. PflugersArch 452:573–588 https://doi.org/10.1007/s00424-006-0067-4
Lupia M, Angiolini F, Bertalot G et al (2018) CD73 regulates stemness and epithelial-mesenchymal transition in ovarian cancer-initiating cells. Stem Cell Reports 10(4):1412–1425 https://doi.org/10.1016/j.stemcr.2018.02.009
Al-Rashida M, Uroos QS, Batool N et al (2017) Ectonucleotidase inhibitors: a patent review (2011-2016). Expert Opinion on Therapeutic Patents 27(12):1291–1304 https://doi.org/10.1080/13543776.2017.1369958
Antonioli L, Pacher P, Vizi ES et al (2013) CD39 and CD73 in immunity and inflammation. Trends Mol Med 19(6):355–367 https://doi.org/10.1016/j.molmed.2013.03.005
David A, Bertrand A, Pierre-Olivier G et al (2016) CD73–adenosine: a next-generation target in immuno-oncology. Immunotherapy. 8(2):145–163 https://doi.org/10.2217/imt.15.106
Bono MR, Fernández D, Flores-Santibáñez F et al (2015) CD73 and CD39 ectonucleotidases in T cell differentiation: beyond immunosuppression. FEBS Lett 589(22):3454–3460 https://doi.org/10.1016/j.febslet.2015.07.027
Almeida SM, Kauffenstein G, Roy C et al (2016) The ectoATPDase CD39 is involved in the acquisition of the immunoregulatory phenotype by M-CSFmacrophages and ovarian cancer tumor-associated macrophages: regulatory role of IL-27. OncoImmunology. 5(7):e1178025 https://doi.org/10.1080/2162402X.2016.1178025
Antonioli L, Blandizzi C, Pacher P et al (2013) Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer 13(12):842–857 https://doi.org/10.1038/nrc3613
Coddou C, Yan Z, Obsil T et al (2011) Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev 63(3):641–683 https://doi.org/10.1124/pr.110.003129
Butler M, Sanmugalingam D, Burton VJ et al (2012) Impairment of adenosine A3 receptor activity disrupts neutrophil migratory capacity and impacts innate immune function in vivo. Eur J Immunol 42:3358–3368 https://doi.org/10.1002/eji.201242655
Ruiz ML, Lim YH, Zheng J (2014) Adenosine A2A receptors as drug discovery target. J Med Chem 57(9):3623–3650 https://doi.org/10.1021/jm4011669
Aghaei M, Karami-Tehrani F, Panjehpour M et al (2012) Adenosine induces cell-cycle arrest and apoptosis in androgen-dependent and-independent prostate cancer cell lines. Prostate. 72(4):361–375 https://doi.org/10.1002/pros.21438
White N, Burnstock G (2006) P2 receptors and cancer. Trends Pharmacol Sci 27(4):211–217 https://doi.org/10.1016/j.tips.2006.02.004
Labelle M, Begum S, Hynes RO (2011) Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 20(5):576–590 https://doi.org/10.1016/j.ccr.2011.09.009
Burnstock G (2006) Purinergic signalling. Br J Pharmacol 147(Suppl. 1):S172–S181 https://doi.org/10.1038/sj.bjp.0706429
Vázquez-Cuevas FG, Martínez-Ramírez AS, Robles-Martínez L et al (2014) Paracrine stimulation of P2X7 receptor by ATP activates a proliferative pathway in ovarian carcinoma cells. J Cell Biochem 115(11):1955–1966 https://doi.org/10.1002/jcb.24867
Tafani M, Schito L, Pellegrini L et al (2011) Hypoxia-increased RAGE and P2X7R expression regulates tumor cell invasion through phosphorylation of Erk1/2 and Akt and nuclear translocation of NF-{kappa} B. Carcinogenesis 32(8):1167–1175 https://doi.org/10.1093/carcin/bgr101
Murdoch WJ, McDonnel A (2002) Roles of the ovarian surface epithelium in ovulation and carcinogenesis. Reproduction. 123(6):743–750
Gu BJ, Wiley JS (2006) Rapid ATP-induced release of matrix metalloproteinase 9 is mediated by the P2X7 receptor. Blood. 107:4946–4953 https://doi.org/10.1182/blood-2005-07-2994
Qiu Y, Li WH, Zhang HQ et al (2014) P2X7 mediates ATP-driven invasiveness in prostate cancer cells. PLoS One 9(12):e114371 https://doi.org/10.1371/journal.pone.0114371
Mangin P, Ohlmann P, Eckly A et al (2004) The P2Y1 receptor plays an essential role in the platelet shape change induced by collagen when TxA2 formation is prevented. J Thromb Haemost 2(6):969–977 https://doi.org/10.1111/j.1538-7836.2004.00722.x
Dorsam RT, Kunapuli SP (2004) Central role of the P2Y12 receptor in platelet activation. J Clin Invest 113(3):340–345 https://doi.org/10.1172/JCI20986
Cho MS, Bottsford-Miller J, Vasquez HG et al (2012) Platelets increase the proliferation of ovarian cancer cells. Blood. 120(24):4869–4872 https://doi.org/10.1182/blood-2012-06-438598
Gungor T, Kanat-Pektas M, Sucak A et al (2009) The role of thrombocytosis in prognostic evaluation of epithelial ovarian tumors. ArchGynecol Obstet 279:53–56
Yan M, Lesyk G, Radziwon-Balicka A et al (2014) Pharmacological regulation of platelet factors that influence tumor angiogenesis. Semin Oncol 41(3):370–377 https://doi.org/10.1053/j.seminoncol.2014.04.007
Wang Y, Sun Y, Li D et al (2013) Platelet P2Y12 is involved in murine pulmonary metastasis. PLoS One 8(11):e80780 https://doi.org/10.1371/journal.pone.0080780
Burnstock G, Di Virgilio F (2013) Purinergic signalling and cancer. Purinergic Signal 9:491–540 https://doi.org/10.1007/s11302-013-9372-5
Ballerini P, Dovizio M, Bruno A et al (2018) P2Y12 receptors in tumorigenesis and metastasis. Front Pharmacol 9:66 https://doi.org/10.3389/fphar.2018.00066
Stone RL, Nick A, McNeish IA et al (2012) Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med 366(7):610–618
Schumacher D, Strilic B, Sivaraj KK et al (2013) Platelet derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell 24(1):130–137 https://doi.org/10.1016/j.ccr.2013.05.008
Damman P, Woudstra P, Kuijt WJ et al (2012) P2Y12 platelet inhibition in clinical practice. J Thromb Thrombolysis 33(2):143–153 https://doi.org/10.1007/s11239-011-0667-5
Cho MS, Noh K, Haemmerle M et al (2017) Role of ADP receptors on platelets in the growth of ovarian cancer. Blood. 130:1235–1242 https://doi.org/10.1182/blood-2017-02-769893
Chi KR (2016) The tumour trail left in blood. Nature. 532(7598):269–271 https://doi.org/10.1038/532269a
Gomathi M, Padmapriya S, Balachandar V et al Drug studies on Rett syndrome: from bench to bedside. J Autism Dev Disord (In Press)
Ohta A, Gorelik E, Prasad SJ et al (2006) A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A 103(35):13132–13137 https://doi.org/10.1073/pnas.0605251103
Vecchio EA, Tan CY, Gregory KJ et al (2016) Ligand-independent adenosine A2B receptor constitutive activity as a promoter of prostate cancer cell proliferation. J Pharmacol Exp Ther 357:36–44 https://doi.org/10.1124/jpet.115.230003
Mebratu Y, Tesfaigzi Y (2009) How ERK1/2 activation controls cell proliferation and cell death: is subcellular localization the answer? Cell Cycle 8:1168–1175 https://doi.org/10.4161/cc.8.8.8147
Wang Y, Zhu Y, Qiu F et al (2010) Activation of Akt and MAPK pathways enhances the tumorigenicity of CD133+ primary colon cancer cells. Carcinogenesis. 31(8):1376–1380 https://doi.org/10.1093/carcin/bgq120
Vinette V, Placet M, Arguin G et al (2015) Multidrug resistance-associated protein 2 expression is upregulated by adenosine 5,-triphosphate in colorectal cancer cells and enhances their survival to chemotherapeutic drugs. PLoS One 10(8):e0136080 https://doi.org/10.1371/journal.pone.0136080
Hlavata I, Mohelnikova-Duchonova B, Vaclavikova R et al (2012) The role of ABC transporters in progression and clinical outcome of colorectal cancer. Mutagenesis. 2:187–196 https://doi.org/10.1093/mutage/ger075
Bookman MA (2003) Developmental chemotherapy and management of recurrent ovarian cancer. J Clin Oncol 21(10 Suppl):149s–167s
Marchini S, Fruscio R, Clivio L et al (2013) Resistance to platinum-based chemotherapy is associated with epithelial to mesenchymal transition in epithelial ovarian cancer. Eur J Cancer 49(2):520–530 https://doi.org/10.1016/j.ejca.2012.06.026
Galluzzi L, Senovilla L, Vitale I et al (2012) Molecular mechanisms of cisplatin resistance. Oncogene. 31(15):1869–1883 https://doi.org/10.1038/onc.2011.384
Shirali S, Aghaei M, Shabani M et al (2013) Adenosine induces cell cycle arrest and apoptosis via cyclinD1/Cdk4 and Bcl-2/Bax pathways in human ovarian cancer cell line OVCAR-3. Tumor Biol 34:1085–1095 https://doi.org/10.1007/s13277-013-0650-1
Sureechatchaiyan P, Hamacher A, Brockmann N et al (2018) Adenosine enhances cisplatin sensitivity in human ovarian cancer cells. Purinergic Signal 14(4):395–408 https://doi.org/10.1007/s11302-018-9622-7
Venugopal A, Mahalaxmi I, Venkatesh B, Balachandar V (2019) Mitochondrial calcium uniporter as a potential therapeutic strategy for Alzheimer’s disease (AD). Acta Neuropsychiatria 26:1–19
Shabbir M, Ryten M, Thompson C et al (2008) Purinergic receptor-mediated effects of ATP in high-grade bladder cancer. BJU Int 101:106–112 https://doi.org/10.1111/j.1464-410X.2007.07286.x
Acknowledgements
We thank Bharathiar University, Coimbatore, Avinashilingam Institute for Home Science and Higher Education for Women, Coimbatore and Mizoram University, Aizwal, Mizoram for providing the necessary help to carry out the article review process.
Funding
No funding was obtained to draft this review article.
Author information
Authors and Affiliations
Contributions
The authors have read and approved the final manuscript. Conceptualization: VB, AN, CN, IM. Literature search: IM, CN. Data analysis: IM, CN. Drafting of manuscript: IM, CN. Manuscript final proof: VB, AN, ZS.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This is a review article; thus, it does not contain any studies with human participants performed by any of the authors.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chandran, N., Iyer, M., Siama, Z. et al. Purinergic signalling pathway: therapeutic target in ovarian cancer. Egypt J Med Hum Genet 21, 23 (2020). https://doi.org/10.1186/s43042-020-00059-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-020-00059-3