Abstract
Background
An arsenic-resistant microbial strain, Micrococcus sp. KUMAs15 isolated from West Bengal, India, has demonstrated high resistance to arsenic due to its arsenic accumulation and adsorption ability, establishing the strain as a potential arsenic bioremediation candidate for arsenic-contaminated niche. The successful field application of the microbe necessitates evaluation of probable immunotoxicological reactions on human cells. The present study determines expression profiles of pro-inflammatory and anti-inflammatory cytokines in cells exposed to KUMAs15.
Results
The present study explored the alterations in expressions of the pro-inflammatory and anti-inflammatory cytokines in two human cell lines exposed to KUMAs15. The expression profile of the cytokine genes demonstrated that Micrococcus sp. KUMAs15 does not significantly induce inflammatory effects in these human cell lines. The upregulated expression of IL-8 and downregulated expression of IL-6 were observed in HaCaT. The HepG2 have shown downregulated IL-12 gene expression. These observations indicate the non-pathogenicity of KUMAs15 on the human cell lines.
Conclusion
The observations from the study extend the applicability of the arsenic-resistant Micrococcus sp. KUMAs15 for environmental arsenic decontamination. The isolate KUMAs15 was observed to be non-pathogenic to the human cell lines, as the strain does not initiate inflammatory reactions in these cell lines.
Similar content being viewed by others
Background
Arsenic (As) is a naturally occurring metalloid often considered as one of the most hazardous chemical pollutants in the contemporary scientific era. The As contamination is now being appropriately called the largest mass poisoning in human history [1]. Arsenic, the most abundant hazardous toxic metalloid [2], is widely distributed in the lithosphere, hydrosphere, and biosphere since the ancient geological period. The anthropogenic activities, together with the spontaneous geological activities like volcanic eruptions and weathering, are responsible for the dissipation of this metalloid throughout the globe [3]. The As removal technologies, combining chemical and biological approaches, are changing the scientific landscape for environmental As decontamination. The microbe-assisted remediation for As-contaminated systems are making significant progress towards the development of sustainable technologies that are applicable to different agro-ecological regions [4, 5].
Microbes possess resistance adaptations to resist high concentrations of heavy metals present in its ecological niche [6,7,8]. The metal tolerance adaptations of bacteria have potentialities for heavy metal bioremediation [9, 10] in different environments. Despite tremendous potentialities for microbial bioremediation, earlier studies reported that heavy metal-tolerant bacteria could be pathogenic to humans [11]. One of the major concerns in the field application of the As-resistant microbial strains for As decontamination of the environment is the probable pathogenic response with other organisms, especially to humans. Thus, for sustainable microbe-assisted bioremediation management, knowledge on probable toxicological or immunogenic reactions with the microbial strain and human cells should be elucidated to establish the isolated microbial strains as non-pathogenic before their application in As-contaminated fields where they might interact with humans.
A highly As-resistant microbial isolate has been previously reported from our laboratory. The microbial isolate is designated as KUMAs15 identified as a strain of Micrococcus [12] following 16SrDNA sequencing and analysis of homology by the NCBI BLAST function [13] and Ribosomal Database Project [14]. The isolated microbial strain has shown arsenite [As(III)] oxidation capability, by accelerating the spontaneous conversion of As (III) to a less toxic form arsenate [As(V)] [15] which could be removed from water with ease due to its lower solubility than As (III) [16, 17]. The microbe Micrococcus sp. KUMAs15 could also remove As from the culture medium in a laboratory condition by surface adsorption as well as arsenic accumulation at the environmentally relevant concentration of As; the results have been earlier reported [12]. The present study aimed to validate the possible pathogenic effect of the isolated arsenic-decontaminating bacterium on human cell lines.
Methods
Cell culture and co-culture with KUMAs15
The present study included two adherent human cell lines, keratinocytes (HaCaT) and liver epithelial cells (HepG2), obtained from the National Centre for Cell Science, Pune, India. The HaCaT cell line was cultured in Dulbecco’s modified Eagle’s medium (DMEM; HiMedia) supplemented with high glucose, sodium pyruvate, and 10% fetal bovine serum (FBS; Gibco, USA), while the HepG2 cell lines were cultured in Eagle’s minimum essential medium (MEM; HiMedia) supplemented with 10% FBS. The cells were inoculated without antibiotics to prevent interference in microbial culture in co-culture experiments in subsequent assays. The cells were maintained at 37 °C with 5% CO2 in a standard CO2 incubator (Thermo Scientific, USA). The present study has been waived from ethical permissions according to the guidelines of the Indian Council of Medical Research (ICMR) for the biomedical research studies conducted in India [18].
Cellular toxicity assay
The cytotoxicity of Micrococcus sp. KUMAs15 on the HaCaT and HepG2 cells was determined by the MTT assay for the analysis of cellular viability. In the experiment, HaCaT and HepG2 cell lines treated only with PBS served as the negative control, whereas cells treated with Triton-X served as the positive control.
Cytokine assay
The inflammatory reactions of the HaCaT and HepG2 after the exposure to KUMAs15 were determined by cellular cytokine assay. ELISA analysis of the pro-inflammatory cytokines TNF-α, IL-8, IL-2, IL-6, IL-10, and IL-12p70 in the human cell lines HaCaT and HepG2 exposed to isolated Micrococcus sp. KUMAs15 was performed in a 4-h and 24-h time span. The PBS-treated human cell line not exposed to KUMAs15 served as the negative control group whereas cells co-cultured with E. coli served as the positive control due to its lipopolysaccharides, which elicit a cytokine response in these cell lines [19].
Transcriptional analysis of cytokine genes
The transcriptional expression of cytokine genes have been determined by semi-quantitative reverse transcriptase PCR. The HaCaT and HepG2 cells have been cultured and subjected to treatment with either PBS, served as the negative control, or the Micrococcus sp. KUMAs15 and have been incubated overnight. Total RNA isolation was carried out using Trizol reagent (Invitrogen, USA) following the standard protocol [20] and was eluted with 20 μL of RNase-free water (Thermo Scientific, USA) and quantified at 260 nm (Evolution 201 UV-Visible Spectrophotometer, Thermo Scientific, USA). The cDNA has been synthesized by reverse transcription, using the total RNA as the template with the RevertAidTM First Strand cDNA Synthesis Kit (Thermo Scientific, USA) following the manufacturer’s protocol, followed by the PCR amplification of the genes of interest using the cDNA as the template and appropriate primers [21] and followed by the agarose gel electrophoresis. The constitutive expression of the housekeeping gene, β-actin, was used as the loading control in this experiment.
Translational analysis of cytokine genes
The expressional analysis at the transcriptional level of cytokine genes was further confirmed at the protein level by western blot analysis. The HaCaT and HepG2 were cultured and exposed to the isolated Micrococcus sp. KUMAs15 for 24 h followed by protein isolation and western blotting.
Statistical analysis
The statistical analysis was performed using GraphPad Prism 6 software. The GraphPad algorithm has been used to perform one-way ANOVA followed by Tukey’s multiple comparison tests. All the experiments were carried out in triplicate, and p < 0.05 was considered as statistically significant.
Results
Cellular toxicity assay
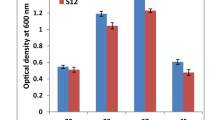
The viability of the HaCaT and HepG2 cells exposed to isolated Micrococcus KUMAs15 was observed to be unaltered in MTT assay. The unaltered survival of the HaCaT and HepG2 cell lines when co-cultured with KUMAs15 showed the non-pathogenicity of the isolated microbe Micrococcus sp. on these cell lines (Fig. 1).
Cytokine profile of the human cell lines
The HaCaT and HepG2 cell lines show differential cytokine profiles when exposed to Micrococcus sp. KUMAs15. The HaCaT cells show no significant alteration of overall cytokine profile when compared to the PBS-treated group, although higher cytokine levels were observed when cells were exposed to E. coli, which served as the positive control. Specifically, the HaCaT cells showed increased IL-2 and IL-6 cytokine levels after 24 h of co-culture with isolated KUMAs15. The HepG2 cells showed no alterations in the majority of the cytokines tested when compared to the PBS group, with higher cytokine levels observed when exposed to E. coli cells. The HepG2 cells showed a higher cytokine level when exposed to KUMAs15 for 4 h, but after 24 h of incubation, the cells showed no alteration in the IL-8 level. The human liver epithelial HepG2 cells also showed increased IL-2 and IL-10 levels in 4 h, which decreased after 24 h of KUMAs15 exposure (Fig. 2).
ELISA analysis of the cytokines in the human cell lines HaCaT (a) and HepG2 (b) exposed to Micrococcus sp. KUMAs15 for 4 h and 24 h. Culture media with PBS as the negative control and all values represent fold change induction of the cytokines relative to the PBS control. Data are the mean of three replications for each treatment regime with ± SD (p < 0.05)
Transcriptional analysis of cytokine genes
The RT-PCR analysis of HaCaT cells (Fig. 3) showed no significant upregulation for the IL-6 gene when exposed to KUMAs15. The transcriptional expression of the IL-8 gene decreased when exposed to KUMAs15 and the downregulation is not very marked, whereas a nominal level of upregulation was observed in the TNF-α cytokine gene in KUMAs15-exposed HaCaT cells. Overall, it appeared from the transcriptional study that KUMAs15 have not significantly induced the cytokine gene expression in HaCaT cells. The HepG2 cells show marked upregulation in IL-10 gene expression at the RNA level when incubated with KUMAs15 culture (Fig. 4). The upregulation of TNF-α gene expression is also evident in the treatment group but not as marked as that of IL-10 expression. The cytokines IL-12 and IL-8 show non-significant downregulation in KUMAs15-treated cells when compared to the PBS-treated negative control group. The observations at the RT-PCR analysis of RNA expression of cytokine genes in these cell lines indicate that KUMAs15 does not initiate an inflammatory reaction in these cell lines.
Transcriptional expression of the cytokine genes in the HaCaT cell line showing no significant difference between the control (PBS treated) and KUMAs15 co-cultured cells. β-actin was considered as the housekeeping gene with constitutive transcriptional expression. Densitometry was done and plotted with ± SD (p < 0.05)
Transcriptional expression of the cytokine genes in the HepG2 cell line showing no significant difference between the control (PBS treated) and KUMAs15 co-cultured cells. β-actin was considered as the housekeeping gene with constitutive transcriptional expression. Densitometry was done and plotted with ± SD (p < 0.05)
Translational analysis of cytokine genes
Western blotting analysis of the protein expression in HaCaT cells showed upregulation of IL-8 whereas significant downregulation was observed in IL-6 protein expression (Fig. 5). When exposed to KUMAs15, HaCaT cells showed no significant alteration in TNF-α protein expression. Thus, protein profiling of HaCaT cells confirms the pattern of cytokine gene expression evident from RT-PCR analysis. The HepG2 (Fig. 6) showed no significant alteration in protein expression for IL-8, IL-10, and TNF-α. The protein expression of IL-12 showed downregulation when HepG2 cells were exposed to KUMAs15, but the decrement was not very marked. Western blotting analysis confirms the expression pattern was evident from the transcriptional study.
Discussion
The eco-friendly microbe-mediated arsenic bioremediation demands suitable microbial candidates for successful field administration with optimum safety to the ecologically interacting organisms, most importantly humans. The essential criteria for any microbial candidate for sustainable heavy metal decontamination are the cytotoxic or immunogenic non-reactivity of the isolated microbial candidate with the human cells [22]. The present study dealt with the effect of isolated microbial strain Micrococcus sp. KUMAs15 on human keratinocytes, HaCaT, and liver epithelial cells, HepG2. The characterization of the isolated microbial strain, KUMAs15, has been earlier reported [12], which also established Micrococcus sp. KUMAs15 as a potential candidate for environmental decontamination of arsenic.
An initial step in understanding the effect of the microbial strain KUMAs15 on the human cells is to examine its cytotoxic or inflammatory effect on the cell lines, representative of human tissues. The cell lines were initially incubated with KUMAs15 followed by analysis of the change in cell viability and inflammatory responses. Following the cytotoxicity assay to ascertain cellular viability, cytokine assay was performed on two human cell lines namely human keratinocyte (HaCaT) and human liver epithelial cell line (HepG2) to ascertain the role of the isolated strain in these cells for finding the suitability of the isolated strain for sustainable microbial bioremediation.
Application of the bacterial isolate to decontaminate ambient arsenic contamination could be limited due to any possible health concern that may emerge during field application if the bacteria are pathogenic to humans [11]. To gain knowledge on toxicological or immunogenic reactions of KUMAs15 exposure, immune-toxicological parameters were studied after the exposure of the isolated microbial strain Micrococcus sp. KUMAs15 on human cell lines. The present study demonstrated that Micrococcus sp. KUMAs15 did not have any significant toxic effect on the viability of the human cells, when compared to the viability of the PBS-treated control cells assessed by the MTT assay (Fig. 1). The human cell lines were directly exposed to the isolates bacteria Micrococcus sp. KUMAs15 in culture condition and then removed by washing as well as antibiotic treatment. The bacterium is completely safe as confirmed by the assay as no increase in cellular apoptosis or necrosis was observed after exposure to the isolated strain. To maintain consistency, the cytokine profile of the cell line after exposure to the isolate was also measured by ELISA; the observation (Fig. 2) was further confirmed by gene expression profiling at the RNA and protein level by RT-PCR (Figs. 3 and 4) and western blotting (Figs. 5 and 6) respectively. The cytokine gene expression shows no alteration in TNF, IL-10, and IL-8 levels. This is due to the suppressive effect of IL-10 on pro-inflammatory cytokine responses [23, 24] in the HepG2 cell line, while the HaCaT cells showed no alteration in TNF expression earlier reported to be induced by GMCSF [25]. It is well evident that exposure to Micrococcus sp. KUMAs15 did not provoke a sustained elevation of any of the inflammatory cytokines in the human cell lines studied. The present observations bring us to the inference that arsenic-resistant Micrococcus sp. KUMAs15 seems to be inherently non-pathogenic to mammalian cells as it does not induce a robust immunogenic response in the human cell lines. The immunotoxicological feature of the isolated strain does not show any alteration in the cytokine profile in human cells in vitro. These results indicate that Micrococcus sp. KUMAs15 is a non-pathogenic strain and along with its potential could be used as a potential arsenic-decontaminating agent.
Conclusion
The cytotoxicity assay, cytokine assay, and gene expression studies of cytokine genes at the transcriptional and translational levels indicate the non-pathogenic nature of the Micrococcus sp. KUMAs15 on the human cell lines at culture conditions. Thus, the present work could be considered as an extension of the previous findings of our laboratory, indicating the extent of safety in applying the Micrococcus sp. KUMAs15 in fields, by establishing it as a non-pathogenic microbial candidate for sustainable As bioremediation.
Availability of data and materials
Not applicable
Abbreviations
- ANOVA:
-
Analysis of variance
- As:
-
Arsenic
- BLAST:
-
Basic Local Alignment Search Tool
- cDNA:
-
Complementary DNA
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- GMCSF:
-
Granulocyte-macrophage colony-stimulating factor
- IL:
-
Interleukin
- MEM:
-
Minimum essential medium
- PBS:
-
Phosphate buffer saline
- PCR:
-
Polymerase chain reaction
- RT-PCR:
-
Reverse transcriptase PCR
- TNF:
-
Tumor necrosis factor
References
Sen P, Biswas T (2013) Arsenic: the largest mass poisoning of a population in history. BMJ 346:f3625
Mandal BK, Suzuki KT (2002) Arsenic round the world: a review. Talanta 58:201–235
Oremland RS, Stolz JF (2005) Arsenic, microbes and contaminated aquifers. Trends Microbiol 13:45–49
Cavalca L, Zanchi R, Corsini A, Colombo M, Romagnoli C, Canzi E et al (2010) Arsenic-resistant bacteria associated with roots of the wild Cirsium arvense (L.) plant from an arsenic polluted soil, and screening of potential plant growth-promoting characteristics. Syst Appl Microbiol 33:154–164
Paul T, Mukherjee SK (2018) Exploration and intervention of geologically ancient microbial adaptation in the contemporary environmental arsenic bioremediation. In: Donati ER (ed) Heavy Metals in the environment: microorganisms and bioremediation. CRC Press, USA
Silver S (1996) Bacterial resistance to toxic metal ions - a review. Gene 179:9–19
Monchy S, Benotmane A, Janssen P, Vallaeys T, Taghavi S, van der Lelie D et al (2003) Ralstonia metallidurans, a bacterium specifically adapted to toxic metals: towards a catalogue of metal resistance genes. FEMS Microbiol Rev 27:385–410
Samanta A, Bera P, Khatun M, Sinha C, Pal P, Lalee A et al (2012) An investigation on heavy metal tolerance and antibiotic resistance properties of bacterial strain Bacillus sp. isolated from municipal waste. J Microbiol Biotechnol Res 2:178–189
Karthikeyan R, Kulakow PA (2003) Soil plant microbe interactions in phytoremediation. Adv Biochem Eng Biotechnol 78:51–74
McIntyre T (2003) Phytoremediation of heavy metals from soils. Adv Biochem Eng Biotechnol 78:97–123
Singh A, Mishra M, Tripathi P, Sachan S (2015) Resistance of heavy metals on some pathogenic bacterial species. Afr J Microbiol Res 9:1162–1164
Paul T, Chakraborty A, Islam E, Mukherjee SK (2018) Arsenic bioremediation potential of arsenite-oxidizing Micrococcus sp. KUMAs15 isolated from contaminated soil. Pedosphere 28:299–310
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Maidack BL, Olsen GJ, Larson N, Overbeek R, McCaughey MJ, Woese CR (1997) The RDP (Ribosomal Database Project). Nucleic Acids Res 25:109–111
Hu S, Lu JS, Jing CY (2012) A novel colorimetric method for field arsenic speciation analysis. J Environ Sci 24:1341–1346
Clifford D (1990) Ion exchange and inorganic adsorption. In: Pontius F (ed) Water Quality and Treatment. McGraw-Hill, New York
Rhine ED, Phelps CD, Young LY (2006) Anaerobic arsenite oxidation by novel denitrifying isolates. Environ Microbiol 8:899–908
Indian Council of Medical Research (2006) Ethical guidelines for biomedical research on human participants. Indian Council of Medical Research, New Delhi
Mogensen TH (2009) Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 22:240–273
Rio DC, MJr A, Hannon GJ, Nilsen TW (2010) Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb Protoc 6:1–3
Curfs JH, Meis JF, Hoogkamp-Korstanje JA (1997) A primer on cytokines: sources, receptors, effects, and inducers. Clin Microbiol Rev 10:742–780
Wall RJ, Shani M (2008) Are animal models as good as we think? Theriogenology 69:2–9
Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A (1991) IL-10 inhibits cytokine production by activated macrophages. J Immunol 147:3815–3822
Malefyt RDW, Abrams J, Bennett B, Figdor CG, de Vries JE (1991) Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med 174:1209–1220
Shi Y, Liu CH, Roberts AI, Das J, Xu G, Ren G et al (2006) Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don’t know. Cell Res 16:126–133
Acknowledgements
Not applicable
Funding
The study was supported by the financial grant received from the extramural project of the Department of Biotechnology (DBT) under the Government of India (No. BT/PR4693/BCE/8/894/2012). The abovementioned project supports all the experimentations and the stipend.
Author information
Authors and Affiliations
Contributions
TP carried out the entire study. TP and SKM participated in the design of the study and performed the statistical analysis. TP and SKM conceived the study, participated in its design and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study has been waived from ethical permissions according to the guidelines of the Indian Council of Medical Research (ICMR) for the biomedical research studies conducted in India.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Paul, T., Mukherjee, S.K. Induction of inflammatory response in human cell lines by arsenic-contaminated soil-isolated bacterium Micrococcus sp. KUMAs15. Egypt J Med Hum Genet 20, 2 (2019). https://doi.org/10.1186/s43042-019-0011-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-019-0011-8