Abstract
Rare earth elements (REE) are essential for the production of technological devices. However, their high demand and low availability, together with an increase in electronic waste generation, compel the development of efficient, economic and green methods for recovering these elements from electronic waste. In this work, a facile method for selective recovering of REE from Liquid Crystal Display (LCD) screen wastes, employing ultrasound assisted leaching is presented. The screen wastes were milled and sieved to pass through a − 325 mesh sieve (44 μm). The milled powder was subjected to ultrasound-assisted leaching in an aqueous medium, at room temperature (25 °C) and pH 6 for 60 min. Subsequently, a magnetic separation was applied to the leach residue. Inductively coupled plasma was employed to quantitatively analyze the composition of the LCD powders and determine the effectiveness of the extraction process. Scanning Electron Microscopy/Energy Dispersive X-Ray Spectroscopy allowed qualitative chemical analysis of the solid materials. The results show that the LCD screen wastes are formed, mainly, by amorphous oxides of Si, Fe, In, Sn and REE. The amount of Gadolinium (Gd) and Praseodymium (Pr) in the wastes were 93 and 24 mg kg− 1, respectively, which justifies their recovery. X-ray diffraction analysis of the magnetic portion of the leached residue, confirmed the presence of an amorphous phase together with crystalline metallic iron alloy. The magnetic behavior, obtained by Vibration Sample Magnetometry, helped to understand the nature of the residues. The formation of this metallic alloy is attributed to the effect of high power ultrasonic during the leach. It was confirmed that the magnetic residue concentrates and recovers 87 wt% of Gd and 85 wt% of Pr contained in the original material. Therefore, ultrasound-assisted leaching is a selective and facile method for recovering Gd and Pr from waste LCD.
Similar content being viewed by others
Introduction
Currently, electronic waste has become one of the major contributors to environmental pollution, mainly due to the politics of programmed obsolescence of many electronic devices over the past 20 yr [1]. Nowadays, the average life of a computer or a mobile phone is between 4 and 5 yr [2], considerably increasing the amount of electronic waste generated [3]. Since the end of the twentieth century, Liquid Crystal Display (LCD) screens have been used in various electronic products due to the advantages of light quality, small volume and low energy consumption; therefore, more than 700 million LCD panels have been produced worldwide in recent years. Considering the average lifespan of 3–8 yr of these panels, large quantities of LCD panels will be reaching their end-of-life in the coming years, tremendously contributing to the generation of electronic waste [4]. Despite the many disadvantages, these wastes contain important amounts of different elements with high commercial value [5, 6]. Among these, the rare earth elements (REE) [7] are considered critical raw materials [8], due to the difficulty of their separation, acquisition and the uncertainty of their disposition [9]. Recent studies have shown the quantities of REE in LCD screens are economically attractive for recovery, however, the proposed methods have focused mainly on the backlight or light-emitting diodes of the LCD screens [10, 11]. In contrast, this investigation studies the recovery of Gadolinium (Gd) and Praseodymium (Pr), among others, contained in the LCD panels, a subject that has not been reported. Furthermore, the importance of the recovery these REEs is implicit not only in their use in new technologies, but also in their current high prices, which are among the REEs with the greatest economic value [12].
The development of REE recovery strategies has been trending in recent years; most of the proposed processes employ inorganic acids such as HCl, H2SO4 or HNO3 for leaching [13,14,15], which can damage the environment and human health, if they are not adequately controlled. Additionally, more environmentally friendly reagents, such as sulfate-roasting followed by water leaching [16] and ionic liquids [17] have also been studied, to leach the REE. However, the chemicals and conditions employed in both proposals are costly and involve complicated downstream separation processes.
In the present investigation, the pyrophosphate ion (PPi) is used as a less hazardous alternative to inorganic acids since it has been employed as a selective ligand for the REE ions in other studies [18]. It is also employed in the preparation of medicines and the preservation of different foods, proving to be safe for the environment and for humans [19, 20]. A thermodynamic study, in the form of species distribution diagrams using the Hydra-Medusa software suite [21] was performed to determine the appropriate leaching conditions. In addition, ultrasound was employed as an enhancement method during the leach, since recent studies have shown that the use of ultrasound accelerates the dissolution of metals [22] and REEs, achieving nearly 100% in 1 to 3 h [23,24,25]. In sonochemistry, molecules undergo chemical reactions promoted by the application of ultrasound radiation (20 kHz–10 MHz) in solid–liquid systems; ultrasound enhances the diffusion of soluble species in the liquid phase and increases the rate of penetration into the solid principally by the cavitation effect, which leads to the creation of many microcracks on the solid surface. Furthermore, if the raw material is a powder, ultrasound energy can cause particle rupture, with a consequent increase in surface area available for reaction [26].
Hence, the present study explores the recovery of the REE compounds (Pr and Gd) from waste LCD screens by ultrasound-assisted leaching, using PPi. This proposal introduces a quick, easy and inexpensive method to recover these valuable elements from wastes.
Materials and methods
The LCD screens were collected from electronic wastes (televisions, cell phones, electronic tablets, laptops and cameras). The total weight of a waste LCD screen was not determined, since it varies according to the type of electronic device. The LCD screens were cleaned by separating components that were not required, such as polarized, adhesives, connectors, diffusive sheets, reflective sheet and, the plastic frame. Subsequently, the LCD were subjected to the leaching assisted by ultrasound process or sono-leaching, followed by a magnetic separation shown in Fig. 1.
First, 500 g of LCD screen wastes were milled for 30 min, using an automatic mortar grinder. The milled powder was sieved to pass through − 325 mesh sieve (44 μm), since the largest quantity of RE elements is recovered by leaching at this particle size [27]. The milling process to obtain the fine particle sizes consumes extra time and energy in the process; however, the use of inexpensive and safe reagents, compensate this expenditure, rendering the process economically feasible.
Subsequently, representative samples of 3 g were taken from the milled and screened powder, using the quartering method. The powder obtained was characterized by X-ray diffraction, XRD, (Equinox 2000 diffractometer), scanning electron microscopy with Energy-dispersive X-ray spectroscopy (SEM-EDS-Jeol, model IT 300) and, by vibration sample magnetometry, VSM (MicroSense EV7) at room temperature (20 °C) and a maximum magnetic field of ± 18 kOe.
In order to quantify the chemical composition of the raw materials, 3 g sample of LCD powders was digested in aqua regia (HCl:HNO3, 3:1) at 90 °C for 2 h. The obtained liquor was filtered. Aliquot of 1.5 mL of the liquor were diluted to 30 mL with deionized water, for their analysis by inductively coupled plasma (ICP-OES, Perkin Elmer, Optima 3000 XL). This process was performed on three different samples of the same material, in order to ensure the repetitively of the data obtained.
For the leaching tests, 3 g of the milled and sieved powder were immersed in a beaker with 150 mL of the solution of 0.05 M PPi (P2O74−) as leaching agent, using a liquid-to-solid ratio of 20 g L− 1. The solution pH was adjusted and maintained at 6, using 1 M sulfuric acid. These experimental conditions were selected in accordance with the results of previous studies [18]. The leaching solution was sonicated during the leaching time, using an Ultrasonic Homogenizer 300VT, equipped with a piezoelectric transducer at a frequency of 90 kHz and a solid titanium tip of 9.5 mm. The experiments were performed at a sonication output power of 120 W. The beaker was placed into an ice bucket, to guarantee that the temperature of the solution in all cases did not exceed room temperature (25 °C). The ultrasound was used to promote the exposure of the rare elements to the leaching agent, through the rupture of the particles, and to increase the rate of penetration into the solid by the cavitation effect [26]. The leaching solution was filtered, obtaining a liquor and a solid phase (leach residue). The leach liquor was characterized by ICP. The solid residue was magnetically separated, into non-magnetic and magnetic powders, after having been air-dried at 80 °C for 15 min. The magnetic and non-magnetic residues were characterized by XRD, SEM-EDS, VSM and ICP. The digestion procedures of the solid residues, for ICP analysis, is the same as that previously described for LCD powders.
Results and discussion
In Fig. 2, the XRD pattern of the milled LCD screen powder is presented. As may be observed, no specific diffraction peaks are exhibited, indicating an amorphous material. This result may be expected, since the main component of the LCD screens is silicon [28], combined with small amounts of different metallic oxides, such as indium, REE and tin (not detectable by XRD due to the detection limit of the diffractometer).
To confirm the presence of REE in the milled and sieved LCD powder, SEM-EDS qualitative elemental analyses were carried out. The results are shown in Fig. 3, where the qualitative chemical distributions of different elements are shown; silicon, aluminum, some REE, indium, tin and iron may be observed. In addition, all the REEs are uniformly distributed. As can be appreciated, the elements with the highest concentrations are silicon, aluminum and oxygen, probably as oxide compounds (SiO2 and Al2O3), whereas the REE are concentrated in the smallest particles. Moreover, the fine particle size ensures the homogeneity of the sample and the percentage of the REEs that can be recovered [29]. For this reason, the powder sieved at − 325 mesh (44 μm) was selected for the leaching study.
The results of the chemical analysis obtained by ICP-OES show the presence of rare earths, such as Pr (24 mg kg− 1), Gd (93 mg kg− 1), Er (477 mg kg− 1) and others elements, such as In (2422 mg kg− 1), Sn (835 mg kg− 1), Fe (2827 mg kg− 1) and Zn (9 mg kg− 1). According to the structural and chemical characterization (SEM-EDS and ICP), the LCD screen waste is composed of a mixture of oxides of Si, Al, Fe, and small amounts of oxides of Gd, In, Pr and Er. It is important to note that these materials are in sufficient quantities to justify their separation [30].
To select the adequate experimental conditions for selectively separating Gd and Pr from the other elements, a thermodynamic analysis using the Hydra-Medusa software [21] was performed; this analysis shows that Gd (III) forms a soluble species Gd2(P2O7)2+ with the PPi up to pH 8, with a log k (complex stability constant) value of 20.5. However, the stability constant for Pr (III) has not been reported. Considering that the log k values for the REE close to Pr are similar for the same type of complexes (Nd (III) = 20, Sm3+ = 20.2, Eu (III) = 20.3, Gd (III) = 20.5) [32], it is possible to infer that the behavior of this element with PPi is comparable. The reaction between the PPi ion and Gd is shown in Eq. (1):
In addition, the interaction between PPi ion and Fe (III) was considered, since it plays an important role in the proposed method, which is based on a final magnetic separation of the leaching residue. The Hydro-Medusa analysis confirms that Fe (III) forms a stable complex, Fe2(P2O7)− up to pH 8, with a log k of 22.2.
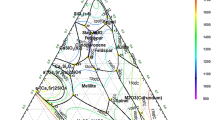
Therefore, based on theoretical analyses, under the experimental conditions selected, the PPi forms stable complexed with Gd (III), Pr (III) and Fe (III). Furthermore, these ions precipitate as hydroxides in alkaline solutions (above pH 8). Species distribution diagrams are constructed from the logarithm of the reaction equilibrium constant (k) of the reagents [31]. The speciation diagram for the Gd is shown in Fig. 4. This analysis helped to establish the adequate leaching conditions: pH values between 4 and 6, room temperature (25 °C), assisted by ultrasound to improve the dissolution process [25, 33].
After performing the ultrasonic-assisted leaching process for 60 min at room temperature, a solid residue was obtained (leached residue), which was analysed by XRD (Fig. 5). As can be appreciated in Fig. 5a, the leached residue consisted of an amorphous material, together with small amount of crystalline Fe, which is identified as a peak near to 2θ of 44°. Due to the presence of metallic iron, the residue was subjected to a magnetic separation, obtaining a magnetic and a non-magnetic solid. Both solids were independently analyzed by XRD (Fig. 5b and c). As can be observed, the non-magnetic residue (Fig. 5 b) shows an XRD pattern typical of an amorphous material, attributed to silica base material, which was not affected by the leaching. In contrast, the magnetic residue (Fig. 5c) is a crystalline iron matrix, probably with small amounts of other metals (Gd, Pr or similar elements), since a slight displacement of the diffraction peak is detected from its theoretical position at 2θ of 44 °. The three residues (combined leach, magnetic and non-magnetic), were qualitatively characterized by SEM, using back-scattered electrons. As can be observed, the powders are composed by irregular and polygonal particles. In addition, there are no differences in contrast in each residue, which indicates that the residues contain a homogenous distribution of atoms along the particles. However, comparing the different residues, the magnetic residue (Fig. 5c) appears brighter, which may be ascribed to the presence of compounds that contain atoms with greater atomic number, such as REE.
To characterize their magnetic behavior, the hysteresis loops of each residue were acquired and are presented in Fig. 6. In this figure, it can be observed that the magnetic residue presents a saturation magnetization of 120 emu g− 1, attributed to the presence of an iron alloy with undefined composition, in good agreement with the XRD pattern show in Fig. 5c. It is known that, pure iron shows a specific saturation magnetization near to 217 emu g− 1, therefore, the reduced magnetization value corresponds to iron, containing very low concentrations of materials that possesses slight magnetization, in accordance with the XRD patterns, since no other phases were detected.
The non-magnetic residue shows ferrimagnetic behavior, with a very low specific saturation magnetization of approximately 0.08 emu g− 1, attributed to the presence of small amounts of ferrimagnetic materials as oxides, although these was not observed in XRD pattern due to the detection limit of the analysis equipment.
In addition, the magnetic hysteresis loop of the combined leach residue shows ferrimagnetic behavior, with a specific saturation magnetization around 0.19 emu g− 1. This confirms mostly amorphous silica and aluminum oxides, together with small quantities of ferrimagnetic materials, as iron and RE metals and/or oxides.
The chemical composition of the leached liquor and the solid residues (magnetic and non-magnetic) was quantified by ICP. The results are shown in Table 1 as the percentages of the total element in the initial LCD powder in each of the following states: present in the leach liquor, remaining in the LCD powder (non-magnetic residue) or recovered in the magnetic residue. According to these results, the magnetic material (0.3 g) is composed mainly of Fe, Pr and Gd, corresponding to about 95, 87 and 85%, respectively, of the total amount of each element contained in the LCD screens; this represents an important concentration of these elements, with a higher recovery compared to conventional leaching [34]. On the other hand, approximately 99% of the In, 74% of the Sn and 84% of the Er remained in the non-magnetic solid (2.58 g). As for the leached liquor, it contained appreciable percentages of Er (12%), Sn (25%) and Zn (91%).
It is worth mentioning that when the leaching process is carried out without PPi, the separation of Gd and Pr was not achieved nor were these elements leached. On the other hand, when the leaching is performed without ultrasound, a magnetic residue is not produced; therefore, the ultrasound radiation promotes the selective separation of Gd and Pr from other REE, as magnetic materials and the PPi maintains the solubility of the REE.
As the magnetic residue showed a selective separation of Gd and Pr, together with iron, an elemental mapping was performed by SEM-EDS analysis, shown in Fig. 7. In this figure, the presence of a homogeneous distribution of Fe, Gd and Pr can be observed, confirming the concentration of these elements into the magnetic residue.
The formation of an iron base alloy containing REEs, as Gd and Pr, is an interesting result in itself, and it can be ascribed to the ultrasound effect during the leaching process. It is well-known that the ultrasound produces mechanical effects, such as micro jets and shock waves, which cause microscopic turbulence in the solution and high-speed collisions between the solids [35]. These effects are difficult to achieve with conventional mechanical agitation [26]. According to some authors [35, 36], sonochemistry or ultrasonic irradiation of water produces the free radicals H· and OH· that can combine to produce H2O2 [35]:
The presence of H2 and H2O2, promote chemical and physical effects since they can act as strong reducing agents, as follows:
In ultrasonic leaching, the formation of these agents promote an iron ion reduction from Fe3+ and/or Fe2+ to Fe0, as shown in Eq. (5), which could incorporate Gd (III) and Pr (III) into its crystal structure or they could be also reduced to metallic phases as an alloy. These solid products can be recovered by applying a magnetic field, obtaining a concentrated magnetic residue composed mainly of Fe, Gd and Pr, as was demonstrated previously. Therefore, the magnetic separation of the residue formed after the ultrasonic-assisted leach is a facile and economic method for concentrating Gd and Pr elements from LCD wastes.
Conclusions
LCD screen wastes were found to contain 93 and 24 mg kg− 1 of Gd and Pr, respectively. To retrieve these REEs, a facile method for selective concentrating of some REEs is proposed. In particular, Gd and Pr from LCD screen wastes can be effectively recovered by ultrasonic-assisted leaching, using pyrophosphate ion as complexing ligand. A retrieval of 85 and 87 wt% of Gd and Pr, respectively, was achieved, using an ultrasound-assisted leaching for 60 min at room temperature. The combination of ultrasound and leaching at room temperature showed positive impacts on enhancing the separation of REEs; substantial physicochemical changes occurred during the leaching assisted with ultrasound, including structural transformations, chemical radical formation, chemical reduction, and even, compound decomposition. Structural analysis and chemical decomposition, promoted by the formation of water radicals, could explain the effectiveness of the ultrasound leaching in improving the recovery of Gd and Pr from LCD screen waste. However, other valuable REEs, such as In and Er, remain in the non-magnetic solid residue.
Availability of data and materials
All data generated or analyzed during this study will be made available on request.
Change history
30 December 2020
The reference list of the original article were incorrectly formatted. The article has been updated to rectify the errors.
References
Sethurajan M, van Hullebusch ED, Fontana D, Akcil A, Deveci H, Batinic B, et al. Recent advances on hydrometallurgical recovery of critical and precious elements from end of life electronic wastes – a review. Crit Rev Env Sci Tec. 2019;49:212–275.
Kumar A, Holuszko M, Espinosa DCR. E-waste: an overview on generation, collection, legislation and recycling practices. Resour Conserv Recy. 2017;122:32–42.
Balde CP, Forti V, Gray V, Kuehr R, Stegmann P. United Nations University (UNU). The Global E-waste Monitor. Bonn, Geneva, Vienna: United Nations University, International Telecommunication Union, International Solid Waste Association; 2017.
Zhang LG, Chen Y, Xu ZM. Controllable formation of carbon fiber in pyrolysis process of liquid crystals from waste LCD panels and indium recovery by vacuum in situ reduction with carbon fiber. ACS Sustain Chem Eng. 2018;6:541–50.
Kanari N, Allain E, Shallari S, Diot F, Diliberto S, Patisson F, et al. Thermochemical route for extraction and recycling of critical, strategic and high value elements from by-products and end-of-life materials, Part I: treatment of a copper by-product in air atmosphere. Materials. 2019;12:1625.
Zhang LG, Xu ZM. Towards minimization of secondary wastes: element recycling to achieve future complete resource recycling of electronic wastes. Waste Manage. 2019;96:175–80.
Binnemans K, Jones PT, Muller T, Yurramendi L. Rare earths and the balance problem: how to deal with changing markets? J Sustain Metall. 2018;4:126–46.
Deloitte Sustainability, British Geological Survey, Bureau de Recherches Géologiques et Minières, Netherlands Organisation for Applied Scientific Research. Study on the Review of the List of Critical Raw Materials. Luxembourg. Luxembourg: Publications Office of the European Union; 2017.
Ganguli R, Cook DR. Rare earths: a review of the landscape. MRS Energy Sustain. 2018;5:E9.
Ruiz-Mercado GJ, Gonzalez MA, Smith RL, Meyer DE. A conceptual chemical process for the recycling of Ce, Eu, and Y from LED flat panel displays. Resour Conserv Recy. 2017;126:42–9.
Yang JX, Retegan T, Steenari BM, Ekberg C. Recovery of indium and yttrium from Flat Panel Display waste using solvent extraction. Sep Purif Technol. 2016;166:117–24.
Berlet CJ. Rare Earth Metals Toronto: MineralPrices.com; 2020.
Paulino JF, Neumann R, Afonso JC. Production of sodium and aluminum chemicals and recovery of rare earth elements after leaching cryolite from Pitinga mine (Amazonas - Brazil) with sulfuric acid. Hydrometallurgy. 2018;180:254–61.
Ribagnac P, Deblonde GJP, Blancher SB, Lengagne L, Donati L, Malimba C, et al. Leaching of niobium- and REE-bearing iron ores: significant reduction of H2SO4consumption using SO2and activated carbon. Sep Purif Technol. 2017;189:1–10.
Balinski A, Atanasova P, Wiche O, Kelly N, Reuter MA, Scharf C. Recovery of REEs, Zr(+Hf), Mn and Nb by H2SO4leaching of eudialyte concentrate. Hydrometallurgy. 2019;186:176–86.
Onal MAR, Binnemans K. Recovery of rare earths from waste cathode ray tube (CRT) phosphor powder by selective sulfation roasting and water leaching. Hydrometallurgy. 2019;183:60–70.
Yin XF, Tian XM, Wu YF, Zhang QJ, Wang W, Li B, et al. Recycling rare earth elements from waste cathode ray tube phosphors: experimental study and mechanism analysis. J Clean Prod. 2018;205:58–66.
Alvarado-Hernández L, Lapidus GT, González F. Recovery of rare earths from waste cathode ray tube (CRT) phosphor powder with organic and inorganic ligands. Waste Manage. 2019;95:53–8.
Dedinszki D, Szeri F, Kozák E, Pomozi V, Tökési N, Mezei TR, et al. Oral administration of pyrophosphate inhibits connective tissue calcification. EMBOMol Med. 2017;9:1463–70.
Cercamondi CI, Duchateau GSMJE, Harika RK, van den Berg R, Murray P, Koppenol WP, et al. Sodium pyrophosphate enhances iron bioavailability from bouillon cubes fortified with ferric pyrophosphate. Brit J Nutr. 2016;116:496–503.
PuigdomenechI. Medusa: Software for Chemical Equilibrium Calculations(Version 1) [Windows]. Stockholm: Royal Institute of Technology;2015.
Liu HL, Chen GH, Liu L, Yan MQ. Influence of ultrasound on the properties of dissolved organic matter with regards to proton and metal ion binding moieties. Water Res. 2018;145:279–86.
Allaedini G, Zhang P. Treatment of phosphoric acid sludge for rare earths recovery II: effect of sonication and flocculant solution temperature on settling rate. Sep Sci Technol. 2019;54:1842–52.
Diehl LO, Gatiboni TL, Mello PA, Muller EI, Duarte FA, Flores EMM. Ultrasound-assisted extraction of rare-earth elements from carbonatite rocks. Ultrason Sonochem. 2018;40:24–9.
Tanaka Y, Zhang QW, Saito F. Sonochemical recovery of metals from recording media. J Chem Eng Jpn. 2002;35:173–77.
Jiang F, Chen YQ, Ju SH, Zhu QY, Zhang LB, Peng JH, et al. Ultrasound-assisted leaching of cobalt and lithium from spent lithium-ion batteries. Ultrason Sonochem. 2018;48:88–95.
Peelman S, Kooijman D, Sietsma J, Yang Y. Hydrometallurgical recovery of rare earth elements from mine tailings and WEEE. J Sustain Metall. 2018;4:367–77.
Choi YS, Yun JU, Park SE. Flat panel display glass: current status and future. J Non-Cryst Solids. 2016;431:2–7.
Lin RH, Howard BH, Roth EA, Bank TL, Granite EJ, Soong Y. Enrichment of rare earth elements from coal and coal by-products by physical separations. Fuel. 2017;200:506–20.
Jakobsson LK, Kennedy MW, Aune RE, Tranell G. Recovery of rare earth elements from the ferrous fraction of electronic waste. In: Kirchain RE, Blanpain B, Meskers C, Olivetti E, Apelian D, Howarter J, et al., editors. REWAS 2016. Cham: Springer; 2016. p. 89–93.
NIST. Critically Selected Stability Constants of Metal Complexes: Version 8.0. Gaithersburg: National Institute of Standards and Technology; 2006.
Qian A, Zhang W, Shi C, Pan C, Giammar DE, Yuan SH, et al. Geochemical stability of dissolved Mn(III) in the presence of pyrophosphate as a model ligand: complexation and disproportionation. Environ Sci Technol. 2019;53:5768–77.
Yin SH, Pei JN, Jiang F, Li SW, Peng JH, Zhang LB, et al. Ultrasound-assisted leaching of rare earths from the weathered crust elution-deposited ore using magnesium sulfate without ammonia-nitrogen pollution. Ultrason Sonochem. 2018;41:156–62.
Cardoso CED, Almeida JC, Lopes CB, Trindade T, Vale C, Pereira E. Recovery of rare earth elements by carbon-based nanomaterials–a review. Nanomaterials-Basel. 2019;9:814-19.
Wood RJ, Lee J, Bussemaker MJ. A parametric review of sonochemistry: control and augmentation of sonochemical activity in aqueous solutions. Ultrason Sonochem. 2017;38:351–70.
Palomino RL, Miro AMB, Tenorio FN, De Jesus FS, Escobedo CAC, Ammar S. Sonochemical assisted synthesis of SrFe12O19 nanoparticles. Ultrason Sonochem. 2016;29:470–75.
Acknowledgements
A.D Toache-Pérez is grateful to CONACyT from Mexico, for the postgraduate scholarship granted to pursue her doctoral studies.
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
Conceptualization, G.T.L.L. and A.M.B.M.; methodology, A.D.T.P. and A.M.B.M.; software, A.D.T.P. and G.T.L.L.; validation, A.M.B.M., F.S.D. and G.T.L.L.; formal analysis, A.M.B.M. and G.T.L.L.; investigation, A.D.T.P.; resources, G.T.L.L. and A.M.B.M.; data curation, A.D.T.P.; writing original draft preparation, A.D.T.P. and A.M.B.M..; writing review and editing, A.M.B.M., G.T.L.L., F.S.D. and A.D.T.P.; visualization, A.D.T.P. and A.M.B.M.; supervision, G.T.L.L.; project administration, G.T.L.L. and A.M.B.M.; funding acquisition, G.T.L.L. and A.M.B.M. All authors have read and agreed to the published version of the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Toache-Pérez, A.D., Bolarín-Miró, A.M., Sánchez-De Jesús, F. et al. Facile method for the selective recovery of Gd and Pr from LCD screen wastes using ultrasound-assisted leaching. Sustain Environ Res 30, 20 (2020). https://doi.org/10.1186/s42834-020-00060-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42834-020-00060-w